-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Eosinophilic enteritis – case report of a rare manifestation and review of updates
Eozinofilní enteritida – kazuistika vzácné manifestace onemocnění a stručný přehled novinek
Souhrn: Eozinofilní enteritida je jednou z eozinofilních gastrointestinálních poruch charakterizovaných nespecifickými klinickými příznaky. Při stanovení dia gnózy hraje zásadní roli histopatologický průkaz husté eozinofilní infiltrace. Bohužel dosud nejsou jednoznačně stanoveny její mezní hodnoty. Léčba je založena na dodržování eliminační diety a podávání kortikosteroidů. Autoři zde uvádějí případ 14letého chlapce trpícího náhle vzniklou bolestí břicha. Vyšetření počítačovou tomografií odhalilo zesílení duodenální stěny s infiltrací přesahující na omentum a žlučník. Histopatologické vyšetření vzorků odebraných laparoskopicky prokázalo eozinofilní infiltraci a fibrózu stěny žlučníku a dvanáctníku. Následné endoskopické vyšetření zažívacího traktu prokázalo rozsáhlé postižení eozinofilním zánětem (ezofagitida, dvanáctníkový vřed a proktitida). Pacientovi byla doporučena eliminační dieta a kortikoidy.
Klíčová slova:
enteritida – eozinofily – dvanáctníkový vřed – cholecystitida – eozinofilní gastroenteritida
Authors: Hloušková E. 1; Bajerová K. 1; Pecl J. 1; Pinkasová T. 1; Jabandžiev P. 1,2; Ježová M. 3; Tůma J. 4
Authors place of work: Department of Paediatrics, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Central European Institute of Technology, Masaryk University, Brno, Czech Republic 2; Department of Pathology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Paediatric Surgery, Orthopaedics and Traumatology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 4
Published in the journal: Gastroent Hepatol 2020; 74(6): 492-496
Category: Kazuistika
doi: https://doi.org/10.48095/ccgh2020492Summary
Summary: Eosinophilic enteritis is one of the eosinophilic gastrointestinal disorders characterised by various clinical symptoms. Histopathologic proof of dense eosinophilic infiltration is the cornerstone for the dia gnosis. There is no consensus on how dense the infiltration should be, and cut-off values of the eosinophilic count in HPF have not been defined yet. Therapy for eosinophilic enteritis is based on an elimination diet and corticosteroid treatment. Herein the authors report a case of 14-year-old boy who suffered from acute abdominal pain. Computer tomography of the abdomen revealed a thickening of the duodenal wall mimicking a tumour, which seemed to infiltrate the gallbladder and omentum. Histopathological examination of samples taken during laparoscopy showed eosinophilic infiltration and sclerotic changes of the gallbladder and duodenum. Follow-up endoscopy proved extensive eosinophilic infiltration of the gastrointestinal tract: eosinophilic esophagitis, duodenal ulcer with dense eosinophilic presence and eosinophilic proctitis. The patient recovered after dietary restrictions and prednisone.
Keywords:
eosinophilic enteritis – duodenal ulcer – eosinophilic cholecystitis – cholecystitida – eozinofily
Introduction
Eosinophilic gastrointestinal disorders (EGID) are a group of entities characterised by mucosal infiltration of eosinophils in the absence of other causes for eosinophilia [1]. Eosinophilic enteritis (EE) rarely, if ever, exists as a solitary form [2]. The disease presents with various gastrointestinal symptoms, such as abdominal pain, dyspepsia, malabsorption, nausea, obstruction and diarrhoea. Clinical manifestation depends on the location of the eosinophilic infiltration in different layers of the intestinal wall (mucosal, muscular and serosal) [3]. The overall prevalence of EE is unknown. In 2017 Mansoor et al. published a population-based study where the prevalence of eosinophilic gastroenteritis was determined to be 5.1/100,000 [4]. The pathophysiology of EGID is still unclear. It seems that food and aeroallergens play an essential role. An inflammatory response is triggered by Th2 cytokines, IL-5 and IL-13, IL-15, IL-18 chemokines and eotaxins [5,6]. The endoscopic appearance of EGID is not specific. The combination of serious clinical suspicion and histopathologically proven dense eosinophilic infiltration of the intestine is the cornerstone of establishing a dia gnosis of EE. Unfortunately eosinophilic infiltration of the gastrointestinal wall is common in all parts except the oesophagus [7]. Furthermore, we can find eosinophils in mucosal specimens in many inflammatory diseases such as coeliac, inflammatory bowel disease (IBD), parasitic infections, lymphomas, connective tissue diseases etc.
The threshold of mucosal eosinophilic gastroenteritis is set to be up to 20/HPF. The presence of intraepithelial eosinophils and eosinophils in Peyer‘s patches favour the dia gnose of EE [8,9]. Abdominal surgery or laparoscopy is required in the case of the muscular or serosal form of EE to obtain specimens for histopathological examination. The treatment of EE is based on an empiric approach, including dietary treatment, corticosteroid therapy and other drugs. Nowadays dietary treatment is based on the results of food allergy tests or the so-called six-food elimination diet, i.e. the elimination of the six most common food allergens (cow‘s milk, eggs, whey, soy, fish and nuts). Corticosteroids are the mainstay of the treatment. Prednisone acts by inducing eosinophil apoptosis and inhibiting chemotaxis [10]. Oral administration of 1 mg/kg/day of prednisone is recommended. In 1–2 weeks after the beginning of the treatment one typically achieves relief from symptoms. In case reports and small case-series, budesonide is reported to be an effective treatment too. Its high metabolism rate leads to a lower risk of long-term treatment side effects. We can often see relapses during or after the withdrawal of corticosteroids. We can consider leukotriene receptor antagonist montelukast a steroid-spearing long-term treatment option. Patients with GI obstruction or perforation need to undergo surgery.
Case report
A 14-year-old boy presented in the emergency room with diffuse abdominal pain lasting one day. The patient denied having diarrhoea, vomiting or fever. His father suffered from hairy cell leukaemia. The patient‘s medical history included one-week hospitalisation for otitis media. He had had no medication to date and reported no allergies. He presented with specific eating manners previously: he was exclusively breastfed for one year because of his refusal to ingest solid foods, later he preferred eating main and side dishes separately. The physical examination showed mild abdominal distension; laboratory analysis showed eosinophilia (0.8 × 109/l), an elevation of IgE (579 kU/l), uric acid (406 mmol/l) and alpha-fetoprotein (476 kU/l).
The serological inflammatory markers and liver enzymes were within the normal range. An abdominal computed tomography, as well as magnetic resonance imaging, revealed thickening of the duodenal wall, which expanded to the gallbladder and splenomegaly (Fig. 1). The oesophagogastroduodenoscopy showed modest deformation of the entrance to the second part of the duodenum. The mucosa appeared normal. The histopathological report proved increased eosinophilic infiltration in one of the mucosal samples from the duodenum. The following endoscopic sonography demonstrated thickening of the duodenal wall with infiltration of the gallbladder and omentum. During the period of examination, the patient presented with temporary and mild abdominal pain. The findings mentioned above gave suspicion of even an oncological dia gnosis. Our patient underwent video laparoscopy, which uncovered mild ascites and extensive inflammation of the gallbladder, which was fast retracted to the duodenal wall and omentum. Histopathological examination of the specimens obtained during surgery verified inflammation without extensive content of eosinophils both the intestine and ascites. We still missed the final dia gnosis, and this was the reason for a second-look cholecystectomy and full-thickness bio psy of the duodenal wall. Finally, dense eosinophilic infiltration of the duodenum and gallbladder serosa was proved microscopically. The presence of Charcot-Leyden crystals in the submucosal part of the duodenal wall supported the dia gnosis of EE. Subsequent complex gastrointestinal endoscopy discovered a large ulcer in the duodenum, probably in the place of the previous incision (Fig. 2), and the pathologist confirmed significant eosinophilic infiltration of the oesophagus, duodenum and rectum with presence of Charcot-Leyden crystals (Fig. 3, 4). We performed prick-to-prick tests and we recommended the elimination diet according to its results. To relieve the inflammation quickly, we decided to use prednisone 1mg/kg/day for four weeks and then to reduce the dose and withdraw gradually within two months. The symptoms in our patient disappeared within a couple of days of treatment. Follow-up endoscopy after three months of therapy confirmed healing of the duodenal ulcer (Fig. 5). Histologically, the absence of eosinophils in the oesophagus and a decrease in the eosinophil count in the colon with maximum 40/HPF was seen. The patient is continuing with the diet and is currently under budesonide medication.
Fig. 1. MRI – marked thickening of the duodenal wall with retraction to the gallbladder.
Obr. 1. MR – zesílení stěny duodena, které je retrahováno ke žlučníku.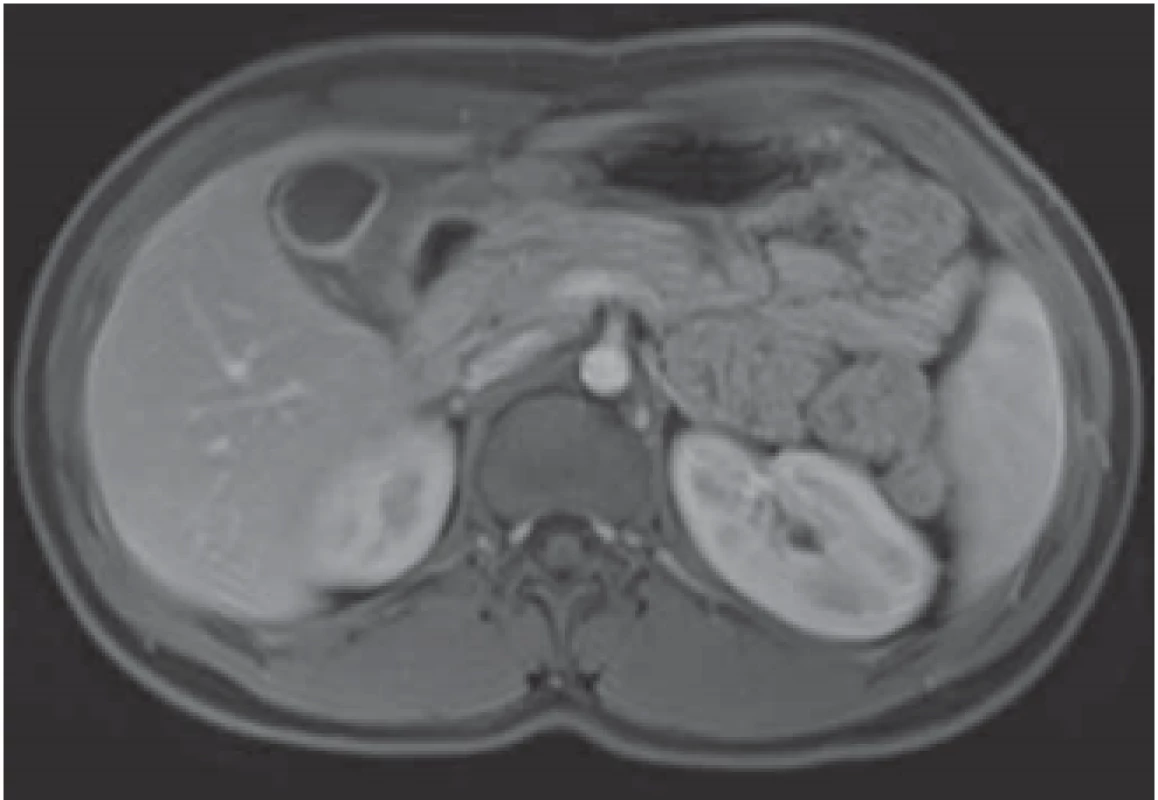
Fig. 2. Duodenal ulcer.
Obr. 2. Vřed ve dvanáctníku.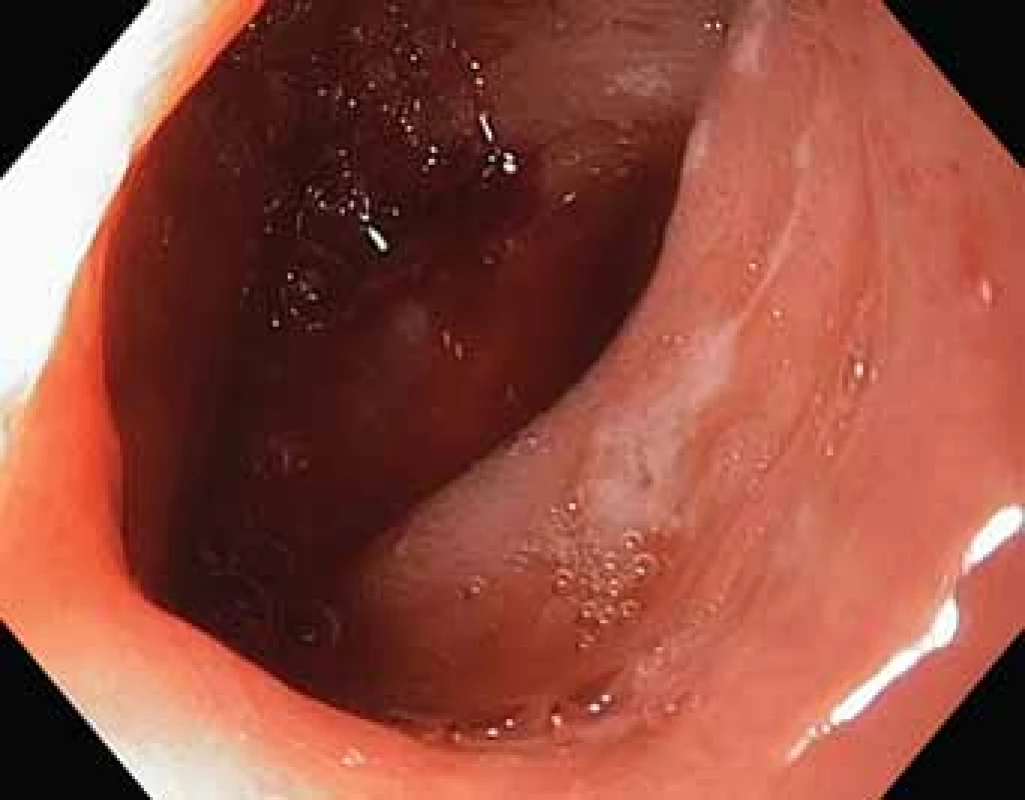
Fig. 3. Full thickness biopsy of duodenum, sheets of degranulated eosinophils in deep duodenal wall, Charcot-Leyden crystals (arrows), hematoxylin and eosin stain, magnifi cation 100×.
Obr. 3. Histopatologické vyšetření incize stěny duodena ukazuje v hlubokých vrstvách degranulované eozinofi ly a Charcot-Leydenovy krystaly (šipky), barvení hematoxylin-eosinem, zvětšení 100×.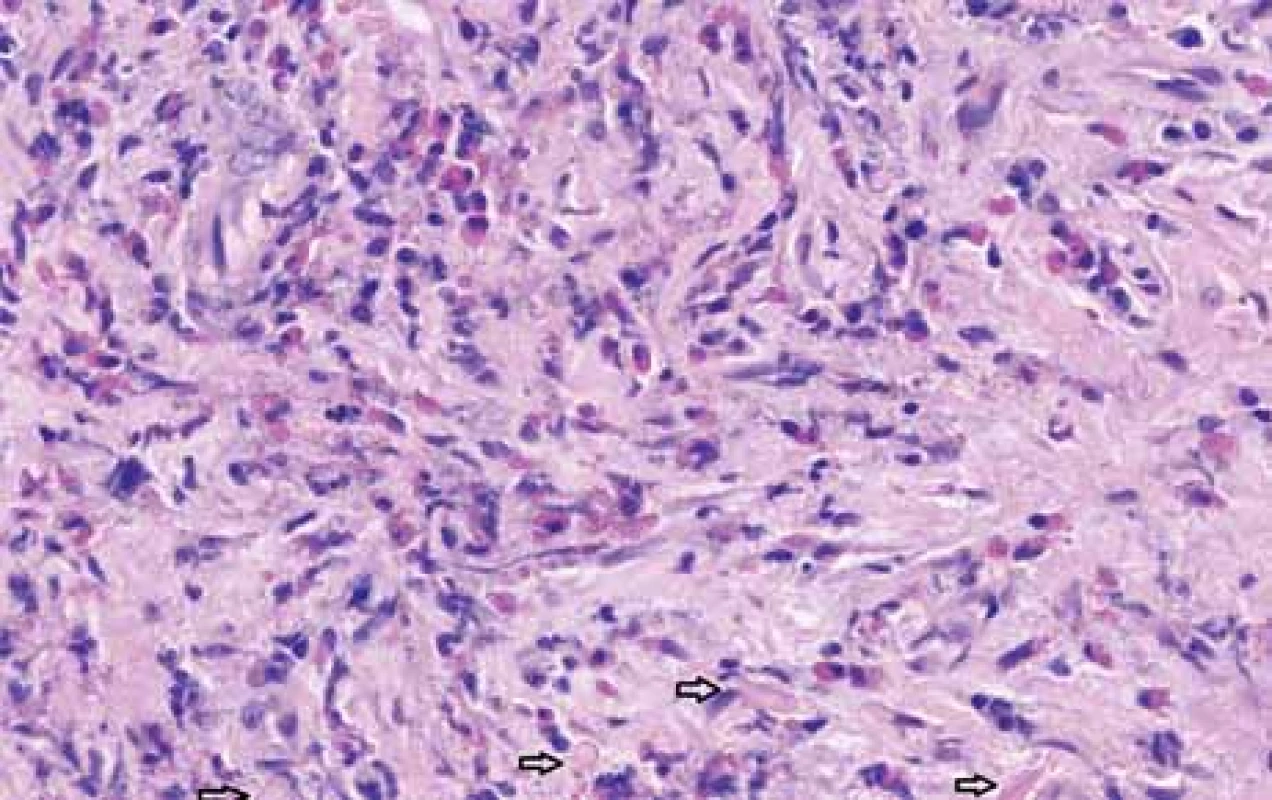
Fig. 4. Biopsy from duodenum shows increased eosinophils, hematoxylin and eosin stain, magnifi cation 200×.
Obr. 4. Histopathologický průkaz zvýšeného počtu eozinofi lů ve sliznici duodena, barvení hematoxylin-eosinem, zvětšení 200×.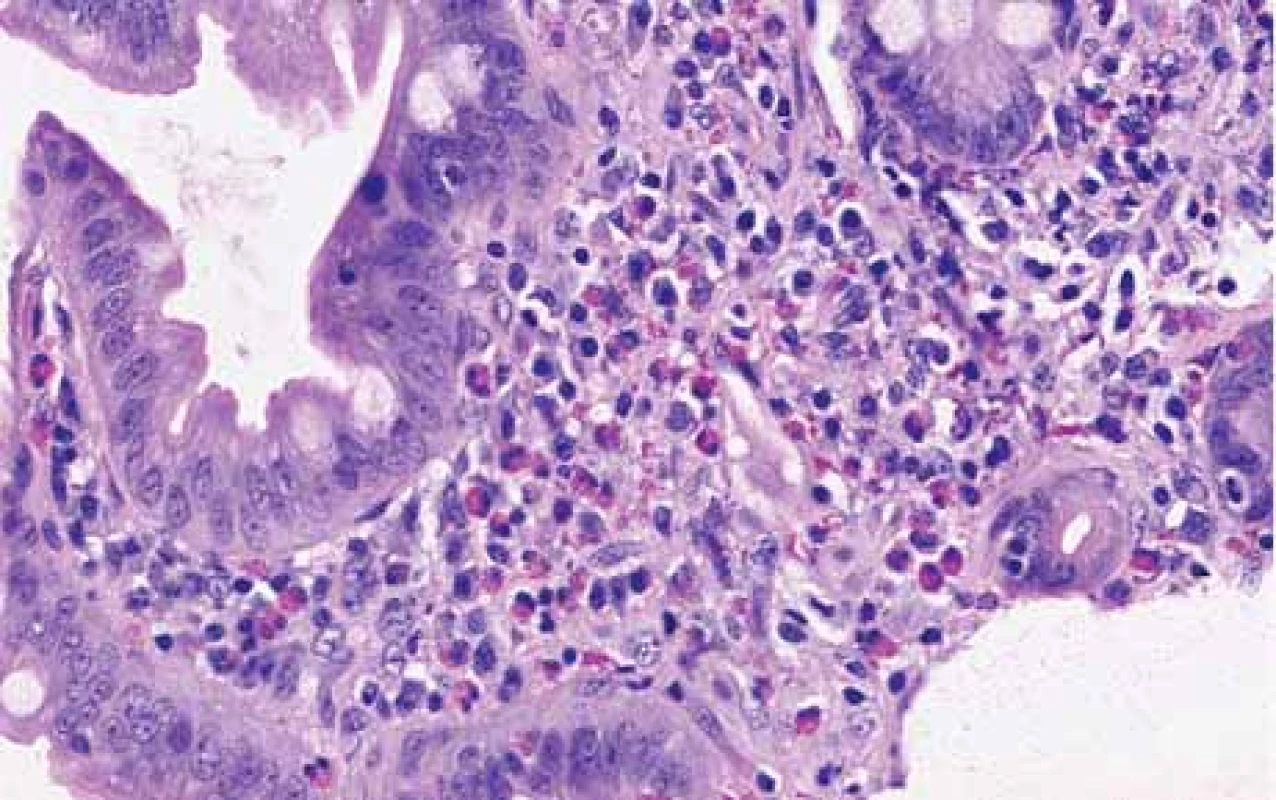
Fig. 5. Healing duodenal ulcer after prednison treatment.
Obr. 5. Kontrolní EGFS na terapii kortikoidy – hojení duodenálního vředu.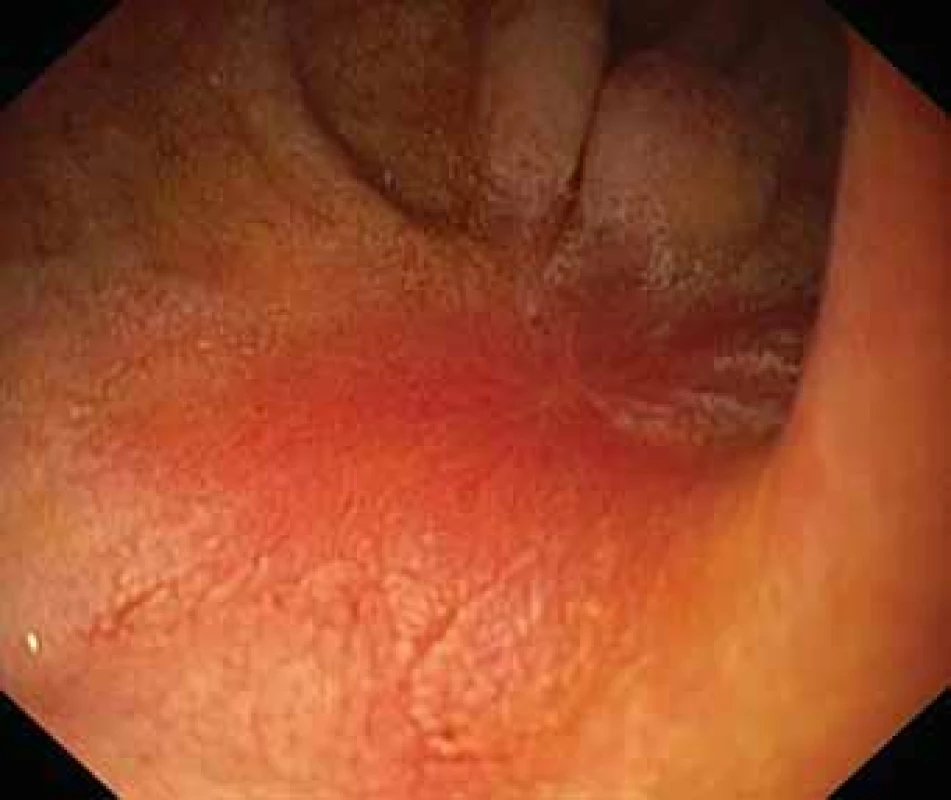
Discussion
Eosinophilic enteritis is a rare disease. The duodenum is the most common localisation. Clinical manifestation varies according to the intestinal wall layer in which the eosinophil infiltration is present (Tab. 1) [11]. Some cases present as an acute bowel obstruction as a combination of both mechanical (due to stricture) and functional (due to inflammation) obstruction, oedema and decreased GI motility [1]. Peripheral eosinophilia is a relevant but not mandatory laboratory sign of EE (our patient presented with only mild eosinophilia – 10%). Imaging tests have low sensitivity or could even be without abnormalities [12]. Moreover, endoscopic findings could be physiological or nonspecific. The eventual endoscopic appearance included erythema, whitish specks, erosions, ulcers, narrowings and strictures. As we show in our case, the serosal type of EE can mimic other dia gnoses, especially the oncological kind. The investigation process could be complicated and might significantly postpone the establishment of the correct dia gnosis. Surgery procedures with full-thickness bio psy are mandatory.
Tab. 1. Clinical manifestation of eosinophilic enteritis according to depth of invasion [12].
Tab. 1. Klinické příznaky eozinofilní enteritidy dle hloubky postižení střevní stěny [12].![Clinical manifestation of eosinophilic enteritis
according to depth of invasion [12].<br>
Tab. 1. Klinické příznaky eozinofilní enteritidy dle hloubky
postižení střevní stěny [12].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image_pdf/f3e42a324ac02574365c17d2fb1af3b0.png)
There are only rare experiences with eosinophilic cholecystitis (EC) in children. Recently Garzón et al. published what is so far a unique paediatric case series report. The authors collected data from 134 children who had undergone a cholecystectomy. Of those, 8.2% had the histological findings of EC [13]. All of them had symptoms of acute cholecystitis with cholecystolithiasis confirmed by ultrasound. Only 27.3% developed peripheral eosinophilia. Unfortunately, there is no information about consequent EGID or other allergic diseases. In our case, we did not discover cholecystolithiasis. Eosinophilic infiltration of the gallbladder was secondary to the natural appearance of the serosal type of EE.
The predominantly serosal pattern of eosinophilic gastroenteritis seems to have a relatively good prognosis presenting usually as a single flar and no continuous chronic course [14]. It reacts rapidly to corticosteroid therapy.
Conclusion
EE is a rare chronic inflammatory disease. Its clinical presentation may be similar to many other gastrointestinal disorders. There are no specific non-invasive explorative tests. Moreover, endoscopic findings could be physiological or nonspecific. Therefore, the investigation process could be complicated. The cornerstone of the dia gnosis is histopathological proof of dense eosinophilic infiltration of the intestine. Because of a risk of patchy eosinophilic infiltration, multiple samples are mandatory. Histopathological confirmation of the muscular or serosal type of EE requires obtaining full-thickness specimens surgically. The treatment is based on diet and corticosteroids. Further studies are needed to evaluate the best dia gnostic and therapeutic options.
Eliška Hloušková, MD
Department of Paediatrics
University Hospital Brno
Faculty of Medicine
Masaryk University
Černopolní 212/9
613 00 Brno
Czech Republic
Submitted/Doručeno: 10. 11. 2020
Accepted/Přijato: 30. 11. 2020
Zdroje
1. Zhang MM, Li YQ. Eosinophilic gastroenteritis: a state-of-the-art review. J Gastroenterol Hepatol 2017; 32 (1): 64–72. doi: 10.1111/1gh.13 463.
2. Collins MH, Capocelli K, Yang GY. Eosinophilic gastrointestinal disorders pathology. Front Med (Lausanne) 2018; 4 : 261. doi: 10.3389/ fmed.2017.00261.
3. Klein NC, Hargrove RL, Sleisenger MH et al. Eosinophilic gastroenteritis. Medicine (Baltimore) 1970; 49 (4): 299–319. doi: 10.1097/000 05792-197007000-00003.
4. Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol 2017; 15 (11): 1733–1741. doi: 10.1016/j.cgh.2017.05.050.
5. Shukla A, Mishra A, Venkateshaiah SU et al. Elements involved in promoting eosinophilic gastrointestinal disorders. J Genet Syndr Gene Ther 2015; 6 (2): 265. doi: 10.4172/2157 - 7412-1000265.
6. Kim HJ, Jung YJ. The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune Netw 2020; 20 (3): e24. doi: 10.4110/in.2020.20.e24.
7. Matsushita T, Maruyama R, Ishikawa N et al. The number and distribution of eosinophils in the adult human gastrointestinal tract: a study and comparison of racial and environmental factors. Am J Surg Pathol 2015; 39 (4): 521–527. doi: 10.1097/PAS.00000000000370.
8. Rothenberg ME, Mishra A, Brandt EB et al. Gastrointestinal eosinophils. Immunol Rev 2001; 179 : 139–155. doi: 10.1034/j.1600-065x. 2001.790114.x.
9. Koutri E, Patereli A, Noni M et al. Distribution of eosinophils in the gastrointestinal tract of children with no organic disease. Ann Gastroenterol 2020; 33 (5): 508–515. doi: 10.20524/aog.2020.0518.
10. Sunkara T, Rawla P, Yarlagadda KS et al. Eosinophilic gastroenteritis: dia gnosis and clinical perspectives. Clin Exp Gastroenterol 2019; 12 : 239–253. doi: 10.2147/CEG.S173130.
11. Rodriguez HE, Djohan RS, Cahill WJ et al. Laparoendoscopic dia gnosis of eosinophilic enteritis. JSLS 1998; 2 (2): 181–184.
12. Abassa KK, Linn XY, Xuan JY et al. Dia gnosis of eosinophilic gastroenteritis is easily missed. World J Gastroenterol. 2017; 23 (19): 3556–3564. doi: 10.3748/wjg.v23.i19.3556.
13. Garzón LNG, Jaramillo LEB, Valero JJH. Eosinophilic cholecystitis in children: case series. J Pediatr Surg 2020; S0022-3468 (20): 30371–30377. doi: 10.1016/j.jpedsurg.2020.05. 039.
14. Pineton de Chambron G, Gonzalez F, Canva JY et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 2011; 9 (11): 950–956. doi: 10.1016/j.cgh.2011.07.017.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článek GNET of small bowellČlánek Recenzia knihy
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2020 Číslo 6- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Pediatric gastroenterology and hepatology
- Eosinophilic esophagitis – 10 years of experience in five Czech pediatric endoscopy centers
- Trichohepatoenteric syndrome in a patient with TTC37 mutations – a case report
- Superior mesenteric artery syndrome in conjunction with Crohn’s disease – a case report
- Eosinophilic enteritis – case report of a rare manifestation and review of updates
- Joint opinion of professional societies on pharmacological treatment of obesity
- Adjustable gastric balloons for weight loss – a higher yield of responders compared to non-adjustable gastric balloons
- Imaging methods in non-traumatic acute abdomen
- Effect of a synbiotic, ColonFit, in patients with irritable bowel syndrome, functional constipation and functional diarrhea
- GNET of small bowell
- Proton pump inhibitors – do we know them well and are they really that safe? – part 2
- Biosimilar adalimumab FKB-327 in the treatment of inflammatory bowel disease
- Subcutaneous vedolizumab treatment for ulcerative colitis and Crohn‘s disease in clinical trial VISIBLE
- The selection from international journals
- What is the significance of covid-19 testing before endoscopic examination?
- Recenzia knihy
- MUDr. Radoslav Pruška zemřel 10. listopadu 2020
- Will gastroenterological-hepatological research in the Czech Republic reach excellence?
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Imaging methods in non-traumatic acute abdomen
- Superior mesenteric artery syndrome in conjunction with Crohn’s disease – a case report
- Eosinophilic esophagitis – 10 years of experience in five Czech pediatric endoscopy centers
- Eosinophilic enteritis – case report of a rare manifestation and review of updates
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



