-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
New pharmaceutical insights related to the pathways of PUFAs
Nové farmaceutické pohľady vzhľadom k metabolickým cestám polynenasýtených mastných kyselín
Acylový zvyšok mastnej kyseliny je hlavnou štrukturálnou zložkou prakticky všetkých lipidov, čím predstavuje jednu zo základných funkčných skupín týchto molekúl. Mastné kyseliny (FAs) sa vzájomne líšia dĺžkou reťazca, počtom násobných väzieb a pozíciou násobnej väzby v reťazci. Podľa počtu násobných väzieb v polynenasýtenej FA (PUFAs) možno rozlíšiť mononenasýtené FAs (MUFAs) a polynenasýtené FAs (PUFAs). V živých bunkách predstavujú PUFAs dominantný substrát pre tvorbu biologicky aktívnych zlúčenín – oktadekanoidov, eikosanoidov a dokosanoidov – klasifikovaných ako oxylipíny alebo PUFAnoidy. Predložená prehľadová práca sa sústreďuje len na skupinu PUFAnoidov, ktorých biologické účinky zahŕňajú “pozitívny efekt” pre bunku. Skupina omega-3 PUFAnoidov pozostáva z lipoxínov, resolvínov a protektínov. Všetky tieto biologicky aktívne lipidy sú prednostne formované metabolickou cestou prostredníctvom LOX. Predstavujú časť bunkových mechanizmov, ktoré prispievajú k odstráneniu zápalových buniek a k obnove integrity tkanív. Nový prístup k protizápalovým modelom je orientovaný na duálnu COX/LOX-inhibíciu a stimuláciu tvorby ochranných eikosanoidov a dokosanoidov, a na ich dôležitý terapeutický potenciál pri riadení molekulárnych mechanizmov v rámci chronických zápalových procesov.
Kľúčové slová:
polynenasýtené mastné kyseliny, lipoxíny, resolvíny, protektíny
Authors: Marek Obložinský; Mária Pekárová; Peter Hoffman; Lýdia Bezáková
Published in the journal: Čes. slov. Farm., 2012; 61, 139-143
Category: Přehledy a odborná sdělení
Summary
The fatty acyl structure represents the major lipid building block of practically all lipids and therefore is one of the most fundamental categories of these molecules. Fatty acids (FAs) differ particularly in their chain length, number of double bonds and position of the bonds in the chain. The number of double bonds in the unsaturated molecule of FA distinguishes monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs). In the living cell PUFAs represent the dominant substrates for the formation of biologically active compounds – octadecanoids, eicosanoids and docosanoids – classified as oxylipins or as PUFAnoids. The present review focuses only on the groups of PUFAnoids which biological activities comprise a “positive effect” for the cell. This group of omega-3 PUFAnoids consists of lipoxins, resolvins and protectins. All these biologically active lipids are formed mainly in the LOX-pathway. They are part of the cell mechanisms that contribute to the removal of inflammatory cells and restoration of tissue integrity. A new approach to an optimal anti-inflammatory model shows orientation to the dual COX/LOX-inhibition and the stimulation of the protective eicosanoids and docosanoids formation and its considerable therapeutic potential in managing of molecular mechanisms of chronic inflammatory processes.
Keywords:
polyunsaturated fatty acids, lipoxins, resolvins, protectinsPUFAs as precursors of biologically active lipids
Fatty acids (FAs) are aliphatic monocarboxylic acids bound through ester linkages in simple lipids (mono-, di, triacyl - glycerols), respectively in complex lipids (phospholipids, sphingolipids). The fatty acyl structure represents the major lipid building block of lipids and therefore is one of the most fundamental categories of biological lipids. FAs differ particularly in their chain length, number of double bonds, a position of the bonds in the chain and stereospecific location of hydrogen atoms around these bonds. FAs can be categorized into groups according to the length of the aliphatic chain, including the short-chain FAs (SCFAs), medium chain FAs (MCFAs), long-chain FAs (LCFAs) and very long chain FAs (VLCFAs). The number of double bonds in the unsaturated molecule of FA distinguishes monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs). An overview of the most important PUFAs (occurring in all kingdoms of life) based on the location of the last double bond counted from the methyl end of FA (omega) is shown in Table 11, 2, 3).
Tab. 1. Overview of the most important C<sub>18</sub>-,C<sub>20</sub>-, C<sub>22</sub>- and C<sub>24</sub>- PUFAs 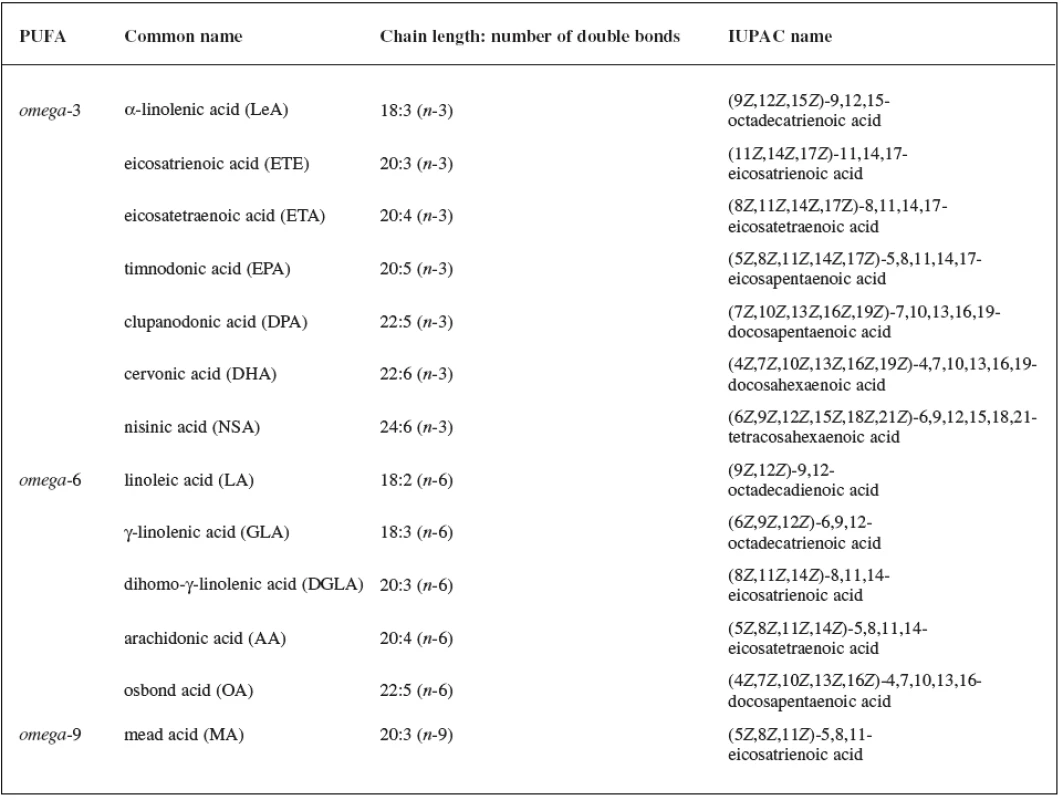
In the living cell PUFAs are not predetermined for energy storage, but they represent the dominant substrates for the formation of biologically active compounds. In plant tissues for the primary substrates stand the 18-carbon PUFAs (especially LA, LeA), from which in several pathways biologically active compounds (octadeconoids) are formed4). In mammals, the dominant substrates represent the 20-carbon PUFAs (mainly AA, EPA and DGLA), whose catalytic conversion by dioxygenases [cyclooxygenase (COX), lipoxygenase (LOX)] and monooxygenase [cytochrom P450 epoxygenase (CYP)] leads to the formation of different groups of eicosanoids5). But, also the 22-carbon PUFAs can play a role in the cascades of polyunsaturated fatty acid (in mammals mainly DPA) – in these pathways docosanoids could be formed6). Octadecanoids, eicosanoids and docosanoids are biologically active lipids that could be classified as oxylipins (due to the dominant oxidative transformation of lipid substrates) or as PUFAnoids (due to the corresponding polyunsaturated FA used as the precursor molecule in the beginning of the pathways).
PUFAnoids with “positive functions” and their biological activities
In mammals, the functions of phospholipase A2 (PLA2), COX, LOX, CYP and the items of information about the formation of corresponding prostaglandins, prostacyclins, tromboxanes and leukotrienes (mainly in the pathway of AA) are well known. Eicosanoids derived from AA represent a large group of biologically active compounds that play an important role in the “keeping up processes” of the animal cell homeostasis (eicosanoids are systemic regulators and universal modulators). But, their increased production is associated with inflammatory processes, fever, allergic reactions and immune response7). In the present review we will focus only on the groups of PUFAnoids whose biological activities comprise a “positive effect” for the cell. This group of omega-3 PUFAnoids (labelled as novel eicosanoids/docosanoids) consists of lipoxins, resolvins and protectins. All these biologically active lipids are formed mainly in the LOX-pathway.
Leukotrienes (especially LTB4) and prostaglandins (PGE2 and PGD2) derived from AA are important in the early stages of the inflammatory process. As tissues return to health, resolvins and protectins, together with lipoxins, promote resolution of the inflammation through the removal of the leukocytes together with cellular debris, ideally without leaving remnants of the host defences or of the invading microorganisms or other inflammatory initiators. Resolvins and protectins are part of the molecular mechanisms that contribute to the removal of inflammatory cells and restoration of tissue integrity once the need for the inflammatory response is over, i.e. they actively assist in the resolution of inflammation, once thought to be a passive process8, 9).
Lipoxins
Lipoxins could be formed by three biosynthetic pathways. The first is through 5-LOX and 12-LOX, the second through 15-LOX and the third is based on the interaction of 5-LOX products with acetylated COX-2, which generates epi-lipoxins (they are also called “aspirin-triggered lipoxins”, because COX-2 is acetylated by acetylsalicylic acid)10, 11). Enzymes of the lipoxin biosynthesis were found in leukocytes and platelets of mammals, but also in other cells of vertebrates and invertebrates. Today, the structure is best known for two lipoxins – lipoxin A4 (LXA4) and lipoxin B4 (LXB4). LXA4 induces rapid arteriolar dilation and may act as an antagonist to vasoconstriction induced by LTD4. LXA4 also blocks LTB4-induced chemotaxis of neutrophils, which suggests that it has an anti-inflammatory potential. LXA4 and LXB4 inhibit cytotoxic activity of NK-cells. They also act as antagonists in the bronchoconstriction process induced by leukotriene C4 (LTC4) and leukotriene D4 (LTD4), so they may modulate the vasoconstriction effects of leukotrienes and have anti-inflammatory properties. In general, LXA4 and LXB4 have an anti-inflammatory potential, they could regulate the termination of the inflammation process and could stimulate the renewal and restructuralization of damaged tissue12, 13).
Resolvins, protectins
The resolvins and protectins possess potent anti-inflammatory and immunoregulatory actions at concentrations in the nanomolar and picomolar ranges. The term “resolvins” or “resolution-phase interaction products” was used, because these PUFAnoids were first encountered in resolving inflammatory exudates. Compounds derived from EPA are designated as resolvins of the E series, while those formed from the precursor DHA are denoted as either resolvins or protectins (neuroprotectins) of the D series14).
18R-resolvins: In vascular endothelial cells derived from blood vessels, acetylated COX-2 introduces an 18R-hydroperoxy-group into the molecule of EPA. The product is reduced to the corresponding hydroxy compound before a 5S-hydroperoxy group is introduced into the molecule by the action of 5-LOX. A further reduction step produces 15S,18R-dihydroxy-EPA or resolvin E2 (RvE2). Alternatively, the 5S-hydroperoxy, 18R-hydroxy-EPA intermediate is converted to a 5,6-epoxy fatty acid in polymorphonuclear neutrophils in humans and eventually to 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z, 16E-EPA (resolvin E1, RvE1) by an enzyme required for the biosynthesis of leukotrienes in leukocytes8).
17S-resolvins: In another pathway 15-LOX generates 17S-hydroxy-DHA as the initial product. This is converted to 7S-hydroperoxy,17S-hydroxy-DHA by the action of a 5-LOX, and thence via epoxy intermediates to resolvin D1 (RvD1, 7S,8R,17S-trihydroxydocosa-4Z,9E,11E, 13Z,15E,19Z-hexaenoic acid) and epimeric resolvin D2 (RvD2, 7S,16R,17S-trihydroxydocosa-4Z,8E,10Z,12E, 14E,19Z-hexaenoic acid), i.e. all contain a 17S-hydroxyl group. A further LOX-generated intermediate from 17S-hydroxy-DHA, i.e. 4S-hydroperoxy, 17S-hydroxy-DHA, is transformed via an epoxide to resolvins D3 (RvD3) and D4 (RvD4)8).
Protectins: The LOX product 17S-hydroperoxy-DHA is converted first to a 16(17)-epoxide and then to the 10,17-dihydroxydocosatriene (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid), denoted as 10R,17S-DT or protectin D1 (PD1). Synthesis of PD1 is induced as a response to oxidative stress and/or activation of neurotrophils. Its highly stereospecific structure is essential for biological activity15).
During inflammation, polymorphonuclear neutrophils are produced which have generally beneficial effects in countering disease, but in the longer term or malfunctioning they may eventually cause trauma and tissue damage through infiltration into tissues. The resolvins, as well as the lipoxins, appear to have an important role in regulating and indeed inhibiting these harmful effects. In so doing they oppose the effects of some of the pro-inflammatory prostanoids. For example, nanomolar concentrations of RvE1 dramatically reduce dermal inflammation, peritonitis, dendritic cell migration and interleukin production. Similarly, RvD2 has extremely potent regulatory actions on neutrophil trafficking in the picogram range in vivo by stimulating resolution and enhancing innate host defense mechanisms via a specific receptor16, 28).
Protectins appear to operate in the same way as the resolvins in brain tissue. Thus, PD1 has anti-inflammatory effects and protects retinal epithelial cells from apoptosis induced by oxidative stress. In addition, it has protective effects in animal models of stroke and of Alzheimer’s disease. Amongst its activities in non-neuronal tissues, it promotes apoptosis of T-cells and it has beneficial effects towards asthma in nanogram amounts. It is evident that such compounds and their metabolism have a considerable potential for therapeutic intervention in acute inflammation or chronic inflammatory disease. They may also mitigate the affects of sepsis17).
Pharmaceutical influencing of PUFAs-pathways
Related to the information mentioned in previous chapters, the products formed by catalytic activity of COX-, LOX - and CYP-pathways show in mammals a number of diverse effects. In the past a lot of attention was devoted to develop PLA2 - or COX-inhibitors of arachidonic acid pathway. The activity and formation of the PLA2 - and COX-enzymes in the AA-pathway is very good described18), whereby the use of molecules inhibiting their catalytic activity is also well established (primarily in the groups of steroidal and non-steroidal anti-inflammatory drugs).
However, a new approach to an optimal anti-inflammatory model shows orientation to different areas. The first one is the dual COX/LOX-inhibition (which is sequentially implicated in some therapeutical models), the second one is the stimulation of the protective eicosanoids and docosanoids formation (which could be discussed today only in theoretical level).
Over the past decade many pharmaceutical companies have developed molecules that inhibit 5-LOX and other enzymes involved in the progression of the inflammatory process. The dual COX/LOX-inhibition seems to be quite effective, because both pathways are interlinked – the inhibition of cyclizing COX-pathway leads to increased preference of linearizing LOX-pathway19). Although leukotrienes generated by 5-LOX play a crucial role in the inflammatory process20), all related studies indicate that the inhibition of 5-LOX represents a non-effective therapeutical model of inflammatory diseases. Based on these results it can be assumed that the simultaneous inhibition of prostanoids and leukotrienes has a synergic effect and represents an optimal anti-inflammatory activity21). Moreover, the dual and specific inhibition COX/5-LOX does not block the activity of 12-LOX - and 15-LOX-isoforms, which contribute to the synthesis of biologically active lipoxins with anti-inflammatory properties22). Drugs capable of inhibition of both COXs (COX-1 and COX-2) and 5 LOX were prepared to retain the activity of non-steroidal anti-inflammatory drugs (NSAIDs), but to miss their side effects. Conventional NSAIDs act mainly through the inhibition of the inflammatory COX-isoform. One of their major side effects is the reduction of gastroprotective prostaglandins production by increasing of the gastrodamaging and bronchoconstricting leukotrienes level. That is why it is advantageous to have a molecule with both activities, because prostaglandins enhance leukotriene-mediated inflammation. Currently, different classes of dual COX/5-LOX-inhibitors are reported in the literature. One of the first dual COX/LOX inhibitors was a modified NSAID – tepoxalin. It inhibits the synthesis of PGE2 and LTB4 in the synovial liquid and shows no significant ulcerogenic effect. Today it is approved for veterinary use and is primarily used to reduce inflammation and as a relief of pain caused by musculoskeletal disorders23). Licofelone is a pyrolizine derivate and substrate analogue of AA. This molecule shows an equilibrating dual inhibitory activity against COX and LOX. Also, it possesses anti-inflammatory, analgetic, antipyretic, anti-asthmatic and antiplatelet properties. Licofelone almost abolished 5-LOX activity by inhibiting LTB4 generation in neutrophils and prevented platelet TXB2 production from whole blood. It reduces neointimal formation and inflammation in an atherosclerotic model more markedly than the selective COX-2 inhibitor rofecoxib. This effect, together with the antiplatelet activity of licofelone, suggests that this drug may have a favorable cardiovascular profile24). Dual COX/LOX-inhibitors represent promising class of drugs, because they could be potentially used in the following areas. COX and LOX together showed a crosslink between PUFAs and carcinogenesis, including, e.g., colon, pancreatic, breast, lung, skin and liver carcinomas25). Another promising therapeutic indication may be age-related degenerative diseases (Alzheimer’s disease, Parkinson’s disease), such as inflammation of the central nervous system in connection to the oxidative stress26). Dual COX/LOX-inhibitors may also help to manage the major side effects of non-selective COX-inhibitors (e.g. long term medication of acetylsalicylic acid at low dose) – renal and abdominal malfunctions associated with blocking of TXA2 production in platelets and non-influenced formation of PGI2 27).
The stimulation of endogenous cell reparation mechanisms represents a new approach in the study of the potential influence of PUFA-pathways. This hypothesis is based on the positive effects of omega-3 PUFAnoids formed from EPA (EPA-eicosanoids) and omega-3 PUFAnoids formed from DHA (DHA-docosanoids), whose biological activities were described in the previous chapter. Selective activation of these pathways could start the endogenous reparative mechanisms of the cell, particularly in the case of chronic inflammatory processes. Research in this area, however, is situated only in the early stages. But, it is evident that these PUFAnoids or their synthetic analogues have considerable therapeutic potential in managing chronic inflammatory diseases, including arthritis, cardiovascular disease, asthma and even cancer. From a nutritional or health standpoint, it has been suggested that dietary supplements of the precursor omega-3 FA may ameliorate the clinical symptoms of many inflammatory disorders by regulating the time course of resolution via the production of resolvins and protectins8, 9).
Conflict of interest: none.
doc. PharmDr. Marek Obložinský, PhD.; M. Pekárová; P. Hoffman; L. Bezáková
Katedra bunkovej a molekulárnej biológie liečiv
Univerzita Komenského v Bratislave, Farmaceutická fakulta
Kalinčiakova 8, 832 32 Bratislava, Slovenská republika
e-mail: oblozinsky@fpharm.uniba.sk
Zdroje
1. Lobb K., Chow Ch. K. Fatty acid classification and nomenclature. In Chow, Ch.K. ed. Fatty acids in foods and their health implications, 3rd ed. Boca Raton: CRC Press 2007.
2. Fahy E., Subramaniam S., Brown H. A., Glass Ch. K., Merrill A. H. Jr., , Murphy R. C., Raetz Ch. R. H., Russell D. W., Seyama Y., Shaw W., Shimizu T., Spener F., van Meer G., van Nieuwenhze M. S., White S. H., Witztum J. L., Dennis E. A. A comprehensive classification system for lipids. J. Lipid Res. 2005; 46, 839–862.
3. Cunnane S. C. Problems with essential fatty acids time for a new paradigm? Prog. Lipid Res. 2003; 42, 544–568.
4. Schaller F. Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J. Exp. Bot. 2001; 52, 11–23.
5. Kulkarni A. P. Lipoxygenase – a versatile biocatalyst for biotransformation of endobiotics and xenobiotics. Cell. Mol. Life Sci. 2001; 58, 1805–1825.
6. Mukherjee P. K., Chawla A., Loayza M. S., Bazan N. G. Docosanoids are multifunctional regulators of neural cell integrity and fate significance in aging and disease. Prostaglandins Leukot. Essent. Fatty Acids 2007; 77, 233–238.
7. Scher J. U., Pillinger M. H. 15d-PGJ2. The anti-inflammatory prostaglandin? Clin. Immunol. 2005; 114, 100–109.
8. Christie W. W. Resolvins and protectins chemistry and biology. (18. 4. 2012).
9. Kohli P., Levy B. D. Resolvins and protectins mediating solutions to inflammation. Brit. J. Pharm. 2009; 158, 960–971.
10. Chiang N., Bermudez E. A., Ridker P. M., Hurwitz S., Serhan C. N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 2004; 101, 15178–15183.
11. Romano M. Lipid mediators lipoxin and aspirin-triggered 15-epi-lipoxins. Inflamm. Allergy Drug Targets 2006; 5, 81–90.
12. Mirossay L., Mojžiš J. a kol. Základná farmakológia a farmakoterapia, Košice: Equilibria, 2006.
13. Ferenčík M., Škárka B., Novák M., Turecký L. Biochémia, Bratislava: Slovak Academic Press, 2000.
14. Serhan C. N., Petasis, N. A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011; 111, 5922–5943.
15. Weylandt K. H., Chiu C. Y., Gomolka B., Waechter S. F., Wiedenmann B. Omega-3 fatty acids and their lipid mediators Towards an understanding of resolvin and protectin formation. Prostagl. Other Lipid Med. 2012; 97, 73–82.
16. Bannenberg G., Serhan C. N. Specialized pro-resolving lipid mediators in the inflammatory response An update. Biochim. Biophys. Acta 2010; 1801, 1260–1273.
17. Niemoller T. D., Bazan N. G. Docosahexaenoic acid neurolipidomics. Prostagl. Other Lipid Med. 2010; 91, 85–89.
18. Seeds M. C., Bass D. A. Regulation and metabolism of arachidonic acid. Clin. Rev. Allergy Immunol. 1999; 17, 5–26.
19. Gambaro G. Strategies to safely interfere with prostanoid activity while avoiding adverse renal effects could COX-2 and COX-LOX dual inhibition be the answer? Nephrol. Dial. Transplant. 2002; 19, 1159–1116.
20. Sala A., Zarini S., Bolla M. Leukotrienes lipid bioeffectors of inflammatory reactions. Biochemistry (Mosc.) 1998; 63, 84–92.
21. Martel-Pelletier J., Lajeunesse D., Reboul P., Pelletier J. P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective nonsteroidal anti-inflammatory drugs. Ann. Rheum. Dis. 2003; 62, 501–509.
22. Serhan C. N., Levy B. D., Clish C. B., Gronert K., Chiang N. Lipoxins, aspirin-triggered 15-epi-lipoxin stable analogs and their receptors in antiinflammation a window for therapeutic opportunity. Ernst Schering Res. Found. Workshop 2000; 31, 143–185.
23. Leone S., Ottani A., Bertolini A. Dual acting anti-inflammatory drugs. Curr. Top. Med. Chem. 2007; 7, 265–275.
24. Vidal C., Gómez-Hernández A., Sánchez-Galán E., González A., Ortega L., Gómez-Gerique J. A., TuĖón J., Egido J. Licofelone, a balanced inhibitor of cyclooxygenase and 5‑lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis. J. Pharmacol. Exp. Therap. 2007; 320, 108–116.
25. Fischer S. M., Klein R. D. Lipoxygenases as targets for cancer prevention. In Kelloff, G.J., Hawk, E.T., Sigman, C.C. eds. Cancer chemoprevention Promising cancer chemopreventive agents. New Jersey: Humana Press, 2004.
26. Kramer B. C., Yabut J. A., Cheong J., Jnobaptiste R., Robakis T., Olanow C. E., Mytilineou C. Toxicity of glutathione depletion in mesencephalic cultures a role for arachidonic acid and its lipoxygenase metabolites. Eur. J. Neurosci. 2004; 19, 280–286.
27. de Gatetano G., Donati M. B., Cerletti C. Prevention of thrombosis and vascular inflamation benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol. Sci. 2003; 24, 245–252.
28. Bannenberg G., Arita M., Serhan C. N. Endogenous receptor agonists resolving inflammation. Sci. World J. 2007; 7, 1440–1462.
Štítky
Farmácia Farmakológia
Článok vyšiel v časopiseČeská a slovenská farmacie

2012 Číslo 4-
Všetky články tohto čísla
- New pharmaceutical insights related to the pathways of PUFAs
- Mitochondrial enzyme ABAD and its role in the development and treatment of Alzheimer’s disease
- Where does the development of new antituberculotics aim at?
-
Standard prescriptions for the formulation of medicinal preparations in pharmacies V
The collection Dermatologische Magistralrezepturen der Schweiz - Magistral prepared lidocaine-gel for topical aplication on skin
- Formulation of salvia-containing gels
-
Our medicinal preparations in the mid-19th century
Part I – Introduction and chemical preparations -
Our medicinal preparations in the mid-19th century
Part II – galenical preparations -
XXXIV. pracovní dny Radiofarmaceutické sekce České společnosti nukleární medicíny ČLS JEP
Mladá Boleslav, 13.–15. června 2012 - Prof. RNDr. Bohumil Sikyta, DrSc. jubiluje
- PharmDr. Jozef Német, jubiluje
- Česká a slovenská farmacie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Magistral prepared lidocaine-gel for topical aplication on skin
-
Standard prescriptions for the formulation of medicinal preparations in pharmacies V
The collection Dermatologische Magistralrezepturen der Schweiz -
Our medicinal preparations in the mid-19th century
Part II – galenical preparations -
Our medicinal preparations in the mid-19th century
Part I – Introduction and chemical preparations
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



