-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Abnormal liver functional tests in children with congenital heart diseases – a cross-sectional study in the south-east of Iran
Abnormální jaterní testy u dětí s vrozenými srdečními vadami – průřezová studie na jihovýchodě Íránu
Východiska: U dětí s vrozenými srdečními vadami (VSV) není klinický význam abnormálních jaterních testů dostatečně studován. Cílem naší studie byl screening jaterních testů u dětí s VSV. Metodika: U 80 dětí s VSV byly měřeny jaterní testy včetně jaterních enzymů (AST, ALT a ALP), přímého bilirubinu (DB), celkového bilirubinu a albuminu. Výsledky: Z našich pacientů mělo 45 (67,4 %) defekt komorového septa (VSD), 3 (3,8 %) Fallotovu tetralogii (TOF), 25 (20 %) defekt septa síní (ASD), 2 (2,5 %) městnavé srdeční selhání (CHF) a 5 (6,2 %) perzistující ductus arteriosus (PDA). U 3 pacientů (3,8 %) bylo nalezeno abnormálně zvýšené ALT, u 21 (26,2 %) AST, u 6 (7,6 %) ALP, u 10 (12,5 %) DB, u 7 (8,8 %) TB a u 43 (53,8 %) snížený celkový protein. Jednotlivé vrozené srdeční vady se v jaterních testech významně nelišily s výjimkou AST (p = 0,04). Průměrná hladina AST byla významně nižší u dětí s VSD (31,4 ± 10,7 IU/l) než u dětí s TOF (48,3 ± 22,8 IU/l; p = 0,03) a s ASD (36,8 ± 9,8 IU/l; p = 0,03). Závěr: Vzhledem k vysoké incidenci abnormálních jaterních testů u dětí s VSV je vhodné tyto hodnoty pravidelně monitorovat, aby se včas zvládla případná progrese do městnavé nebo ischemické hepatitidy.
Klíčová slova:
srdeční selhání – vrozená srdeční vada – defekt komorového septa – ischemická hepatitida
Authors: I. Shahramian 1
; A. Bazi 2
; E. Akhlaghi 3
; N. M. Noori 4
; F. Parooie 5
; M. Tahani 5
Authors place of work: Pediatric ward, Amir-Al-Momenin Hospital, Zabol University of Medical Sciences, Zabol, Iran 1; Faculty of Allied Medical Sciences, Zabol University of Medical Sciences, Zabol, Iran Iran 2; Student Research Committee, Zabol University of Medical Sciences, Zabol, Iran 3; Children and Adolescent Health Research Center, Resistant Tuberculosis Institute, Zahedan University of Medical Sciences and Health Services, Zahedan, Iran 4; Pediatric Gastroenterology and Hepatology Research Center, Zabol University of Medical Sciences, Zabol, Iran 5
Published in the journal: Gastroent Hepatol 2022; 76(6): 485-491
Category:
doi: https://doi.org/10.48095/ccgh2022485Summary
Background: The clinical implication of abnormal liver functional tests (LFTs) is not well studied in children with congenital heart diseases (CHDs). We aimed to screen LFTs in children with CHDs. Methods: LFTs including liver enzymes (AST, ALT and ALP), as well as direct bilirubin (DB), total bilirubin and albumin were measured in 80 children with CHDs. Results: Ventricular septal defect (VSD), tetralogy of Fallot (TOF), atrial septa defect (ASD), congestive heart failure (CHF) and Patent ductus arteriosus (PDA) accounted for 45 (67.4%), 3 (3.8%), 25 (20%), 2 (2.5%) and 5 (6.2%) of our patients, respectively. Abnormally elevated ALT, AST, ALP, DB, TB and reduced total protein were identified in 3 (3.8%), 21 (26.2%), 6 (7.6%), 10 (12.5%), 7 (8.8%) and 43 (53.8%), respectively. There was no significant difference in LFTs among various CHDs except for AST (P = 0.04). The mean level of AST was significantly lower in children with VSD (31.4 ±10.7 IU/l) than those of TOF (48.3 ±22.8 IU/l, P = 0.03) and ASD (36.8 ±9.8 IU/l, P = 0.03). Conclusion: Considering the respectively high incidence of abnormal LFTs in children with CHDs, it is advisable to regularly monitor these tests to timely manage any progressions toward congestive or ischaemic hepatitis.
Keywords:
congenital heart disease – heart failure – ventricular septal defect – ischaemic hepatitis
Introduction
Congenital heart disorders (CHDs) are the most frequent inherited abnormalities responsible for a significant mortality rate within the first year of life [1]. CHDs encompass a wide variety of ventricular, arterial and septal defects. For the majority of these defects, however, the aetiological features are largely unknown [2].
Patients with progressive cardiac conditions are commonly encountered with hepatic dysfunction [3]. Liver involvement is a part of a variety of heart syndromes including cardiogenic shock (CS), [4] acute [5] and chronic [6] heart failure, as well as ischaemia/perfusion heart injury [7]. Acute cardiogenic liver injury (ACLI) also known as ischaemic hepatitis (IH), is characterised with markedly elevated liver enzymes and hepatic necrosis in the context of cardiac dysfunction [8]. Another condition, known as congestive hepatopathy (CH), has been increasingly reported in the context of cardiac anomalies [9]. CH is characterised by prominent elevated direct bilirubin (DB) and alkaline phosphatase (ALP) accompanying histopathological changes in hepatocytes (i.e. atrophy, sinusoidal dilation, fibrosis and finally, cirrhosis) [8,10]. On the other hand, increased liver enzymes (usually >1,000 IU/l) have been noted as a predisposing factor to coronary atherosclerosis [11,12]. Patients with cardiac diseases may further be at risk of liver injury secondary to medications such as blockers of calcium-channels and amiodarone, which have been associated with liver fibrosis [8]. Likewise, elevated liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) have been indicated in ischaemic/reperfusion induced cardiac damage [7]. Mutually, liver diseases themselves can augment cardiac dysfunction, leading to systolic and diastolic abnormalities [13–16]. Elevation in proinflammatory cytokines and fibrogenic factors have been implicated in cardiac structural and functional abnormalities in the context of liver diseases [16]. Nevertheless, underlying mechanisms are poorly divulged.
The prognosis of CHDs can be significantly influenced by extracardiac manifestations [17]. Hepatic involvement along with co-existent cardiac pathology may further progress to multi-organ dysfunctions shortening life expectancy [12]. Accordingly, patients with CHDs complicated with CH after surgical intervention have been at a higher risk of hepatic carcinoma [3,8]. Also, children with CHDs who have undergone the Fontan procedure had a higher risk of liver pathologies [18]. Besides, multiorgan dysfunctions are major contributors to compromised growth parameters in childhood [19]. As hepatic dysfunction, especially when associated with clinical and histopathological changes accompanied with elevated liver enzymes, is a major prognostic factor in patients with cardiac abnormalities [3,5,20–22], screening liver function tests (LFTs) in such patients can help to appropriately manage the cardio-hepatic condition. To our knowledge, there have been no reports on LFTs in children with CHDs. Here, we aimed to determine LFTs and their potential association with the different aetiologies of CHDs in children.
Methods
Participants
The patients included 80 children with CHDs admitted to Amir-Al-Momenin Hospital of Zabol city, Sistan and Baluchestan Province in the south-east of Iran. The study was conducted in 2017–2018. The study was conducted according to the ethical guidelines of the Declaration of Helsinki (2013) and approved by the ethics committee of Zabol University of Medical Sciences (IR.ZBMU.REC.1399.060). Informed consent was obtained from the participants’ parents.
Inclusion and exclusion criteria
Children with no signs of serious systemic disorders or other comorbidities (i.e. coeliac disease, Wilson disease, thyroid diseases and collagen vascular disease) were included. Those with a current hepatic disorder or a family history of hepatic disorders, and those under treatment with medications affecting hepatic function, were excluded.
Demographic variables
The demographic variables were gathered by a checklist containing information regarding sex, age and weight. For children <2 years old, weight was measured using a lying scale (Iran) with ±10 grams accuracy. For children >2 years old, weight was determined by a standing scale (Japan) with ±100 grams accuracy.
Assessing LFTs
Blood samples were obtained from the participants in the morning. The sample for analysis of cell blood counts was gathered in EDTA. The serum samples were separated from blood gathered in serum-separator tubes by standard protocol and stored at –80 °C until use. LFTs including liver enzymes (AST, ALT and ALP), as well as direct bilirubin (DB), total bilirubin and albumin were measured by specific ELISA kits (Pars Azmoon, Iran).
Statistical analysis
Statistical analyses were performed in SPSS 19 software. Distribution of the data was checked by the Kolmogorov-Smirnov test. Descriptive statistics were used as frequencies and means ± standard deviations. The chi-square test and student sample t-test were used for univariate inferential analysis.
Results
Of the total of 80 children with CHDs, 37 (46.2%) were males. The mean age and weight were 4.89 ±4.6 years old (the range of 1–18) and 14.42 ±11 kg (3–63), respectively. Ventricular septal defect (VSD, 45, 67.4%) and congestive heart failure (CHF, 2, 2.5%) comprised the most and the least common disorders, respectively. Tab. 1 shows the distribution of different CHDs in this study.
Tab. 1. Diff erent congenital heart disorders in 80 children.
Tab. 1. Různé vrozené srdeční vady u 80 dětí.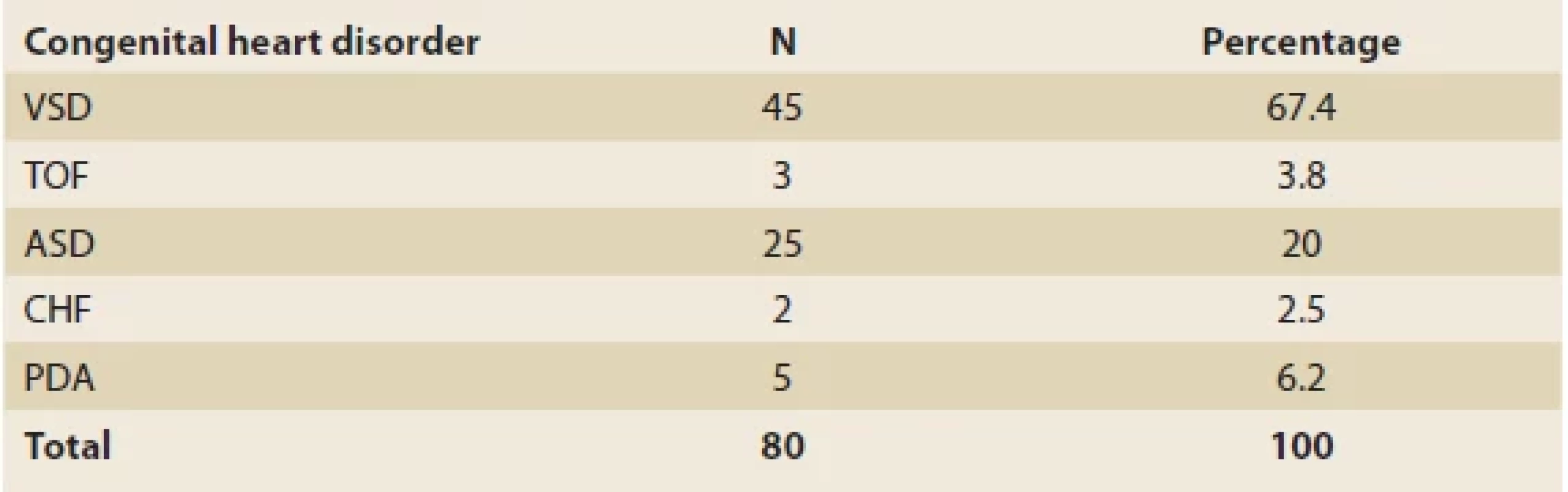
VSD – ventricular septal defect, TOF – tetralogy of fallot, ASD – arterial septal defect, CHF – congestive heart failure, PDA – patent ductus arteriosus The mean levels of ALT, AST and ALP were 24 ±35.5 IU/l, 35.6 ±10.9 IU/l and 601.5 ±367.2 IU/l, respectively (Tab. 2). Overall, abnormally elevated ALT, AST, ALP, DB, TB and total protein were identified in 3 (3.8%), 21 (26.2%), 6 (7.6%), 10 (12.5%), 7 (8.8%) and 43 (53.8%). Hypoalbuminemia was not encountered in our patients. There was no significant difference among various CHDs regarding the ratio of patients with elevated LFTs except for AST (P = 0.04) (Tab. 3). The mean level of AST was significantly lower in children with VSD (31.4 ±10.7 IU/l) than those of TOF (48.3 ±22.8 IU/l) and ASD (36.8 ±9.8 IU/l, P = 0.03 for both comparisons) (Fig. 1).
Tab. 2. Biochemical and hematological parameters in 80 children diagnosed with congenital heart disorders.
Tab. 2. Biochemické a hematologické parametry u 80 dětí s diagnózou vrozených srdečních vad.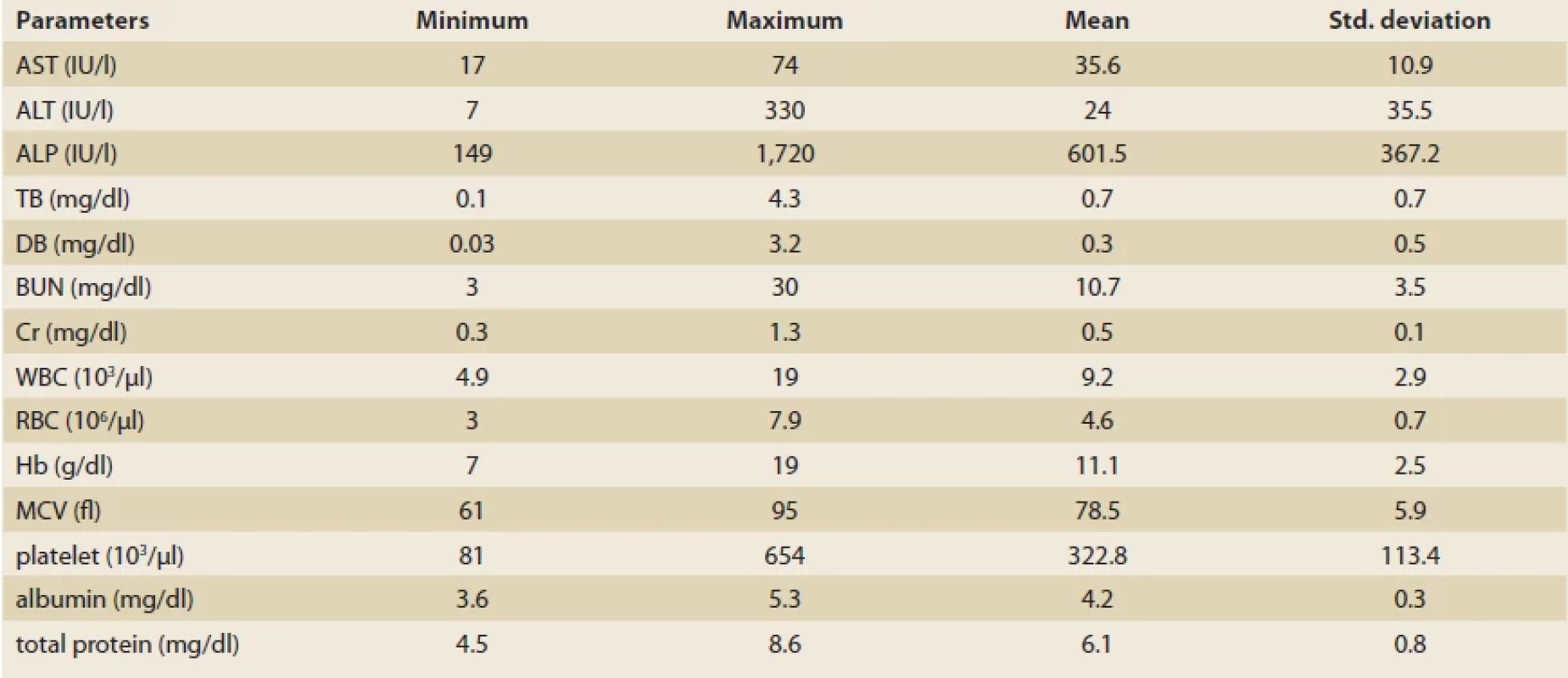
AST – aspartate aminotransferase, ALT – alanine aminotransferase, ALP – alkaline phosphatase, TB – total bilirubin, DB – direct bilirubin, BUN – blood urea nitrogen, Cr – creatinine, WBC – white blood cell count, RBC – red blood cell count, Hb – hemoglobin, MCV – mean corpuscular volume Tab. 3. Liver functional tests in 80 children diagnosed with congenital heart disorders.
Tab. 3. Funkční jaterní testy u 80 dětí s diagnózou vrozených srdečních vad.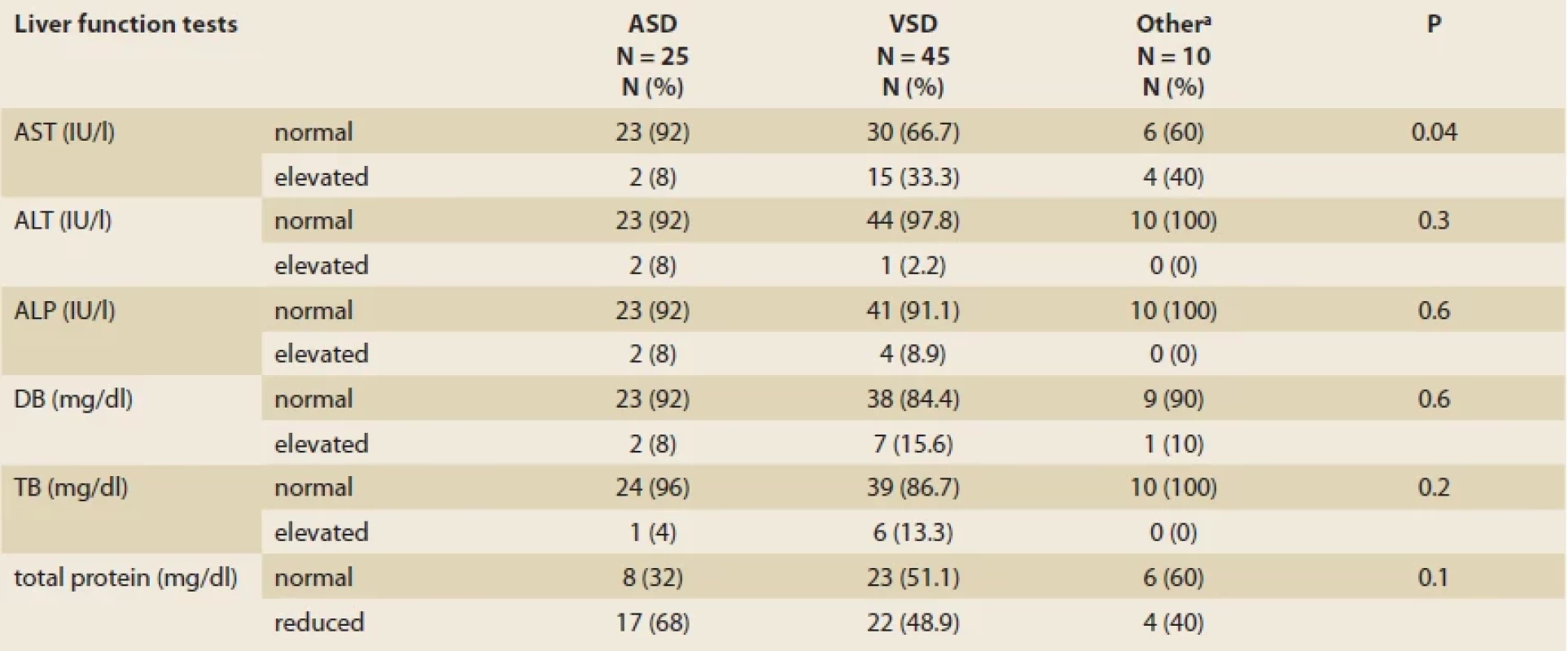
VSD – ventricular septal defect, ASD – arterial septal defect, AST – aspartate aminotransferase, ALT – alanine aminotransferase, ALP – alkaline phosphatase, TB – total bilirubin, DB – direct bilirubin a – tetralogy of fallot (N = 3), congestive heart failure (N = 2), and patent ductus arteriosus (N = 5) Fig. 1. Comparison of liver functional tests between various congenital heart disorders in children; aspartate aminotransferase (A), alanine aminotransferase (B), total bilirubin (C), and alkaline phosphatase (D).
Obr. 1.Porovnání funkčních jaterních testů u různých vrozených srdečních vad u dětí; aspartátaminotransferáza (A), alaninaminotransferáza (B), celkový bilirubin (C) a alkalická fosfatáza (D).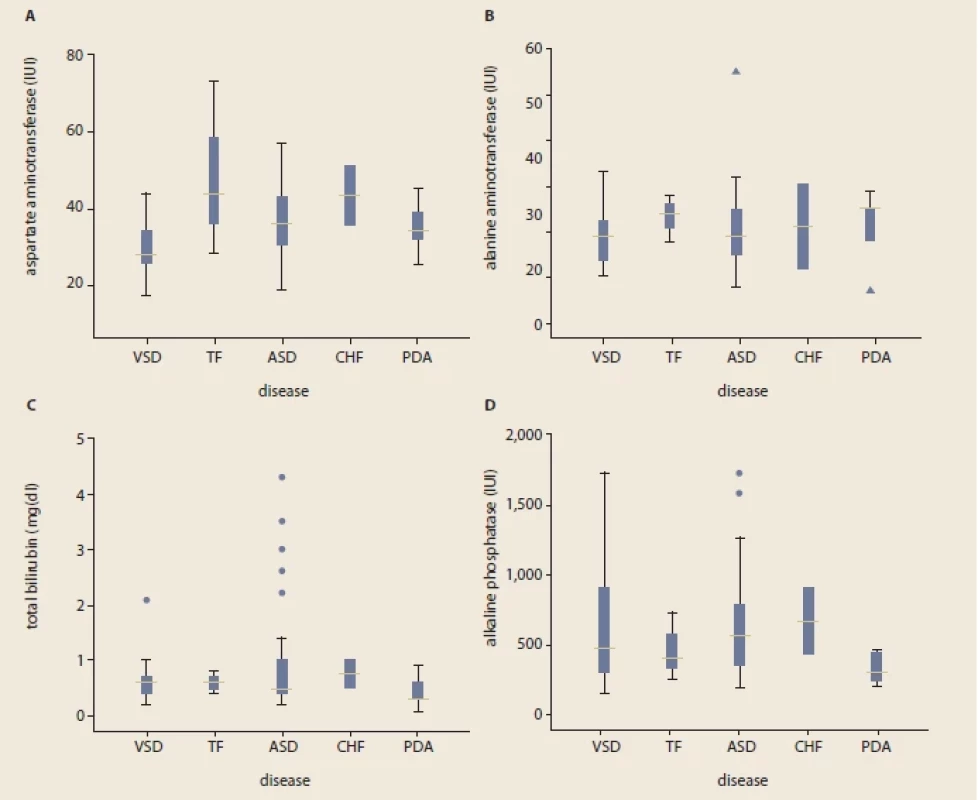
Outliers’ implications: *indicate a child with AST and ALT of 74 IU/l and 55 IU/l respectively. Δ; indicates ALT of 55 IU/l (the child with ASD) and 7 IU/l (the child with PDA). Total bilirubin levels were 2.2, 2.6, 3, 3.5 and 4.3 mg/dl in children with ASD, while 2.1 in the child with VSD. Alkaline phosphatase outlier values were 1,579 and 1,720 IU/l. The only signifi cant diff erence was observed when comparing the mean AST level in VSD children with either TOF (P = 0.03) or ASD (P = 0.03). VSD – ventricular septal defect, TOF – tetralogy of fallot, ASD – arterial septal defect, CHF – congestive heart failure, PDA – patent ductus arteriosus Discussion
The clinical implications of abnormal LFTs in children with CHDs has been poorly characterised. Out of the 80 children with CHDs enrolled in the present study, the majority of the patients were diagnosed with VSD and ASD (67.4% and 20%), respectively. Other diagnoses included PDA (N = 5), TOF (N = 3) and CHF (N = 2). The most frequent abnormality in LFTs was low total serum protein (43 out of 80, 53.8%). On the other hand, no patient was diagnosed with hypoalbuminemia. This may be explained by the fact that albumin, comprising 50% of plasma proteins, is produced by hepatocytes at the rate of 10–15 g/day and attains a relatively long half-life of 21 days [23]. Liver enzymes ALT, AST and ALP were elevated in 3 (3.8%), 21 (26.2%) and 6 (7.6%) of the patients, respectively.
As a major recipient of blood flow directed from the heart, the liver is a susceptible target for any functional deterioration inflicting the myocardium [24]. Hepatic involvement in children with CHDs delivers a threatening clinical condition requiring urgent medical intervention. Nevertheless, there is a shortage of studies on LFTs in children with CHDs. In patients with CS, 58% of the patients revealed high ALT levels [4]. Furthermore, ALT levels rendered prominent predictive value for the risk of mortality in CS, while other biochemical markers of hepatic dysfunction (i.e. ALP, g-glutamyltransferase and TB) have not been associated with mortality risk [4]. Other conditions characterising with cardio-hepatic complications such as myocardial ischaemia/reperfusion [7], coronary heart disease [25] and acute heart failure [5] have also been accompanied by elevated levels of AST and ALT. In patients with acute cardiac arrest, AST and ALT enzymes were elevated in 42% and 35% of the patients, respectively. [5] Also, TB and ALP were elevated in 34% and 20%, respectively, of the individuals suffering from acute heart failure [5]. In another study, 17–26% of the patients with acute heart failure exhibited abnormal baseline values for albumin, AST, ALT, ALP and TB levels [6]. In individuals suffering from chronic heart failure, increased levels of ALT and TB were reported in 3.1% and 13%, respectively [22]. Furthermore, hypoalbuminemia was recorded in 18.3% of the patients in the recent study [22]. In atrial fibrillation, ALT levels were elevated (>40 IU/l) in 27.6% of the patients [26]. In the study by Shteyer et al, in 384 children undergoing surgical intervention for CHDs, abnormal LFTs were more significantly reported among children with TOF than other anomalies [21]. In the present study, 2 out of 3 children with TOF had elevated AST levels and one of them also showed low total protein serum. However, the number of children with TOF was not sufficient for conducting statistical analysis for seeking a significant relationship. Overall, 11.9% of the children demonstrated elevated AST and ALT levels in the recent report [21]. Reports on LFTs in children with CHDs are very limited. However, the highly variable ratios of patients with abnormal LFTs obtained in different cardiological conditions may lie in the different pathologies of the underlying conditions.
In general, ACLI or IH (also known as hypoxic hepatitis) underlies abnormal LFTs in patients with acute or chronic cardiac conditions. Heart failure, either acute or chronic, comprises around one-half to two-thirds of IH cases [27,28]. In addition to cardiac failure, IH has been reported in the context of viral hepatitis, septic shock, drug-induced liver injury, respiratory insufficiency and systemic hypotension [27–31]. The exact pathophysiological processes leading to IH and hepatocyte necrosis are yet to be divulged. However, systemic hypotension seems to be the main contributing factor [32]. The outcomes of IH in patients with CHDs is not well studied and characterised [33]. Generally, IH is warranted in the context of an appropriate clinical setting in patients with >1,000 IU/l of liver enzymes [29]. However, the definition is not universal, with some suggesting the diagnosis of IH at liver enzymes >8,000 IU/l. [34] In a meta-analysis on 1,782 cases with IH, the maximum levels of LFTs (AST, ALT and total bilirubin) were reported as 2,423 IU/L, 1,893 IU/L and 2.55 mg/dL, respectively [33]. However, no patient in the present study demonstrated such elevations. Although an elevated lactate dehydrogenase (LDH) level has been reported as the primary sign of IH [34], a concise diagnosis requires incorporating haemodynamic characteristics of cardiac and hepatic blood circulations [27]. In histopathological studies, IH is characterised by marked necrosis of hepatocytes in the centrilobular region.
In patients with CHDs, IH may precede with CH [8]. CH is more commonly observed in right-sided cardiac defects and is characterised by hepatic centrilobular congestion [27,35]. Normal hepatic blood circulatory system depends on regulated arterial, portal and hepatic vein blood flow, as well as on intact mesenteric circulation [27,36]. Hepatocellular hypoxia as the end outcome of CH leading to hepatic necrosis results from three consecutive events including compromised hepatic blood flow, liver congestion and finally, systemic hypoxia [27,37]. As CH and IH can be associated with a high mortality rate, timely diagnosis of these conditions by assessing LFTs can be an effective way to suitably manage them.
A multifactorial approach is the most plausible explanation as molecular underlying mechanisms for elevated LFTs in cardiac disorders. Besides hepatic necrosis in ACLI [8], elevated liver enzymes in an animal model of myocardial ischaemia was accompanied with higher hepatic concentrations of inflammatory markers including TNFa, IL-1 and IL-6 [7]. Furthermore, altered immune reactivity toward hepatocytes has been suggested as a contributor to liver injury [38]. The immunological mediator and inflammatory markers, in particular TNFa and IFN g, have been implicated in hepatic infiltration by immune cells and following dilated cardiomyopathy [39]. Furthermore, it has been suggested that intrinsic apoptotic, as well as Akt and ERK1/2 pathways may participate in liver injury induced by myocardial ischaemia [7]. Volume overload has been implicated in CH patients with a direct correlation between bilirubin level and this condition [40]. The reduced synthetic potential associated with hepatocellular damage is the main involved mechanisms in hypoalbuminemia and hypoproteinaemia in liver diseases [41]. Hypoxic liver tissue, on the other hand, is known to result in robust elevation of ALT and AST levels [21]. Intact blood circulation within the hepatic vasculature is important in regulating various liver functions, particularly in neonates [42].
The prognostic value of elevated LFTs in various cardiac functional and structural abnormalities has been controversial. In subclinical heart damage, ALT and AST levels have been correlated with the cardiac injury marker, highly sensitive cardiac troponin T [14]. The mortality rate of cardio-hepatic conditions such as CS has been higher in individuals with higher baseline ALT levels [4]. In those with heart failure, abnormal initial LTFs predicted lower ejection fraction and higher mortality rates [6]. The prognostic implications of subclinical elevated LFTs in children with CHDs, however, is yet to be divulged.
Subclinical deranged LFTs is a respectively common phenomenon in children with CHDs. As abnormal LFTs may herald serious hepatic conditions such as IH and CH, it is highly recommended to routinely screen LFTs in children with CHDs.
Since we could not measure GGT levels in this study, it is suggested that the level of this enzyme be evaluated in future studies.
Acknowledgments
Thanks to the patients’ families.
ORCID authors
I. Shahramian ORCID 0000-0002-4134-1405,
A. Bazi ORCID 0000-0003-4872-1352,
E. Akhlaghi ORCID 0000-0003-2483-5937,
N. M. Noori ORCID 0000-0002-0732-6412,
F. Parooie ORCID 0000-0002-2367-3780,
M. Tahani ORCID 0000-0002-0213-9087.
Submitted/ Doručeno: 17. 2. 2022
Accepted/ Přijato: 16. 3. 2022
Noor Mohammad Noori, MD
Department of Pediatrics
Children and Adolescents Health Research
Resistant Tuberculosis Institute
Zahedan University of Medical Sciences
and Health Services
Sistan and Baluchestan Province
9816743463 Zahedan, Iran
zblresearchcenter@gmail.com
Zdroje
1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39 (12): 1890–1900. doi: 10.1016/s0735-1097 (02) 01886-7.
2. Richards AA, Garg V. Genetics of congenital heart disease. Curr Cardiol Rev 2010; 6 (2): 91–97. doi: 10.2174/157340310791162703.
3. Goncalvesova E, Kovacova M. Heart failure affects liver morphology and function. What are the clinical implications? Bratisl Lek Listy 2018; 119 (2): 98–102. doi: 10.4149/BLL_2018_018.
4. Jantti T, Tarvasmaki T, Harjola VP et al. Frequency and prognostic significance of abnormal liver function tests in patients with cardiogenic shock. Am J Cardiol 2017; 120 (7): 1090–1097. doi: 10.1016/j.amjcard.2017.06.049.
5. Vyskocilova K, Spinarova L, Spinar J et al. Prevalence and clinical significance of liver function abnormalities in patients with acute heart failure. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015; 159 (3): 429–436. doi: 10.5507/bp.2014.014.
6. Ambrosy AP, Vaduganathan M, Huffman MD et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 2012; 14 (3): 302–311. doi: 10.1093/eurjhf/hfs007.
7. Lai CC, Huang PH, Yang AH et al. Baicalein reduces liver injury induced by myocardial ischemia and reperfusion. Am J Chin Med 2016; 44 (3): 531–550. doi: 10.1142/S0192415X16500294.
8. Koehne de Gonzalez AK, Lefkowitch JH. Heart disease and the liver: pathologic evaluation. Gastroenterol Clin North Am 2017; 46 (2): 421–435. doi: 10.1016/j.gtc.2017.01.012.
9. Megalla S, Holtzman D, Aronow WS et al. Predictors of cardiac hepatopathy in patients with right heart failure. Med Sci Monit 2011; 17 (10): CR537–CR541. doi: 10.12659/msm.881 977.
10. Gopanpallikar AM, Rathi PM, Sawant P et al. Cardiac anomalies associated with extrahepatic portal venous obstruction. Indian Pediatr 1998; 35 (11): 1143–1144.
11. Jung DH, Lee YJ, Ahn HY et al. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med 2010; 48 (12): 1829–1834. doi: 10.1515/CCLM.2010.349.
12. Raurich JM, Llompart-Pou JA, Ferreruela M et al. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth 2011; 25 (1): 50–56. doi: 10.1007/s00540-010-1058-3.
13. Karabulut A, Iltumur K, Yalcin K et al. Hepatopulmonary syndrome and right ventricular diastolic functions: an echocardiographic examination. Echocardiography 2006; 23 (4): 271–278. doi: 10.1111/j.1540-8175.2006.00210.x.
14. Lazo M, Rubin J, Clark JM et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol 2015; 62 (4): 841–847. doi: 10.1016/j.jhep.2014.11.024.
15. Lemasters JJ, Stemkowski CJ, Ji S et al. Cell surface changes and enzyme release during hypoxia and reoxygenation in the isolated, perfused rat liver. J Cell Biol 1983; 97 (3): 778–786. doi: 10.1083/jcb.97.3.778.
16. Markus MR, Meffert PJ, Baumeister SE et al. Association between hepatic steatosis and serum liver enzyme levels with atrial fibrillation in the general population: The Study of Health in Pomerania (SHIP). Atherosclerosis 2016; 245 : 123–131. doi: 10.1016/j.atherosclerosis.2015.12. 023.
17. Lui GK, Saidi A, Bhatt AB et al. Diagnosis and management of noncardiac complications in adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation 2017; 136 (20): e348–e392. doi: 10.1161/CIR.0000000000000535.
18. Sugimoto M, Oka H, Kajihama A et al. Non-invasive assessment of liver fibrosis by magnetic resonance elastography in patients with congenital heart disease undergoing the Fontan procedure and intracardiac repair. J Cardiol 2016; 68 (3): 202–208. doi: 10.1016/j.jjcc.2016.05. 016.
19. Zemel BS. Influence of complex childhood diseases on variation in growth and skeletal development. Am J Hum Biol 2017; 29 (2). doi: 10.1002/ajhb.22985.
20. Adachi K, Toyama H, Kaiho Y et al. The impact of liver disorders on perioperative management of reoperative cardiac surgery: a retrospective study in adult congenital heart disease patients. J Anesth 2017; 31 (2): 170–177. doi: 10.1007/s00540-017-2308-4.
21. Shteyer E, Yatsiv I, Sharkia M et al. Serum transaminases as a prognostic factor in children post cardiac surgery. Pediatr Int 2011; 53 (5): 725–728. doi: 10.1111/j.1442-200X.2011.033 56.x.
22. Allen LA, Felker GM, Pocock S et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009; 11 (2): 170–177. doi: 10.1093/eurjhf/hfn031.
23. Gounden V, Jialal I. Hypoalbuminemia. StatPearls Publishing 2018.
24. Birgens HS, Henriksen J, Matzen P et al. The shock liver. Clinical and biochemical findings in patients with centrilobular liver necrosis following cardiogenic shock. Acta Med Scand 1978; 204 (5): 417–421.
25. Feitosa MF, Reiner AP, Wojczynski MK et al. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis 2013; 227 (2): 420–424. doi: 10.1016/j.atherosclerosis.2013.01.013.
26. Makar GA, Weiner MG, Kimmel SE et al. Incidence and prevalence of abnormal liver associated enzymes in patients with atrial fibrillation in a routine clinical care population. Pharmacoepidemiol Drug Saf 2008; 17 (1): 43–51. doi: 10.1002/pds.1514.
27. Ciobanu AO, Gherasim L. Ischemic hepatitis – intercorrelated pathology. Maedica 2018; 13 (1): 5–11.
28. Van den Broecke A, Van Coile L, Decruyenaere A et al. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care 2018; 8 (1): 15. doi: 10.1186/ s13613-018-0356-z.
29. Breu AC, Patwardhan VR, Nayor J et al. A multicenter study into causes of severe acute liver injury. Clin Gastroenterol Hepatol 2019; 17 (6): 1201–1203. doi: 10.1016/j.cgh.2018.08. 016.
30. Raurich JM, Perez O, Llompart-Pou JA et al. Incidence and outcome of ischemic hepatitis complicating septic shock. Hepatol Res 2009; 39 (7): 700–705. doi: 10.1111/j.1872-034X.2009. 00501.x.
31. Waseem N, Chen PH. Hypoxic hepatitis: a review and clinical update. J Clin Transl Hepatol 2016; 4 (3): 263–268. doi: 10.14218/JCTH. 2016.00022.
32. Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol 2017; 33 (3): 158–163. doi: 10.1097/MOG. 0000000000000355.
33. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med 2015; 128 (12): 1314–1321. doi: 10.1016/j.amjmed.2015.07.033.
34. Aboelsoud MM, Javaid AI, Al-Qadi MO et al. Hypoxic hepatitis – its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J Crit Care 2017; 41 : 9–15. doi: 10.1016/j.jcrc.2017.04.040.
35. Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med 2000; 109 (2): 109–113. doi: 10.1016/s0002-9343 (00) 00461-7.
36. Lautt WW, Greenway CV. Conceptual review of the hepatic vascular bed. Hepatology 1987; 7 (5): 952–963. doi: 10.1002/hep.1840070 527.
37. Weisberg IS, Jacobson IM. Cardiovascular diseases and the liver. Clin Liver Dis 2011; 15 (1): 1–20. doi: 10.1016/j.cld.2010.09.010.
38. Roth GA, Zimmermann M, Lubsczyk BA et al. Up-regulation of interleukin 33 and soluble ST2 serum levels in liver failure. J Surg Res 2010; 163 (2): e79–e83. doi: 10.1016/j.jss.2010.04. 004.
39. Torzewski M, Wenzel P, Kleinert H et al. Chronic inflammatory cardiomyopathy of interferon gamma-overexpressing transgenic mice is mediated by tumor necrosis factor-alpha. Am J Pathol 2012; 180 (1): 73–81. doi: 10.1016/j.ajpath.2011.09.006.
40. Samsky MD, Patel CB, DeWald TA et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61 (24): 2397–2405. doi: 10.1016/j.jacc.2013.03.042.
41. Myers RP, Cerini R, Sayegh R et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 2003; 37 (2): 393–400. doi: 10.1053/jhep.2003.50062.
42. Murayama K, Nagasaka H, Tate K et al. Significant correlations between the flow volume of patent ductus venosus and early neonatal liver function: possible involvement of patent ductus venosus in postnatal liver function. Arch Dis Child Fetal Neonatal Ed 2006; 91 (3): F175–F179. doi: 10.1136/adc.2005.079822.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2022 Číslo 6- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Dětská gastroenterologie a hepatologie
- Cesta k úspěšné léčbě obezity – multidisciplinární a multimodální přístup
- Endoscopic findings in cirrhotic children candidates for liver transplantation
- Abnormal liver functional tests in children with congenital heart diseases – a cross-sectional study in the south-east of Iran
- Bariatrické restrikční výkony a výživa
- „Vanishing bile duct syndróm“ ako prejav poliekového poškodenia pečene u pacienta po polytraume
- Perforace sigmatu jako pozdní komplikace ERCP
- Kvalita života pacientov s Crohnovou chorobou v Českej republike
- Komentář ke článku „Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission“ (Clinical Infectious Diseases)
- Dificlir – fidaxomicin – efektivní varianta léčby infekcí C. difficile u dětí
- Titanlax – profil zdravotnického prostředku
- Výběr z mezinárodních časopisů
- Terapeutická endosonografie Jablonec nad Nisou 2022
- Prof. MUDr. Aleš Hep, CSc.
- 1. Slovenský gastroenterologický kongres 2022
- 17. vzdělávací a diskuzní gastroenterologické dny
- Kreditovaný autodidaktický test
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dificlir – fidaxomicin – efektivní varianta léčby infekcí C. difficile u dětí
- „Vanishing bile duct syndróm“ ako prejav poliekového poškodenia pečene u pacienta po polytraume
- 17. vzdělávací a diskuzní gastroenterologické dny
- Titanlax – profil zdravotnického prostředku
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



