-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Viral gastroenteritis in Eastern Bohemia Region of the Czech Republic
Virové gastroenteritidy v regionu východních Čech
Cíl:
Akutní gastroenteritidy celosvětově patří mezi nejčastější choroby v lidské populaci, a představují tak důležitou příčinu mortality a morbidity. Jak se ukazuje v posledním desetiletí, uplatňují se v etiologii akutních gastroenteritid stále více viry. Několik publikovaných studií se zabývalo prevalencí virových původců gastroenteritid v evropských zemích, ale žádná z České republiky.Materiál a metodika:
Celkem 107 vzorků stolice od pacientů hospitalizovaných ve Fakultní nemocnici Hradec Králové bylo vyšetřeno imunochromatografickým testem ROTA-ADENO Card Rapid – Viditest (VIDIA, Česká republika) a RidaQick Norovirus (R-Biopharm, Německo) a současně in-house real-time PCR panelem.Výsledky:
Celkově byl metodou PCR detektován ve 29,9 % vzorků rotavirus, ve 14,0 % norovirus a v 5,0 % adenovirus. Imunochromatografickými testy detekce virových antigenů byl poté zachycen ve 28,0 % rotavirus, ve 4,7 % norovirus a ve 2,0 % adenovirus. Naše nálezy také demonstrovaly nižší senzitivitu imunochromatografických testů v porovnání s údaji od výrobců.Závěr:
Naše studie ukázala limitace imunochromatografických testů, zejména v jejich nízké senzitivitě, a potvrdila tak nezbytnost rutinního používání jiných konfirmačních metod. V naší nemocnici byla jako konfirmační metoda zavedena do rutinního využívání metoda PCR.Klíčová slova:
virové gastroenteritidy – PCR – imunochromatografické testy
Authors: M. Fajfr 1,2; V. Štěpánová 2; L. Plíšková 3; J. Fajfrová 1
Authors place of work: Faculty of Military Health Sciences, University of Defence, Hradec Králové 1; Institute of Clinical Microbiology, University Hospital Hradec Králové, Hradec Králové 2; Department of molecular biology, University Hospital Hradec Králové 3
Published in the journal: Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 2, s. 88-91
Category: Souhrnná sdělení, původní práce, kazuistiky
Summary
Purpose:
Acute gastroenteritis is one of the most common diseases in humans worldwide and represents a significant cause of mortality and morbidity. The majority of cases are of viral aetiology, evidence for which has been increasing in the past decade. Several studies on the prevalence in European countries of viral aetiology of gastroenteritis have been published in the last decade, but none from the Czech Republic.Material and methods:
In total 107 faeces samples obtained from patients hospitalised in the University Hospital in Hradec Králové were examined by immunochromatographic tests using ROTA-ADENO Card Rapid-Viditest (VIDIA, Czech Republic) and RidaQuick Norovirus (R-Biopharm, Germany), and by an in-house Real-time PCR panel.Results:
Overall findings of viruses detected by PCR in the tested faeces samples were: rotaviruses in 29.9%, noroviruses in 14.0% and adenoviruses in 5.0%. Immunochromatographic antigen detection performed at lower sensitivity compared with PCR: rotaviruses in 28.0%, noroviruses in 4.7% and adenoviruses in 2.0%. Our findings demonstrate even lower sensitivity of the used immunochromatographic tests compared with manufacturers’ data.Conclusion:
Our study has revealed limitations in immunochromatographic tests, especially in their sensitivity and the necessity for using another confirmatory method. We have set up real-time PCR in routine diagnosis of viral gastroenteritis in our hospital.Keywords:
viral gastroenteritis – PCR – immunochromatographic assayINTRODUCTION
Gastroenteritis is a large clinical unit with both infectious and non-infectious aetiology. It is characterised by epigastric pain, diarrhoea, nausea and vomiting, fever and prostration. In small children and elderly patients there is a risk of life-threatening dehydration. In the past bacteria were most commonly identified as the causative agents of gastroenteritis, but in the last decade the importance of viral pathogens as a cause of gastroenteritis has increased. The progress in laboratory diagnostic methods has improved the ability to identify viruses more frequently as causative agents of gastroenteritis. In the main, RNA viral families play the predominant role in the aetiology of viral gastroenteritis, e.g. Astroviridae (human astrovirus), Caliciviridae (human norovirus and sapovirus), Reoviridae (rotavirus) and Coronaviridae (human torovirus). The role of parechovirus (Picornaviridae) has also been discussed [1]. Amongst the DNA viruses are members of Adenoviridae (human adenovirus 40 and 41) [2]. The pathogenic possibility of several other viruses, especially bocavirus (Parvoviridae) has also been discussed [3, 4].
In routine laboratory practice the diagnostics of viral gastroenteritis are based mainly on immunochromatographic or EIA detection of viral antigen in faeces samples. According to some clinical studies the sensitivity of some of these tests is inadequate and inferior (75.4–96.6%) compared with that of polymerase chain reaction (PCR) [5, 6, 7, 8]. The culture of viruses which cause gastroenteritis is problematic because of their difficult growth in cell cultures, their low cytopathogenic effect, and the absence of a universal cell culture suitable for all main viral pathogens [9]. PCR has been evaluated as the most sensitive and specific method for viral gastroenteritis virus detection. Recent studies have presented several in-house singleplex or multiplex PCR tests that are able not only to detect the virus itself but also to determine its genogroup [10, 11, 12, 13, 14].
Viral gastroenteritides are monitored in the Czech Republic by National Institute of Public Health (NIPH) for their epidemiological importance. All cases are recorded into the system for reporting infectious diseases – EpiDat. The importance of viral gastroenteritis for public health is supported by data from EpiDat that show an increase in the absolute number of reported cases from 2528 in 2004 to 7174 in 2011. Also, the proportion of viral aetiology in gastroenteritis increased from 9.7% in 2004 to 31.6% in 2011 [15].
In this work we present the results of our unicentric study. Our aim was to determine the proportional representation of viruses in the aetiology of gastroenteritis in Eastern Bohemia region of the Czech Republic. Results from immunochromatographic tests for antigen detection of norovirus, adenovirus and rotavirus, and those from an in-house PCR panel for detection of norovirus, adenovirus, rotavirus, sapovirus and astrovirus were compared in order to evaluate the sensitivity of the immunochromatographic tests. All samples were obtained from patients hospitalized in University Hospital in Hradec Králové.
MATERIAL AND METHODS
Material
In total 107 random faeces samples were examined from patients with gastroenteritis symptoms hospitalised in University Hospital in Hradec Králové. Samples were collected over 5 months (January – May) in 2011. All samples were also examined by standard culture for the presence of bacterial pathogens (Salmonella, Shigella, Campylobacter, enteropathogenic Escherichia coli, Yersinia enterocolitica).
Immunochromatographic tests
For detection of adenoviruses and rotaviruses dual test ROTA-ADENO Card Rapid-Viditest (VIDIA, Czech Republic) was used, able to detect rotavirus and gastrointestinal adenoviruses 40, 41 from faeces samples with manufacturer-declared sensitivity and specificity of more than 99% compared to non-specific commercial immunochromatographic tests. For norovirus detection the commercial test Rida Quick Norovirus (R-Biopharm, Germany) was used, able to detect genogroup I and genogroup II of noroviruses from stool samples. Declared manufacturer sensitivity was 92% and specificity 98% in comparison with RT-PCR. Both tests were used according to the manufacturer manual, and both tests had a control line for validity evaluation.
Nucleic acid preparation
Nucleic acid was extracted from 10–20% stool suspension in PBS (phosphate buffer solution) using QIAamp DNA Stool Mini Kit (QIAGEN) for DNA and QIAamp Viral RNA Mini Kit (QIAGEN) for RNA according to the manufacturer manual, and with addition of recommended RNase inhibitors [16, 17]. Transcriptor First Strand cDNA Synthesis Kit (Roche) was used for reverse transcription in RNA viruses. As positive controls, purified nucleic acids of adenovirus (genotype 40), rotavirus (strain RV4 and F45) and astrovirus plasmid control were employed, all purchased from the National Collection of Pathogenic Viruses (Health Protection Agency, Porton Down, UK). As a norovirus-positive control, a clinical sample with confirmed positivity (by commercial PCR kit and by electron microscopy identification) was used.
Real-time PCR
Primers and probe sequences were used from adopted published in-house real time PCR (Table 1) [11, 14, 18, 19, 20]. TaqMan real-time PCR was performed using LightCycler FastStart DNA Master Plus HybProbe Kit (Roche®). Briefly, the reaction mix contained 1 µl of sample (isolated DNA or transcribed cDNA), 1 µl each of primer and probe (0.5 µM), 4 µl of master mix, and PCR grade water up to total volume of 20 µl. Reaction conditions were: 95 °C for 10 minutes (1 cycle); 95 °C for 15 sec, 55 °C for 15 sec and 72 °C for 25 sec (45 cycles); 40 °C for 30 sec (1cycle). A single fluorescence was taken at the end of each annealing step. All reactions were performed using LightCycler 1.5 (Roche®) and the data were analysed by LightCycler software, ver. 3.5 (Roche®).
Tab. 1. Primers and probe sequences of the PCR panel Tabulka 1. Sekvence primerů a sond použitého in-house real-time PCR panelu 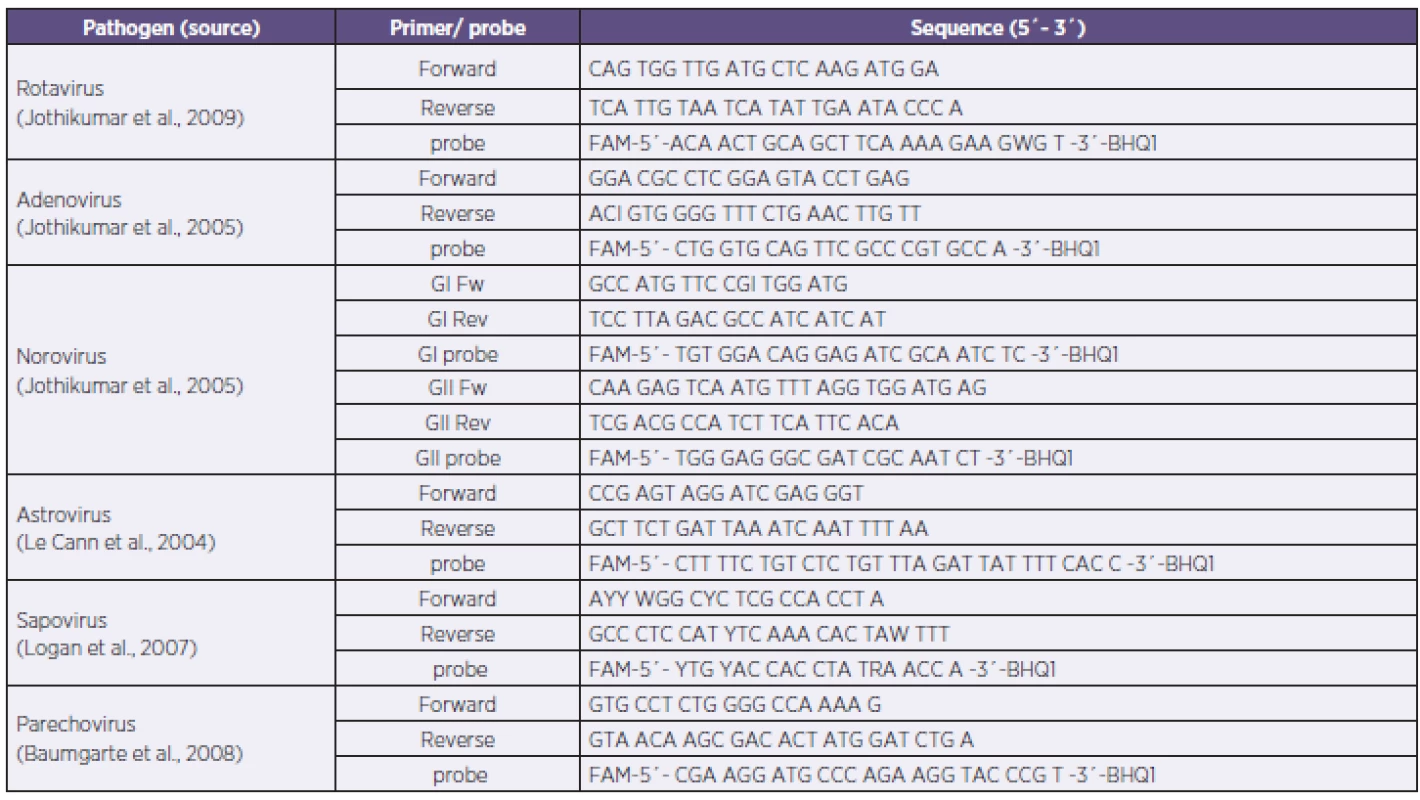
RESULTS
A total of 107 samples from 105 patients were examined: 53.3% (57/107) were from males and 46.7% (50/107) from females, with an age of 1 month to 94 years, average 30.4 years and median 13 years.
Immunochromatographic tests detected positive in 28.0% (28/100) for rotavirus, 16.0% (4/25) for norovirus and 2.0% (2/100) for adenovirus (Table No 2). No dual positives were obtained by immunochromatographic tests. In-house real-time PCR showed overall positivity of 48.6% among all samples. Rotavirus was detected in 29.9% (32/107) of the samples, norovirus in 14.0% (15/107), and adenovirus in 4.5% (5/107). No sapovirus, astrovirus or parechovirus were found. In five stool samples PCR detected positive for two viral pathogens (4.5%, 5/107): three times for rotavirus with adenovirus, and two times for rotavirus with norovirus. In one sample (0.9%, 1/107), both rotavirus and enteropathogenic Escherichia coli were detected. Bacteriological examination of stool samples found Salmonella spp. in 4.1% (4/98), Campylobacter jejuni in 3.1% (3/98) and enteropathogenic Escherichia coli in 1.0% (1/98). The majority of positive samples belonged to patients in the age group 0–9 years in all three PCR-detected viruses (Graph1).
Tab. 2. Results of rapid antigen detection tests (RAT) and PCR (NA – data not applicable for negative results) Tabulka 2. Výsledky v záchytu pozitivit u použitých imunochromatografických testů (RAT) a PCR (NA – data nedostupná pro záchyt pouze negativních nálezů) 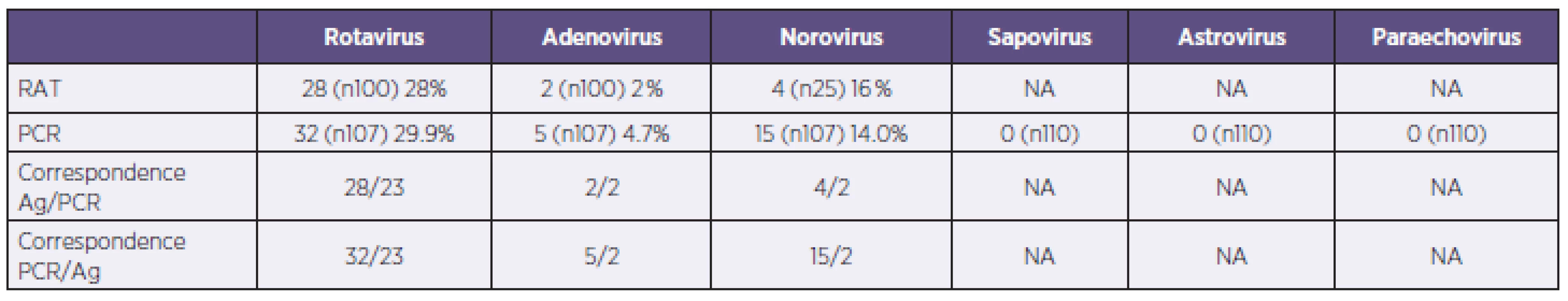
Graph 1. Number of PCR positive patients by age group Graf 1. Počty pozitivních nálezů u jednotlivých věkových skupin 
In comparison with immunochromatographic tests PCR had a higher detection rate. Altogether there were 52 positive detections by in-house PCR (from 107 samples examined by PCR) compared to 34 by immunochromatographic tests (from 100 samples examined for rotavirus and adenovirus, and 25 samples examined for norovirus) – Table 2. The sensitivity and specificity of the used immunochromatographic tests compared with PCR in our tested samples group were determined by standard calculation. For rotavirus sensitivity was 75.0% and specificity 92.9%; assay for adenovirus detection showed sensitivity of 40.0% and specificity 100%; for norovirus sensitivity was 66.7% and specificity 90.9%.
Rotavirus PCR-positive samples were diagnosed mainly in patients with age median of 4 years, and there were similar findings in adenovirus detection (age median 3 years). Noroviruses were positive in older patients, with age median of 36 years.
DISCUSSION
The sensitivity and specificity of immunochromatographic tests render such tests suitable for the first screening examination. According to the cited studies, the real sensitivity of these tests was lower than that presented in manufacture manuals compared with PCR. Khamrin et al. declared the sensitivity of the used immunochromatographic test for norovirus antigen detection as 78.9% [5, 21]. Very similar results were published also for other gastroenteritis viruses. Bon et al. found the sensitivity of various tested immunochromatographic kits for rotavirus detection from 70.0 to 98.8% [22]. These published data are in concordance with our findings, namely low sensitivity of the used immunochromatographic tests, at 40.0% to 75%, and specificity 90.9% to 100.0%. PCR was able to detect positivities in more samples than the immunochromatographic tests in all three compared viruses. PCR determined better results in samples of stool suspensions in PBS compared with those in PCR grade water – the positive curve started 1–3 cycles earlier for the same samples (data not shown).
The overall findings for viruses as aetiological agents in our samples by PCR were: rotavirus in 29.9%, norovirus in 14.0% and adenovirus in 5%. Our data were in concordance with published data from other European countries despite the relatively small sample numbers. Tran et al. published data from hospitalised children in the northern region of France (from 973 stool samples) and the evaluated prevalence was 21% for rotavirus, 13% for norovirus, 5% for adenovirus and 1.8% for astrovirus [23]. Somewhat different data were obtained in Germany from adult hospitalised patients, where the most frequent viral pathogen was norovirus (23%); rotavirus was second (15%) [24]. Studies from other countries distant from central Europe also gave very similar results. Koh et al. published the results from a previous study on hospitalised Korean children, in which rotavirus, norovirus, adenovirus and astrovirus were detected at 25.7%, 13.7%, 3.0% and 0.6% respectively [16]. In our study no astroviruses, sapoviruses or parechoviruses were found by PCR despite using probes for these pathogens. The reason for the absence of these pathogens in our results could be because of the small number of tested samples. The prevalence of astroviruses in stool samples may be 0.6–1.7% according to publications mentioned above. Our tested group included only 107 samples, and this number was probably too low. It is also possible that the territory may influence the incidence of these viruses; e.g. sapoviruses are more common pathogens in South-East Asia.
In our study co-infections were found in 5.5%; two viruses occurred in 4.5% of samples and one virus and bacteria in 1% of samples. In other cited studies co-infections were more frequent – between 3.2% and 36% [16, 23, 24]. Our findings on the median age of rotavirus - and adenovirus-infected patients (median age 3 years) accord with the knowledge that these viruses are predominantly pathogens of young children. On the contrary, noroviruses were found mainly in our adult patients (median age 36 years), also in accordance with existing knowledge [2].
CONCLUSION
Our study showed the necessity of using PCR for confirmatory assay because of the inadequate sensitivity of immunochromatographic rapid antigen tests. We have already set up in our hospital confirmatory PCR diagnosis of viral gastroenteritis as a routine method for severe or epidemiologically important cases.
We acknowledge the financial support by grant OVUVZU2009001 of the Ministry of Defence of the Czech Republic and Development project of Faculty Hospital No.61/2012.
Do redakce došlo dne 4. 9. 2014.
Adresa pro korespondenci:
MUDr. Miroslav Fajfr
Ústav klinické mikrobiologie
Fakultní nemocnice Hradec Králové
Sokolská 581
500 05 Hradec Králové
e-mail: fajfrmiroslav@seznam.cz
Zdroje
1. Tauriainen S, Martiskainen M, Oikarinen S et al. Human parechovirus 1 infections in young children – no association with type 1 diabetes. J Med Virol, 2007;79 : 457–462.
2. Eckardt AJ, Baumgart DC. Viral gastroenteritis in adults. Recent Pat Antiinfect Drug Discov, 2011;6(1):54–63.
3. Yu JM, Li DD, Xu ZQ et al. Human bocavirus infection in children hospitalized with acute gastroenteritis in China. J Clin Virol, 2008;42(3):280–285.
4. Chieochansin T, Thongmee C, Vimolket L et al. Human bocavirus infection in children with acute gastroenteritis and healthy controls. Jpn J Infect Dis, 2008;61 : 479–481.
5. Khamrin P, Takanashi S, Chan-It W et al. Immunochromatography test for rapid detection of norovirus in fecal specimen. J Virol Methods, 2009;157(2):219–222.
6. Khamrin P, Nguyen TA, Phan TG et al. Evaluation of immunochromatography and commercial enzyme-linked immunosorbent assay for rapid detection of norovirus antigen in stool samples. J Virol Methods, 2008;147(2):360–363.
7. Téllez CJ, Montava R, Ribes JM et al. Evaluation of two immunochromatography kits for rapid diagnosis of rotavirus infections. Rev Argent Microbiol, 2008;40(3):167–170.
8. de Rougemont A, Kaplon J, Billaud G et al. Sensitivity and specificity of the VIKIA(®) Rota-Adeno immuno-chromatographic test (bioMérieux) and the ELISA IDEIA trade mark Rotavirus kit (Dako) compared to genotyping. Pathol Biol (Paris), 2009;57(1):86–89.
9. Fong TT, Lipp EK. Enteric Viruses of Humans and Animals in Aquatic Environments: Health Risks, Detection, and Potential Water Quality Assessment Tools. Microbiol Mol Biol Rev, 2005;69(2):357–371.
10. Logan C, O’Leary JJ, O’Sullivan N. Real-Time Reverse Transcription-PCR for Detection of Rotavirus and Adenovirus as Causative Agents of Acute Viral Gastroenteritis in Children. J Clin Microbiol, 2006;44(9):3189–3195.
11. Logan C, O’Leary JJ, O’Sullivan N. Real-time reverse transcription PCR detection of norovirus, sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J Virol Methods, 2007;146(1-2):36–44.
12. Hoehne M, Schreier E. Detection of Norovirus genogroup I and II by multiplex real-time RT - PCR using a 3'-minor groove binder-DNA probe. BMC Infectious Diseases, 2006;6 : 69.
13. Zeng SQ, Halkosalo A, Salminen M et al. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J Virol Methods, 2008;153(2):238–240.
14. Jothikumar N, Cromeans TL, Hill VR et al. Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl Environ Microbiol, 2005;71(6):3131–3136.
15. National Institute of Public Health [online], EpiDat, [citated 2013-08-30], available on: http://www.szu.cz/publikace/data/vybrane-infekcni-nemoci-v-cr-v-letech-2003-2012-absolutne.
16. Koh H, Baek SY, Shin JI, Chung KS, Jee YM. Coinfection of viral agents in Korean children with acute watery diarrhea. J Korean Med Sci, 2008;23(6):937–940.
17. Dreier J, Stormer M, Made D et al. Enhanced reverse transcription-PCR assay for detection of norovirus genogroup I. J Clin Microbiol, 2006;44(8):2714–2720.
18. Jothikumar N, Kang G, Hill VR. Broadly reactive TaqMan((R)) assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples. J Virol Methods, 2009;155(2):126–131.
19. Le Cann P, Ranarijaona S, Monpoeho S, et al. Quantification of human astroviruses in sewage using real-time RT-PCR. Res Microbiol, 2004;155(1):11–15.
20. Baumgarte S, de Souza Luna LK, Grywna K et al. Prevalence, Types, and RNA Concentrations of Human Parechoviruses, Including a Sixth Parechovirus Type, in Stool Samples from Patients with Acute Enteritid. J Clin Microbiol, 2008;46(1):242–248.
21. Kirby A, Gurgel RQ, Dove W et al. An evaluation of the RIDASCREEN and IDEIA enzyme immunoassays and the RIDAQUICK immunochromatographic test for the detection of norovirus in faecal specimens. J Clin Virol, 2010;49(4):254–257.
22. Bon F, Kaplon J, Metzger MH, Pothier P. Evaluation of seven immunochromatographic assays for the rapid detection of human rotaviruses in fecal specimens. Pathol Biol, 2007;55(3-4):149–153.
23. Tran A, Talmud D, Lejeune B et al. Prevalence of Rotavirus, Adenovirus, Nororvirus and Astrovirus infections and coinfections among hospitalised children in northern France. J Clin Microbiol, 2010;48(5):1943–1946.
24. Jansen A, Stark K, Kunkel J et al. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infecious Disseases, 2008;8 : 143.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Mikrobiológia
Článok vyšiel v časopiseEpidemiologie, mikrobiologie, imunologie
Najčítanejšie tento týždeň
2014 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Predicted strain coverage of a new protein-based meningococcal vaccine in the Czech Republic
- A point prevalence survey of healthcare-associated infections in the Slovak Republic – a part of the EU project
- Nosocomial transmission of listeriosis
- Diversity of human Salmonella isolates in the South Moravian Region in 2009–2012
- Post-mortem analysis of Candida albicans breakthrough infection during echinocandin treatment in haematopoietic stem cell transplant recipient
- Candida dubliniensis in clinical specimens and possibilities for identification
- Natural antibodies against α(1,3) galactosyl epitope in the serum of cancer patients
- Cytolethal distending toxins
- Evaluation of the importance of a ready-made, gentamicin-impregnated spacer in relation to bacteriological findings in patients with periprosthetic joint infections
- Q fever – an occupational disease leading to disability – case report
- Measles re-emerging in the Ústí Region
- Vzpomínka na nedožité 90. narozeniny MUDr. Miroslava Přívory, CSc.
- Vzpomínka na nedožité 90. narozeniny prof. MUDr. Bohumila Ticháčka, DrSc.
- Phylogenetic and molecular analysis of A/H1N1pdm influenza viruses isolated in the epidemic season 2012/2013 from hospitalised patients with symptoms of influenza-like illness
- Viral gastroenteritis in Eastern Bohemia Region of the Czech Republic
- Seroprevalence study of hepatitis E virus infection in two districts of the Czech Republic
- An increase in the prevalence of syphilis in women in Eastern Bohemia – 30 years of surveillance
- Diagnosis of Clostridium difficile infections: Comparative study of two immuno enzyme assays with confirmation by PCR and culture followed by PCR ribotyping
- Epidemiologie, mikrobiologie, imunologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Candida dubliniensis in clinical specimens and possibilities for identification
- A point prevalence survey of healthcare-associated infections in the Slovak Republic – a part of the EU project
- Q fever – an occupational disease leading to disability – case report
- Diagnosis of Clostridium difficile infections: Comparative study of two immuno enzyme assays with confirmation by PCR and culture followed by PCR ribotyping
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



