-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Post-mortem analysis of Candida albicans breakthrough infection during echinocandin treatment in haematopoietic stem cell transplant recipient
Post-mortem analýza průlomové infekce Candida albicans při léčbě echinokandiny u pacienta po transplantaci kmenových buněk krvetvorby
Představujeme případ úmrtí dívky na průlomovou infekci Candida albicans při léčbě echinokandiny po transplantaci kmenových buněk krvetvorby pro relaps akutní myeloidní leukémie. Hemokultivace prokázaly opožděně přítomnost rezistence na echinokandiny u kmene Candida albicans, která je obvykle považována za dobře citlivou k antimykotické terapii. Echinokandin podávaný v profylaxi nebyl z důvodu závažných orgánových komplikací (játra, ledviny) změněn na preparát z jiné skupiny ani v léčbě invazivní infekce. Později provedené molekulární analýzy prokázaly přítomnost mutace S645P, popsané jako příčiny rezistence k echinokandinům. Dále byla provedena post-mortem analýza nálože kvasinkové infekce ve tkáních, která prokázala velmi vysoké kvantity kvasinkové DNA v zažívacím traktu, játrech, slezině a ledvinách.
Klíčová slova:
invazivní kandidová onemocnění – transplantace kmenových buněk krvetvorby – rezistence na echinokandiny
Authors: V. Chrenkova 1; P. Hubacek 1,2; P. Sedlacek 2; P. Riha 2; D. Kodetová 3; E. Bébrová 4
Authors place of work: Department of Medical Microbiology nd Faculty of Medicine, Charles University in Prague and Motol University Hospital 1; Department of Paediatric Haematology and Oncology, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital 2; Department of Pathology and Molecular Medicine, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital 3; Department of Medical Microbiology, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital 4
Published in the journal: Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 2, s. 121-124
Category: Souhrnná sdělení, původní práce, kazuistiky
Summary
We present case of a girl deceased due to Candida albicans breakthrough invasive infection during the echinocandin treatment after undergoing allogeneic haematopoietic stem cell transplant for relaps of acute myeloid leukaemia. Candida albicans generally susceptible to all antifungal drugs wasn't considered for potential resistance and conventional blood culture positivity was too late to reveal the resistance to echinocandins. Due to severe organ toxicities (liver, kidneys) she received echinocandin as an antifungal prophylaxis, no change was made for the treatment of Candida albicans infection. Later, the molecular analysis proved the mutation S645P known as being responsible for the echinocandin resistance. The post mortem analysis of fungal burden in autopsy samples showed very high levels of Candida DNA in gut, liver, spleen and kidneys.
Keywords:
invasive candida dinase – haematopoietic stem cell transplant – echinocandin resistanceINTRODUCTION
Invasive Candida disease remains an important cause of morbidity in allogeneic hematopoietic stem cell transplant recipients [3, 9, 19]. Candida albicans is still the most frequently isolated causative agent [10, 16, 26] and echinocandins are the first-line agents for the treatment of Candida albicans sepsis according to European council for infection in leukaemia (ECIL3) guidelines [18] and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2012 paediatric guidelines [12]. The resistance to echinocandins is rare [6], caused by mutations in the glucan synthase complex catalytic subunit (GSC1) [7, 22] and clinical failure of echinocandin treatment in case of the detected reduced susceptibility or resistance was observed [1, 11, 14, 17, 21, 23, 25]. Our report presents a case of proven breakthrough invasive Candida disease rapid onset during echinocandin therapy with subsequent proof of resistance mechanism and post-mortem fungal burden determination.
PATIENT
We present a case of 17 years old girl diagnosed with the acute myeloid leukaemia (AML) with FLT3/ITD fusion gene positive detected in May 2007. She had been treated according to the appropriate protocol (AML BFM 2004) and achieved the complete remission of the disease. During the treatment, she had developed a possible pulmonary mycosis [2] treated successfully with the amphotericin B lipid complex (ABLC) and posaconazole.
In June 2009, leucocytopenia and thrombocytopenia as first symptoms of the AML relaps were detected; therefore hematopoietic stem cell transplantation (HSCT) from an unrelated donor was indicated. Twelve days before HSCT (D-12), a relapse of AML with detection of leukemic cells in the peripheral blood was proven. She was front-line treated with one short course of idarubicin, fludarabine, cytarabine, granulocyte colony stimulating factor (IDA-FLAG) and shortly after bone marrow transplanted in aplasia after conditioning of Melphalan (140 mg/m2) and anti-thymocyte globulin (20 mg/kg of the body weight (bw)) by 10/10 HLA (human leukocyte antigen) identical unrelated donor. There were 10.2x108 nucleated cells per kg of bw, 6.96x106 CD34+ cells/kg bw and 3,38x107 CD3+ cells/kg bw in the peripheral blood stem cell (PBSC) graft.
The standard antifungal prophylaxis started on D-49 with itraconazole 5 mg/kg/day p.o. in two daily doses (div q12h). Due to the nausea and vomiting as known adverse effects of itraconazole, the treatment was switched to voriconazole 12 mg/kg/day p.o. div q12h on D-40. Due to suspected toxic hepatopathy and colonization by Saccharomyces cerevisiae, echinocandin application was started on D-6 with micafungin 100 mg i.v. once daily (q24h). First week after HSCT patient got neutropenic enterocolitis with GIT bleeding, melena and severe anaemia. She developed fluidothorax in both pleural cavities shortly after. During second week after HSCT patient remained sub febrile with CRP about 100 mg/l and fluidothorax increased more on the right side and was drained without any microbiological culture finding.
On D+15, Candida albicans was detected in the non-invasive body-samples: oral cavity swab, nasogastric aspiration, stool, genital swab and urine and at that time no susceptibility testing was done. On D+17 the patient presented toxic hepatopathy and renal insufficiency and yeasts were detected in the blood culture. The antifungal treatment was continued by echinocandins (caspofungin 50 mg i.v. q24h). Central venous catheter (CVC), chest drains and nasogastric tube were extracted and short term CVC and two peripheral catheters were applied. Furthermore we found a positivity of mannan antigen (Platelia® Candida Ag, Bio-Rad, Prague, Czech Republic) in the blood sample on D+16. On D+19 the resistance to caspofungin was detected by phenotypic testing. One day later, patient was transferred to the Intensive Care Unit (ICU) presenting a renal failure, toxic hepatopathy and coagulopathy and deceased on D+21 due to the multi-organ failure. The autopsy findings confirmed disseminated candidiasis with macroscopic presence of Candida foci massively in spleen, liver, heart and kidney. FLT3/ITD detection used for monitoring of minimal residual disease [4] in the patient confirmed high levels of this fusion gene in the patient (positive in bone marrow, liver and spleen).
Tab. 1. Candida DNA quantity in tissues Tabulka 1. Nálož kvasinkové DNA ve tkáních 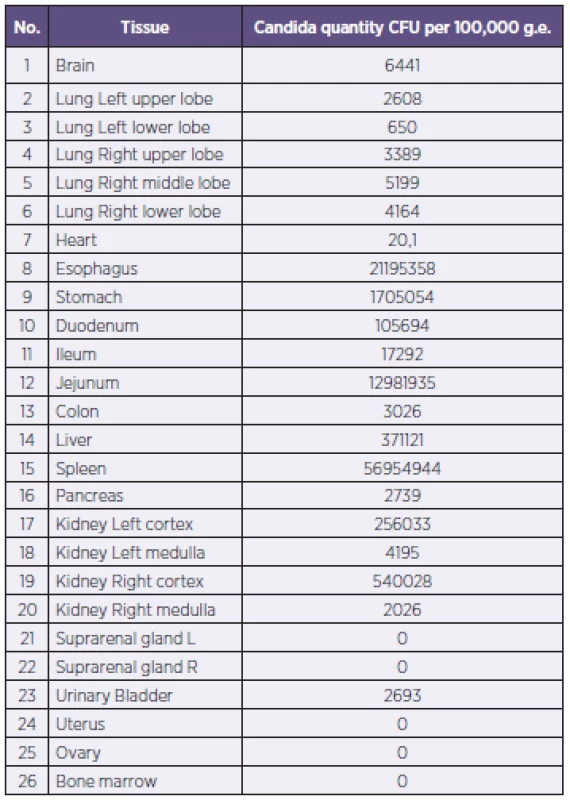
METHODS AND RESULTS
We verified the identification of Candida albicans isolated from three consecutive blood cultures by Auxacolor2 (Bio-Rad, Prague, Czech Republic) and ID 32C (bioMérieux, Prague, Czech Republic). The susceptibility testing was carried out by E-tests (bioMérieux, Prague, Czech Republic) using RPMI-1640 agar (Trios, Olomouc, Czech Republic) and by Sensititre YeastOne (Trek Diagnostik, BioVendor, Brno, Czech Republic) following the manufacturer's instructions. The obtained minimum inhibitory concentrations (MIC) were >32 mg/l for caspofungin, 1.0 mg/l for micafungin, 1.5 mg/l for anidulafungin, 0.125 mg/l for fluconazole, 0.006 mg/l for voriconazole, 0.023 mg/l for posaconazole and 0.5 mg/l for amphotericin B. The reference mycological laboratory confirmed both Candida albicans identification and the susceptibility testing.
Graph 1. Candida DNA quantities post-mortem (numbers correspond to tissues in Table 1) Graf 1. Nálož kvasinkové DNA post-mortem (číslice odpovídají číslování tkání v tab. 1) 
As consequence of the depicted resistance to caspofungin we performed a sequence analysis of the beta-D-glucan synthase catalytic subunit (GSC1/FKS1), known as the target place of echinocandins antifungal activity [7]. The DNA was isolated from the culture by the use of a protocol for yeasts of Qiagen DNA Mini Kit (Qiagen, Hilden, Germany). We used previously described primers [17] for the PCR reaction. This PCR reaction consisted of 1x Long Range PCR buffer (Qiagen, Hilden, Germany), 3.5 mM MgCl2, 100 µM of each dNTP (Sigma-Aldrich, Prague, Czech Republic), 500 nM primers and 0.5 U Qiagen HotStar Taq polymerase (Qiagen, Hilden, Germany). The samples were tested in duplicates and the total volume of PCR reaction was 25 µl, containing 5 µl of extracted DNA. The temperatures of the thermal protocol started at 93 °C for the first 15 min, followed by 35 cycles of 93 °C for 15 s, 57 °C for 30 s and 68 °C for 2.5 min. By this reaction we obtained 2.548 bp long amplicon. On this amplicon we applied the sequence analysis using the ABI Genetic Analyzer 3130 (Applied Biosystems, Prague, Czech Republic). The comparison of the obtained sequence to wild-type strain Candida albicans ATCC 90028 proved the presence of previously described mutation S645P [21]. Niimi in his article describes [20] heterogeneity of yeast mutations based on the susceptibility testing results. Following his logic, in our case, the complete resistance to caspofungin and the reduced susceptibility to micafungin and anidulafungin with MIC < 2 mg/l, lead us to the conclusion that our strain of Candida albicans shows a heterozygosity of mutation in GSC1/FKS1 hot spot 1.
Fig 1. Miliary haematogenous Candida sepsis in kidney both in macroscopic and microscopic examination In microscopic picture, there are clearly visible kidney glomeruli and tubular system close to the local Candida proliferation. Obr. 1. Miliární hematogenní rozsev kandidové sepse v ledvinách v makroskopickém i mikroskopickém zobrazení V mikroskopii jsou viditelné ledvinné glomeruly a tubulární systém v těsném sousedství míst kvasinkové proliferace. 
Besides this complex analysis we performed also retrospective PCR testing on Candida DNA quantity of the stored samples used for standard viral surveillance during post-transplant period and samples from the autopsy. DNA was extracted from the whole blood and the tissues by Qiagen DNA Blood Mini Kit and Qiagen DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. We used the previously described real-time PCR (RQ-PCR) approach [15]. PCR reaction consisted of 1x Qiagen PCR buffer, 4.0 mM MgCl2, 5% of glycerol, 100 µM of each dNTP (Sigma-Aldrich, Prague, Czech Republic), 1.6 µM ROX-6 passive reference dye, 500 nM primers, 200 nM probe and 0.5 U Qiagen HotStar Taq polymerase (Qiagen, Hilden, Germany). Samples were tested in duplicates and PCR reaction was performed on ABI 7500 machine in the total volume of 15 µl, containing 2 µl of extracted DNA. The temperatures of the thermal protocol started at 95 °C for first 15 min, followed by 50 cycles of 94 °C for 15 s and 60 °C for 1 min. The standard curve was constructed on the basis of the serial decadic dilution of suspension of known CFU concentration. For a better comparison of the yeast DNA quantity in different tissues, the Candida quantity was normalized to 100.000 human genome equivalents (g.e.) obtained by the quantification of the albumin gene in the sample [24].
RQ-PCR on Candida detected a massive affection of the yeast infection in the spleen, liver, renal cortex and mucosa of proximal gastrointestinal tract (5.6x107; 3.7x105; 2.6 – 5.4x105 and 0.2 – 2.1x107 CFU normalized to 100.000 g.e. respectively) compared to the rest of the tissues such as brain and heart (6x103 and 2x104 CFU normalized to 100.000 g.e.). We found no Candida DNA in the blood one week before the decease.
DISCUSSION
Our case shows the possible complications with management of invasive Candida infection. Our experience shows that in rare cases, using of broad-spectrum antifungal drugs in the prophylaxis can fail with all clinical consequences.
Phenotypic testing of the colonizing strains including the antifungal susceptibility profile can be fundamental in such cases, even if this testing is rather costly. Therefore, we changed our strategy of microbiological screening in haematology and oncology patients. Regular susceptibility testing of Candida albicans colonization strains in the high-risk, severely immunocompromised patients is included in our guidelines.
However, choose of antifungals in our patient was limited by concomitant diseases. Since renal insufficiency, subsequent renal failure and hepatopathy with veno-occlusive disease of the liver cannot be excluded, treatment with voriconazole or amphotericin B wasn’t possible and the echinocandins were continued. Hepatopathy could also reflect dissemination of Candida.
The post-mortem analysis detected high level of minimal residual disease (MRD), this indicates non controlled leukaemia. At the same time the patient was transplanted in aplasia but wasn’t in remission of the AML as known risk factor of infectious complications – especially of invasive Candida disease – during post-transplant period [13].
Our strain presents heterozygous form of resistance mutation leading to the complete resistance to caspofungin and the reduced echinocandin susceptibility in case of micafungin and anidulafungin. That time, official break-point of 2 mg/l for echinocandins [5] could lead to omitting the resistance in case that only micafungin or anidulafungin is tested, so we are in agreement with published data leading to the break-point change [6, 8].
The Candida DNA detected quantity corresponds to the most frequently touched locations – gut mucosa, hepatosplenic affection and haematogenous dissemination in the human body. The detected quantities will serve us as reference for PCR detection in samples of patients with suspected invasive Candida disease. Our analysis of the DNA quantity in the blood before and during post-transplant period shows that no Candida DNA was found in the blood one week before the decease. This fact could be explained by possibly inadequate way of DNA extraction for Candida detection but no other method was available at that time and there is no serum left for further analysis. In this case no supplement chemical or mechanical cytolysis was done in these extractions as would be recommended for Candida DNA detection [28] because we performed routine DNA extraction protocol for blood used for viral DNA surveillance in patient.
The described method of detection of the mutations in GSC1/FKS1 with molecular microbiology-based methods and also new developed method [27] might help us in the future with a rapid diagnosis in such cases. Together with further detailed phenotypic testing it can be the main evidence for changes in the antifungal treatment or prophylaxis.
Acknowledgement: We want to acknowledge Dr. Ivana Masova from Institute of Public Health, Prague, Czech Republic and miss Dana Michalska for the verification of identification and susceptibility testing in our patient and to Miss Alena Augustinakova and Dr. Marketa Kalinova for help with the sequence analysis.
Supported by MH CZ – DRO, University Hospital Motol, Prague, Czech Republic 00064203.
This study was performed in Motol University Hospital, Prague, Czech Republic.
Do redakce došlo dne 17. 10. 2013.
Adresa pro korespondenci:
MUDr. Vanda Chrenková
Ústav lékařské mikrobiologie FN v Motole
V Úvalu 84
150 06 Praha 5
e-mail: vanda.chrenkova@fnmotol.cz
Zdroje
1. Arendrup MC, et al. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob Agents Chemother, 2009;53(3):1185–1193.
2. Ascioglu S, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis, 2002;34(1):7–14.
3. Benjamin DK Jr. et al. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J, 2002;21(3):227–234.
4. Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol, 2013;162(2):147–161.
5. Canton E, Espinel-Ingroff A, Peman, J. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev Anti Infect Ther, 2009;7(1): 107–119.
6. Castanheira M, et al. Low Prevalence of fks1 Hotspot 1 Mutations in a Worldwide Collection of Candida spp. Antimicrob Agents Chemother, 2010;54(6):2655–2659.
7. Douglas CM, et al. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother, 1997;41(11):2471–2479.
8. Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother, 2009;53(1):112–122.
9. Hale KA, et al. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol, 2010;149(2):263–272.
10. Herbrecht R, et al. Indications and outcomes of antifungal therapy in French patients with haematological conditions or recipients of haematopoietic stem cell transplantation. J Antimicrob Chemother, 2012;67(11):2731–2738.
11. Hernandez S, et al. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother, 2004;48(4):1382–1383.
12. Hope WW, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect, 2012;18(Suppl 7):38–52.
13. Horn DL, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis, 2009;48(12):1695–1703.
14. Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother, 2006;50(8): 2892–2894.
15. Khlif M, et al. Evaluation of nested and real-time PCR assays in the diagnosis of candidaemia. Clin Microbiol Infect, 2009;15(7):656–661.
16. Kocmanova I, et al. Invasive candidiasis in selected heamatology departments in the Czech Republic and Slovakia – microbiological results of the CAN CELL project. Klin Mikrobiol Infekc Lek, 2011;17(1):5–10.
17. Laverdiere M, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother, 2006;57(4):705–708.
18. Maertens J, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3-2009 Update. Bone Marrow Transplant, 2011;46(5):709–1718.
19. Mor M, et al. Invasive fungal infections in pediatric oncology. Pediatr Blood Cancer, 2011;56(7):1092–1097.
20. Niimi K, et al. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J Antimicrob Chemother, 2010;65(5):842–852.
21. Park S, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother, 2005;49(8):3264–3273.
22. Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat, 2007;10(3):121–130.
23. Pfeiffer CD, et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol, 2010;48(7):2373–2380.
24. Pongers-Willemse MJ, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia, 1998;12(12):2006–2014.
25. Slater JL, et al. Disseminated candidiasis caused by Candida albicans with amino acid substitutions in Fks1 at position Ser645 cannot be successfully treated with micafungin. Antimicrob Agents Chemother, 2011;55(7):3075–3083.
26. Steinbach WJ, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J, 2012;31(12):1252–1257.
27. Vella A, et al. Rapid antifungal susceptibility testing by matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis. J Clin Microbiol, 2013;51(9):2964–2969.
28. White PL, Archer AE, Barnes RA. Comparison of non-culture-based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. J Clin Microbiol, 2005;43(5):2181–2187.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Mikrobiológia
Článok vyšiel v časopiseEpidemiologie, mikrobiologie, imunologie
Najčítanejšie tento týždeň
2014 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Predicted strain coverage of a new protein-based meningococcal vaccine in the Czech Republic
- A point prevalence survey of healthcare-associated infections in the Slovak Republic – a part of the EU project
- Nosocomial transmission of listeriosis
- Diversity of human Salmonella isolates in the South Moravian Region in 2009–2012
- Post-mortem analysis of Candida albicans breakthrough infection during echinocandin treatment in haematopoietic stem cell transplant recipient
- Candida dubliniensis in clinical specimens and possibilities for identification
- Natural antibodies against α(1,3) galactosyl epitope in the serum of cancer patients
- Cytolethal distending toxins
- Evaluation of the importance of a ready-made, gentamicin-impregnated spacer in relation to bacteriological findings in patients with periprosthetic joint infections
- Q fever – an occupational disease leading to disability – case report
- Measles re-emerging in the Ústí Region
- Vzpomínka na nedožité 90. narozeniny MUDr. Miroslava Přívory, CSc.
- Vzpomínka na nedožité 90. narozeniny prof. MUDr. Bohumila Ticháčka, DrSc.
- Phylogenetic and molecular analysis of A/H1N1pdm influenza viruses isolated in the epidemic season 2012/2013 from hospitalised patients with symptoms of influenza-like illness
- Viral gastroenteritis in Eastern Bohemia Region of the Czech Republic
- Seroprevalence study of hepatitis E virus infection in two districts of the Czech Republic
- An increase in the prevalence of syphilis in women in Eastern Bohemia – 30 years of surveillance
- Diagnosis of Clostridium difficile infections: Comparative study of two immuno enzyme assays with confirmation by PCR and culture followed by PCR ribotyping
- Epidemiologie, mikrobiologie, imunologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Candida dubliniensis in clinical specimens and possibilities for identification
- A point prevalence survey of healthcare-associated infections in the Slovak Republic – a part of the EU project
- Q fever – an occupational disease leading to disability – case report
- Diagnosis of Clostridium difficile infections: Comparative study of two immuno enzyme assays with confirmation by PCR and culture followed by PCR ribotyping
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



