-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Ciliary Beating Recovery in Deficient Human Airway Epithelial Cells after Lentivirus Gene Therapy
Primary Ciliary Dyskinesia is a heterogeneous genetic disease that is characterized by cilia dysfunction of the epithelial cells lining the respiratory tracts, resulting in recurrent respiratory tract infections. Despite lifelong physiological therapy and antibiotics, the lungs of affected patients are progressively destroyed, leading to respiratory insufficiency. Recessive mutations in Dynein Axonemal Intermediate chain type 1 (DNAI1) gene have been described in 10% of cases of Primary Ciliary Dyskinesia. Our goal was to restore normal ciliary beating in DNAI1–deficient human airway epithelial cells. A lentiviral vector based on Simian Immunodeficiency Virus pseudotyped with Vesicular Stomatitis Virus Glycoprotein was used to transduce cultured human airway epithelial cells with a cDNA of DNAI1 driven by the Elongation Factor 1 promoter. Transcription and translation of the transduced gene were tested by RT–PCR and western blot, respectively. Human airway epithelial cells that were DNAI1–deficient due to compound heterozygous mutations, and consequently had immotile cilia and no outer dynein arm, were transduced by the lentivirus. Cilia beating was recorded and electron microscopy of the cilia was performed. Transcription and translation of the transduced DNAI1 gene were detected in human cells treated with the lentivirus. In addition, immotile cilia recovered a normal beat and outer dynein arms reappeared. We demonstrated that it is possible to obtain a normalization of ciliary beat frequency of deficient human airway epithelial cells by using a lentivirus to transduce cells with the therapeutic gene. This preliminary step constitutes a conceptual proof that is indispensable in the perspective of Primary Ciliary Dyskinesia's in vivo gene therapy. This is the first time that recovery of cilia beating is demonstrated in this disease.
Published in the journal: Ciliary Beating Recovery in Deficient Human Airway Epithelial Cells after Lentivirus Gene Therapy. PLoS Genet 5(3): e32767. doi:10.1371/journal.pgen.1000422
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000422Summary
Primary Ciliary Dyskinesia is a heterogeneous genetic disease that is characterized by cilia dysfunction of the epithelial cells lining the respiratory tracts, resulting in recurrent respiratory tract infections. Despite lifelong physiological therapy and antibiotics, the lungs of affected patients are progressively destroyed, leading to respiratory insufficiency. Recessive mutations in Dynein Axonemal Intermediate chain type 1 (DNAI1) gene have been described in 10% of cases of Primary Ciliary Dyskinesia. Our goal was to restore normal ciliary beating in DNAI1–deficient human airway epithelial cells. A lentiviral vector based on Simian Immunodeficiency Virus pseudotyped with Vesicular Stomatitis Virus Glycoprotein was used to transduce cultured human airway epithelial cells with a cDNA of DNAI1 driven by the Elongation Factor 1 promoter. Transcription and translation of the transduced gene were tested by RT–PCR and western blot, respectively. Human airway epithelial cells that were DNAI1–deficient due to compound heterozygous mutations, and consequently had immotile cilia and no outer dynein arm, were transduced by the lentivirus. Cilia beating was recorded and electron microscopy of the cilia was performed. Transcription and translation of the transduced DNAI1 gene were detected in human cells treated with the lentivirus. In addition, immotile cilia recovered a normal beat and outer dynein arms reappeared. We demonstrated that it is possible to obtain a normalization of ciliary beat frequency of deficient human airway epithelial cells by using a lentivirus to transduce cells with the therapeutic gene. This preliminary step constitutes a conceptual proof that is indispensable in the perspective of Primary Ciliary Dyskinesia's in vivo gene therapy. This is the first time that recovery of cilia beating is demonstrated in this disease.
Introduction
Primary Ciliary Dyskinesia (PCD, OMIM #242650) is an inherited disease mainly characterized by dysfunction of airways' motile cilia. The prevalence is approximately 1 in 12,000–20,000 [1]–[3]. About 50% of patients affected by PCD have a situs inversus which results from monocilia dysfunction at the embryonic node [4]. This association is referred to as Kartagener's syndrome (OMIM #244400) [5]. PCD causes chronic sinus and bronchial respiratory infections that begin early in life, leading to nasal polyps and bronchiectasis. Males are frequently sterile due to dysfunctional spermatozoa flagella [6]. Other symptoms can also be associated with PCD like hydrocephalus, anosmia, retinitis pigmentosa and congenital heart diseases [7]–[10].
The disorder is genetically heterogeneous and in most cases, inheritance is autosomal recessive but X-linked inheritance patterns were also described [11]. Several loci and some genes have been identified, as DNAI1 (Ensembl ENSG00000122735), DNAH5 (ENSG00000039139), DNAH11 (ENSG00000105877), RPGR (ENSG00000156313), TXNDC3 (ENSG00000086288), OFD1 (ENSG00000046651), DNAI2 (ENSG00000171595) and KTU (alias C14orf104) (OTTHUMG00000152331) genes [10], [12]–[19]. The first gene described to be responsible for PCD and Kartagener syndrome was DNAI1 gene [18],[20]. Eighteen mutations in DNAI1 gene were reported, and Zariwala et al. evidenced a founder effect for the most frequent mutation (c.48+2_48+3insT) [21]. Moreover, the authors estimated that mutations in DNAI1 gene represent about 10% of PCD cases.
DNAI1 encodes an axonemal dynein intermediate chain, a component of the outer dynein arm (ODA). Dyneins are molecular motors which produce energy for microtubules doublets sliding in the axoneme. To date, no etiologic treatment of PCD is available and on the long range, PCD leads to respiratory insufficiency and lung transplant.
We hypothesized that gene therapy could restore ciliary function in DNAI1-mutated airway epithelial cells to prevent patients from infectious complications. To introduce genetic material into cells we focused on lentiviral gene transfer because lentivirus has the property to integrate its genetic material into host cell genome even in non-replicating cell [22]. Moreover, lentivirus is weakly immunogenic unlike recombinant adenovirus which efficiency was reported to decrease after several administrations in a clinical study of patients suffering from cystic fibrosis [23]. Lentiviral-derived vectors used in gene therapy were principally based on SIV (simian immunodeficiency virus) or HIV (human immunodeficiency virus). For this latter one, gene transfer efficiency into bronchial epithelium in mice was already demonstrated [24].
We decided to modify a SIV-based vector, previously described by Negre et al. to efficiently transduce mature human dendritic cells [25],[26], and to transduce human airway epithelial cells (HAECs) cultured as described by Jorissen et al. [27]. First, we showed here that normal HAECs were efficiently transduced by SIV-based vector containing eGFP gene. Then, we validated lentiviral vectors' constructions containing DNAI1 cDNA sequence and showed that transduced DNAI1 is transcribed and expressed. Finally, we demonstrated that transduction of DNAI1-mutated HAECs with wild-type DNAI1 can restore ciliary beating and that ODA are binding again to microtubules.
Results
Transduction of HAECs with pGFP
To estimate whether HAECs could be transduced by a SIV-based lentivirus pseudotyped with VSV-G, normal HAECs were infected with pGFP a vector containing eGFP as a reporter gene in a variety of conditions [25],[26]. Three parameters were investigated: (1) the multiplicity of infection (MOI), (2) the moment of infection and (3) whether using a polycation, Polybrene, or not. Two different moments of infection during Jorissen's culture were tested: at J+1, cells were ciliated and in suspension or at J+3, cells were de-differentiated and adherent. Two days post-infection, reporter gene expression was analyzed by FACS (Figure 1). In any of the selected conditions, HAECs were transduced but the proportion of transduced cells seemed to be dependant on MOI irrespective of the other parameters, and apparently higher at MOI 75. Then, transduction of cells infected at J+1 seemed more efficient compared to cells infected at J+3. At MOI 75, approximately 38% of cells infected at J+1 were transduced versus 20% for cells infected at J+3. Moreover, these results seemed to be improved by the use of Polybrene. Finally, at MOI 75, transduction efficiency was quantified at about 38% for cells infected at J+1 without Polybrene compared to approximately 50% for cells infected at J+1 with the use of Polybrene. These experiments which were not repeated, demonstrated that HAECs could be transduced in a variety of conditions and that J+1 with Polybrene at a MOI of 75 were presumably the best experimental conditions for HAECs infection. Therefore, we selected these conditions for further experiments.
Fig. 1. Transduction efficiency analysis by FACS two days after HAECs infection with pGFP vector. 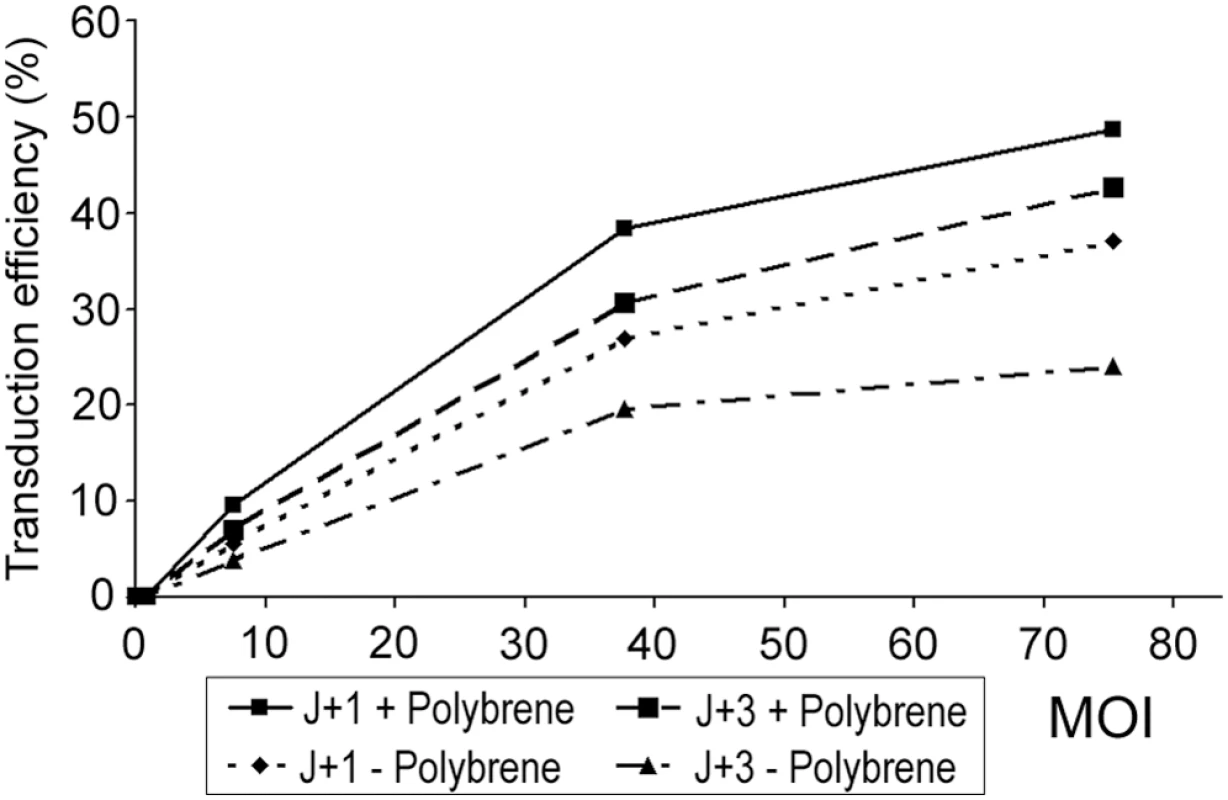
MOI, multiplicity of infection (3 values of MOI were tested 7, 35, and 75); J+1, one day after biopsy, ciliated cells in suspension; J+3, three days after biopsy, de-differentiated and adherent cells; +, with use of Polybrene; −, without use of Polybrene. These results were obtained from a single experiment in each condition. MOI is the ratio of infectious agents (e. g. lentivirus) to infection targets (e. g. cells). DNAI1 cDNA Cloning in Lentiviral Vectors
Full-length DNAI1 cDNA was cloned into the lentiviral vector in place of eGFP gene. Two constructions were obtained using BamH I and Xho I restriction sites (pK-DNAI1) or Nco I and Xho I (pK+DNAI1), and resulted respectively in an intact DNAI1 cDNA associated with a modified Kozak sequence which could prevent normal translation, or an intact Kozak sequence associated with a modified DNAI1 cDNA which could result in a dysfunctional protein (Figure 2A). Then, to differentiate endogene from exogene DNAI1, a hemagglutinin tag (HA) was added at the 3′ side of DNAI1 cDNA sequence in each plasmid which resulted in two additional vectors: pK-HA or pK+HA (Figure 2B).
Fig. 2. Lentiviral vectors containing DNAI1 cDNA sequence with or without HA tag. 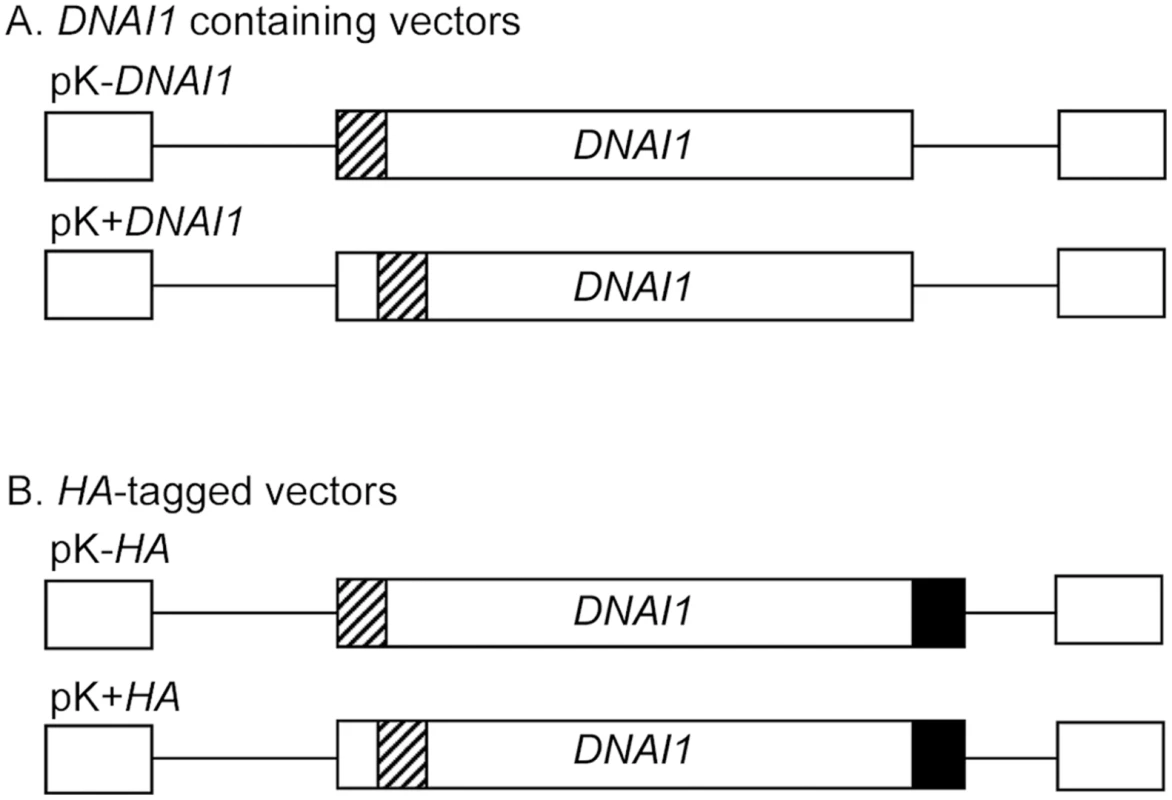
(A) pK-DNAI1 and pK+DNAI1 were derived from pGFP, with DNAI1 cDNA sequence in place of eGFP gene. pK-DNAI1 contains an intact DNAI1 cDNA sequence associated with a modified Kozak sequence (atccccaccauga), whereas pK+DNAI1 contains an intact Kozak sequence associated with a modified DNAI1 cDNA sequence (c.4A>G; p.I2V). (B) HA tag addition at 3′ side of DNAI1 cDNA sequence in pK-DNAI1 and pK+DNAI1 vectors forms pK-HA and pK+HA vectors, respectively. Striped box: site of sequence modification; filled box: HA tag sequence. Empty boxes located 5′ and 3′ to cDNA sequence represent 5′ and 3′ LTR (Long Terminal Repeat), respectively. Detection of DNAI1 Gene Expression in Transduced Normal HAECs
Normal HAECs were transduced at J+1 with Polybrene at a MOI of 75 since these conditions gave satisfactory results as evaluated with eGFP. First, mRNA extracts from transduced HAECs were controlled by PCR with alpha-tubulin specific primers for absence of genomic DNA (gDNA) contamination (not shown). Second, we confirmed that non-ciliated HAECs (NC) do not express DNAI1 because DNAI1 specific RT primer (P5) led to an absence of amplification by contrast to re-ciliated HAECs (RC) template (Figure 3, lanes 1 and 2). Third, DNAI1 mRNA was not amplified using a HA-tag RT specific primer (HA) from non-infected re-ciliated HAECs (Figure 3, lane 3). To test DNAI1 transcription from lentiviral vectors, HA-tagged DNAI1 gene transcription was revealed by RT-PCR using HA-DNAI1 specific primers. Re-ciliated HAECs infected by particles containing HA-tagged DNAI1 with either an exact Kozak sequence (pK+HA) or a modified Kozak sequence (pK-HA) both transcribed HA-tagged DNAI1 (Figure 3, lanes 4 and 5). Non-ciliated HAECs infected by pK-HA particles also expressed HA-tagged DNAI1 (Figure 3, lane 6). In conclusion, DNAI1 transcription is efficient from vectors containing a conserved or a modified Kozak sequence and transcription of the transduced DNAI1 cDNA driven by Elongation Factor-1 promoter is active in non-ciliated and re-ciliated HAECs.
Fig. 3. HA-tagged DNAI1 gene transcription analysis by RT–PCR, in transduced normal HAECs. 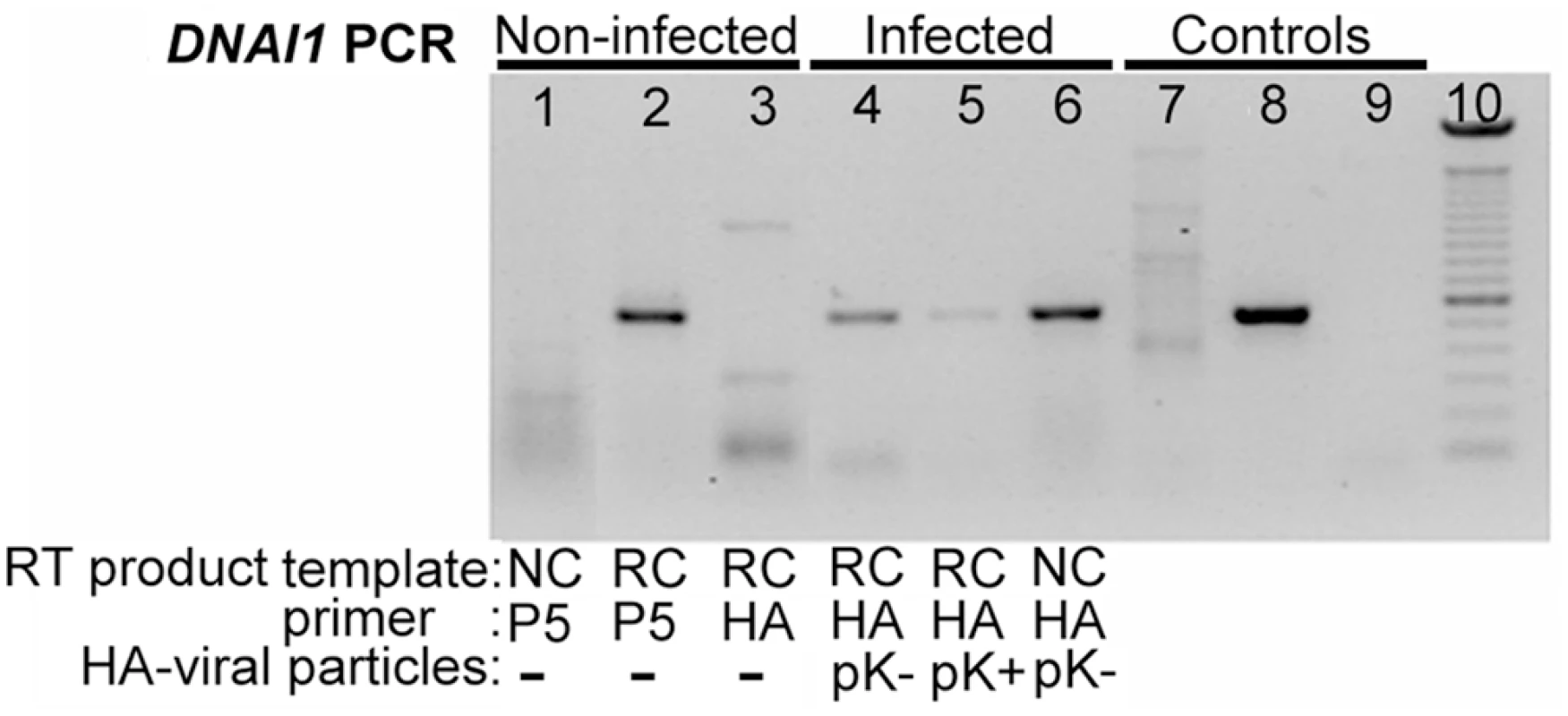
RT products were obtained using non-ciliated (NC) or re-ciliated (RC) HAECs mRNA and DNAI1 (P5) or HA-tag (HA) specific primers. All PCR products were obtained using DNAI1 cDNA specific primers. (1–3) Non-infected cells: (1) NC HAECs and P5 primer, (2) RC HAECs and P5 primer, (3) RC HAECs and HA primer; (4–6) Infected cells with HA-tagged DNAI1 and RT with HA primer: RC HAECs infected with (4) pK-HA or (5) pK+HA, (6) NC HAECs infected with pK-HA; (7–9) PCR control templates (no RT) were (7) genomic DNA, (8) pK-HA vector, (9) no DNA; (10) 100 bp DNA ladder. The symbol “-” means no infection by viral particles. Detection of DNAI1 Proteins in Transfected 293Bosc Cells
To detect DNAI1 protein translation from lentiviral vectors, 293Bosc cell line which naturally does not express DNAI1 was transfected by each lentiviral vector using ExGen500.
DNAI1 protein was detected by Western blot with specific anti-DNAI1 antibodies after 293Bosc cells' transfection with the Kozak modified (pK-DNAI1) or Kozak conserved (pK+DNAI1) DNAI1 sequence (Figure 4, lane 1 and 2). DNAI1 protein was also detected after 293Bosc cells' transfection with the vectors containing HA appended to the 3′ end of DNAI1 sequence (Figure 4, lane 3: modified Kozak and lane 5: conserved Kozak sequence). By contrast, no DNAI1 protein could be detected by Western blot in protein lysate of non-transfected 293Bosc cells with anti-DNAI1 antibodies (Figure 4, lane 4).
Fig. 4. DNAI1 protein detection with the use of DNAI1 anti-dyn69 antibody. 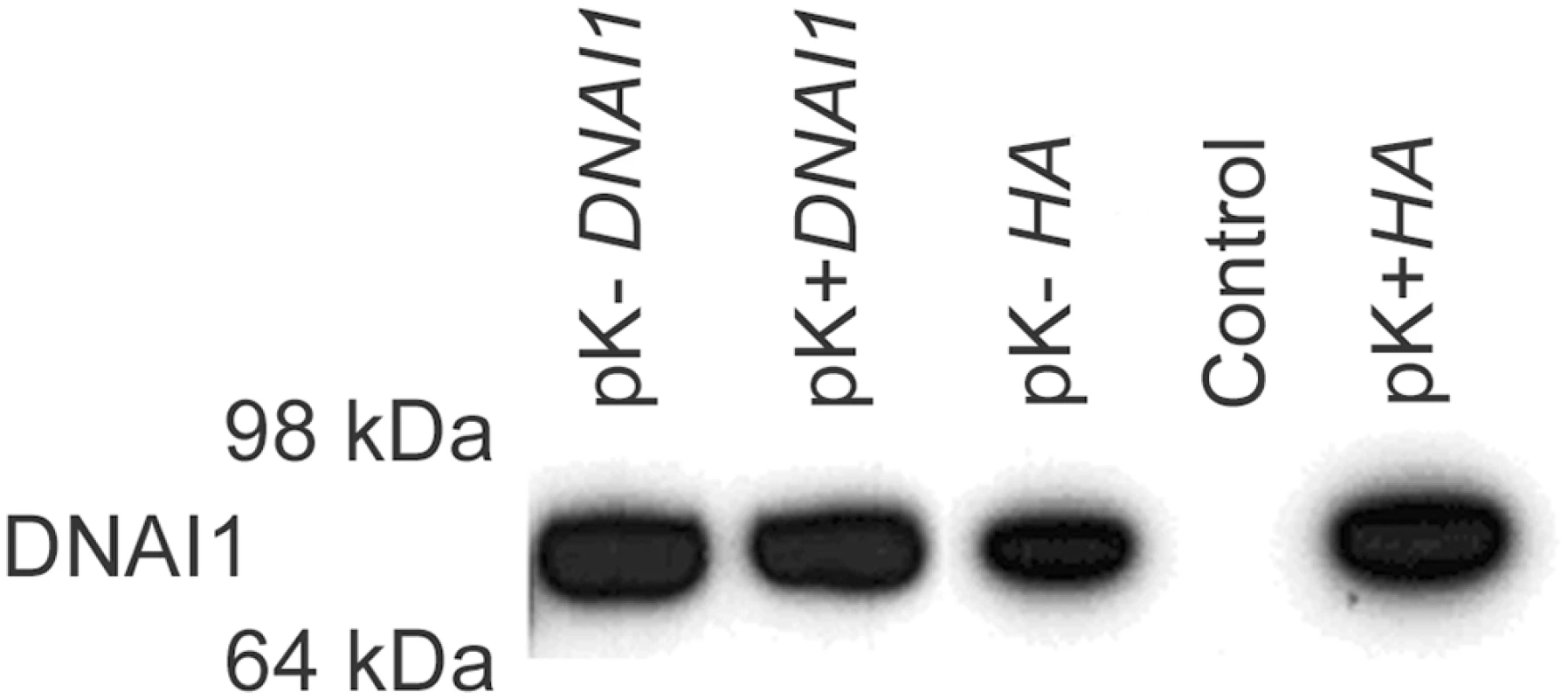
Western blot analysis of proteins lysates from transfected 293Bosc cells with (1) pK-DNAI1, (2) pK+DNAI1, (3) pK-HA, (5) pK+HA, vectors; (4) control of transfection. Moreover, immunofluorescence assays showed that in pK-HA transfected 293Bosc cells, cytoplasmic HA-tagged DNAI1 proteins could be specifically detected by HA antibodies (Figure S1).
Transduction of DNAI1–Mutated HAECs
In the second set of experiments, transduction was performed on DNAI1-mutated HAEC at J+1 and cells were cultured to generate ciliated vesicles. DNAI1-mutated HAECs were transduced with pK-DNAI1 or pK-HA to determine if immotile cilia might recover a beat. The same protocol of transduction was used as for normal HAECs. We confirmed DNAI1-mutated HAECs transduction efficiency with pGFP vector, as de-differentiated cells at J+3 and re-ciliated cells at J'+17 (17 days post-collagen digestion) expressed GFP protein (Figure S2).
After re-differentiation, GFP transduced HAECs were covered with cilia (Figure 5A-A) but these cilia were immotile. The variation of optic signal along a line crossing the cilia during 400 msec does not show any movement (Figure 5A-B). This immotily is also visible on video recording (Video S1). By contrast, ciliary beating was recorded on DNAI1-mutated HAECs transduced with either pK-DNAI1 or pK-HA vector (Figure 5A-C). This beat is demonstrated by recording the variation of optic signal along a line crossing cilia. Waves are clearly visible (Figure 5A-D) that evidenced the periodic beat of cilia. This active beat is also visible on video recording (Video S2). At J'+30, a ciliary beat frequency (CBF) was measured for pK-DNAI1 and pK-HA transduced cells. CBF of HAECs with beating cilia were 9.95±1.23 Hz and 11.31±0.85 Hz, for pK-DNAI1 and pK-HA treated cells, respectively. These values fall into the range of control HAECs (from 7 to 11 Hz). Cilia length in DNAI1-mutated HAECs was estimated at 6 µm in all cases and did not depend on DNAI1 treatment and cilia beating.
Fig. 5. Images of transduced DNAI1–mutated HAECs and electron microscopic sections of cilia. 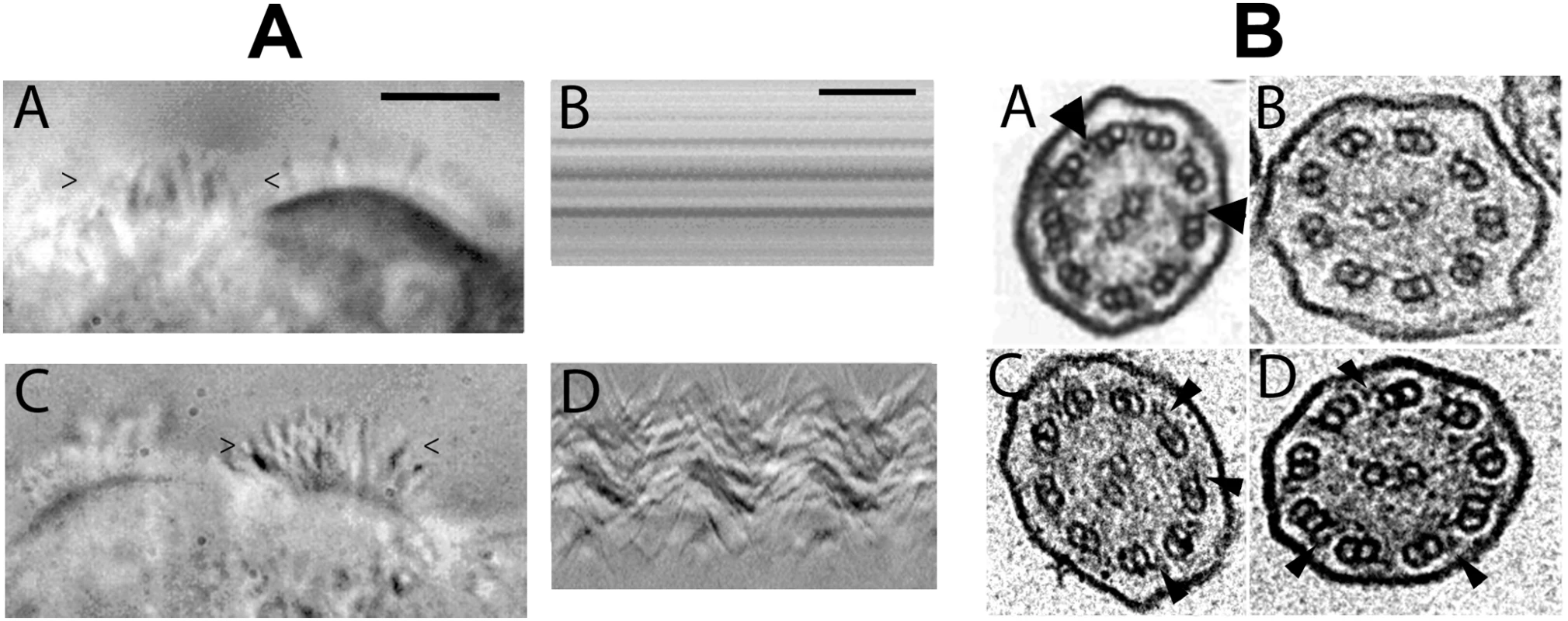
(A) At J'+31, vesicles had cells with cilia transduced with either pGFP (A-A) or pK-DNAI1 vectors (A-C). However, cilia of cells transduced with pGFP were immotile whereas cells transduced with pK-DNAI1 vectors were beating. Figures (A-B) and (A-D) represent the variations of the video signal during the time of recording (400 msec) along a virtual line delimited by the “>” and “<” signs on figures (A-A) and (A-C), respectively. The periodic beat of cilia is clearly visible on figure (A-D) compared to the immotility of cilia on figure (A-B). Black bar on figure (A-A): 10 µm. Black bar on figure (A-D): 100 msec. DNAI1–mutated HAECs transduced with pK-HA vector containing HA-tagged DNAI1 cDNA sequence gave identical results (data not shown). See Video S1 and Video S2. (B) Axoneme ultrastructural analysis by TEM (A-D): (B-A) Normal HAEC. (B-B) DNAI1–mutated HAEC treated with pGFP. (B-C) DNAI1–mutated HAEC treated with pK-DNAI1 vector. (B-D) DNAI1–mutated HAEC treated with pK-HA vector. Arrowheads indicate ODA. Axoneme diameter is about 0.2 µm. In DNAI1-mutated HAECs' axoneme, ultrastructure analysis by TEM showed that ODA were absent or shorter than in normal HAECs (Figure 5B-B). TEM on axonemes of DNAI1-treated cells were analyzed and some cilia had normal amounts of ODA, while in other cilia the ODA were partially absent (Figure 5B-C and 5B-D). The average number of ODA per axoneme was 3.29±1.53 in pGFP infected DNAI1-mutated HAECs. ODA increased significantly to 5.67±1.83 (p<0.0001) and 5.73±2.10 (p<0.002) in DNAI1-mutated HAECs treated with pK-DNAI1 and pK-HA, respectively. By contrast, there was no significant difference between DNAI1-mutated HAECs treated by pK-DNAI1 or pK-HA. The distribution of the number of ODA per axoneme presented a single peak in pGFP infected DNAI1-mutated HAECs but two peaks in pK-DNAI1 and pK-HA treated cells (Figure 6). IDA analysis showed no difference between control and DNAI1-treated cells (data not shown).
Fig. 6. Distribution of the number of ODA per axoneme in DNAI1–mutated infected HAECs. 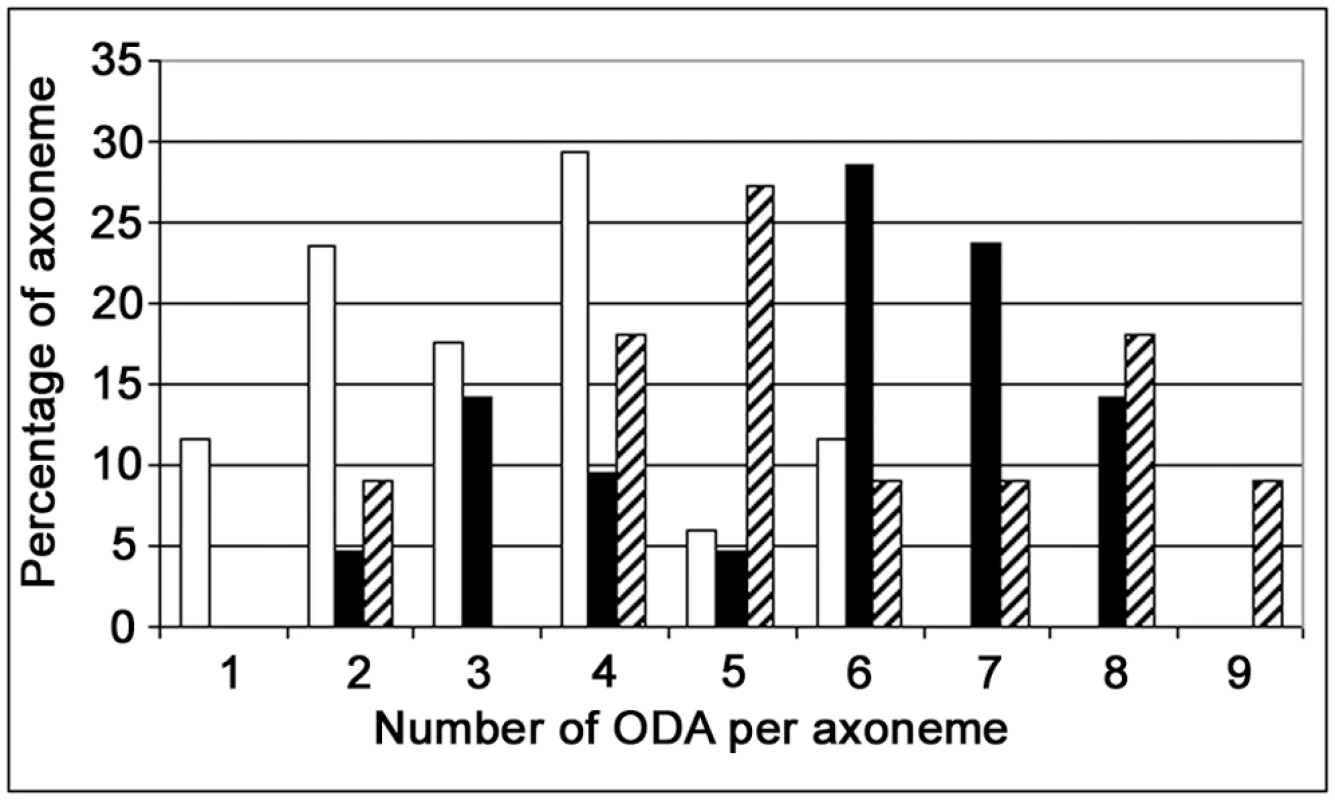
X axis, number of ODA per axoneme; Y axis, percentage of axonemes with a given number of ODA. Empty bars, DNAI1–mutated HAECs infected with pGFP; filled bars, cells infected with pK–DNAI1; striped bars, cells infected with pK-HA. Discussion
In the present study, we demonstrated that a SIV-based vector pseudotyped with VSV-G protein, previously described to efficiently transduce human dendritic cells [25],[26], could also efficiently infect normal cultured HAECs [27]. Previous studies showed that Murine Leukemia virus (MuLV) [28] and Feline Immunodeficiency Virus pseudotyped with VSV-G envelope [29] could transduce HAECs in culture. Though, this transduction was only possible from the basolateral surface of polarized HAECs. We did not know whether a Simian Immunodeficiency Virus would transduce although we could foresee that transduction would be more efficient from the basolateral surface of HAECs since it was pseudotyped with VSV-G. We did not know also whether de-differentiated cells would be transduced. It appeared that whatever the conditions used in this study, a certain proportion of cultured HAECs were infected. We then selected the conditions that seemed to provide the higher percentage of infected cells in culture. In any case, these conditions are strikingly different from in vivo situation where HAECs are a component of a complex epithelium recovered by a thin layer of mucus. Moreover, Polybrene which seemed to improve viral infection in cultured cells cannot be used in vivo due to side-effects. In a next step, we demonstrated transcription and translation of the transduced DNAI1 cDNA. Finally, we showed that DNAI1-mutated HAECs treated by DNAI1 gene transfer recovered ciliary beating whereas GFP-treated cells' cilia remained immotile, thus supporting our concept that PCD gene therapy was possible using DNAI1 gene. This conceptual proof is essential in the perspective of a human gene therapy.
Since it was impossible to obtain a vector containing a conserved Kozak sequence associated with a normal DNAI1 cDNA sequence, we decided to construct two vectors: one with a modified Kozak sequence upstream a conserved DNAI1 cDNA sequence (pK-DNAI1), and another one with a conserved Kozak sequence but associated with a modified DNAI1 cDNA sequence (pK+DNAI1). However, both vectors turned out to be equally efficient in terms of transcription and translation. Moreover, the addition of a HA tag at the 3′ end did not alter the transcription neither the translation of both vectors. Consequently, we pursued the infection experiments on DNAI1-mutated HAECs with the Kozak modified/DNAI1 conserved pair of vectors (with and without HA tag).
We were unable to detect endogenous DNAI1 protein and DNAI1 HA-tagged protein on Western blot of cultured normal HAECs presumably because the amount of extracted proteins from cultured HAECs was too low. However, translation from the various vectors was confirmed in transfected 293Bosc cells.
We also failed to label HA-tagged DNAI1 protein in the cilia of infected HAECs. However, the observation of the rescue of ciliary beating after DNAI1 treatment of DNAI1-mutated HAECs with either one of the selected vectors supported the idea that normal DNAI1 proteins, HA-tagged or not, were efficiently localized to cilia. This functional evidence was reinforced by ultrastructural analysis showing an increased number of ODA in axonemes with tagged and untagged DNAI1 proteins. Beside these observations, no difference in the number of IDA was detected between control and DNAI1-treated HAECs, in agreement with the knowledge that DNAI1 proteins specifically belong to ODA.
In DNAI1-mutated HAECs treated by DNAI1, a partially uncoordinated ciliary beating was observed, probably because as we demonstrated in early experiments, about half of infected cells were not transduced. As a consequence, HAECs aggregates are mosaics composed of cells with immotile cilia and other cells with beating cilia. The number of ODA per axoneme in treated DNAI1-mutated HAECs is lower than normal because these values reflected a mix population of HAECs: efficiently transduced cells and untransduced cells, as shown in the bimodal distribution of ODA per axoneme in Figure 6. In this respect, our results are consistent with the recessive inheritance type of PCD disease for which a normal copy of the DNAI1 gene is sufficient to an absence of symptoms.
In order to improve the number of rescued HAECs, we need to improve transduction efficiency, but respiratory epithelium is not exclusively composed of ciliated cells and at least seven other cell types are present [30]. To produce viral particles which would preferentially target human airway ciliated cells, the VSV-G large cell tropism protein could be replaced by another envelope protein from one of the various viruses known to infect airway epithelium, ie. coronavirus or Influenza virus [31],[32]. Influenza virus receptors, as α2,3-linked sialic acid receptors, were described to be specifically localized on ciliated airway and type II alveolar epithelial cells in both mouse and human respiratory epithelium, and on a subpopulation of basal cells in humans [32]. By contrast, α2,6-linked sialic acid receptors are expressed on human ciliated and goblet cells but not in the mouse. Therefore, it should be interesting to evaluate on our current culture model, the use of hemagglutinin (HA) from selected Influenza viruses which preferentially bind to ciliated cells. Finally, Szecsi et al. demonstrated that HA envelope protein of H7N1 and H5N1 avian viruses was successfully associated with retroviral-based vectors resulting in higher titres of infected cells than with VSV-G [33].
Airway ciliated cells are differentiated cells which do not proliferate and their cycle life span is supposed to be relatively short. In the mouse model, ciliated cells renewal was reported to be provided by Clara cells which are also essential in the alveolar type II cells generation [34]. Nevertheless, Park et al. reported that after an injury event, murine ciliated epithelial cells could transdifferentiate into other cell types and restored the airway epithelium [35]. In the human airway epithelium, Hajj et al. demonstrated that basal cells were able to regenerate as well as to differentiate into secretory and ciliated cells [36]. Targeting these basal cells with a lentiviral vector would result in the stable integration of the therapeutic gene with no need to repeat infections. The specificity of cells' targeting has been recently improved by taking into account the presence of proteases at the cell surface [37]. Thus, cell transduction can be achieved only in cell types that have the capacity to bind viral surface glycoprotein and to cleave the protease substrate because the surface glycoprotein of several enveloped viruses are expressed as precursor proteins that need to be cleaved into two subunits by cellular proteases to promote cell entry.
Finally, DNAI1 gene transfer could be applied to other genes causing PCD, as DNAH5 mutations are responsible for at least 28% of PCD cases [21]. However, another vector should be considered for this gene because of size limitation of lentivirus. As an alternative, AAV vectors offer a good safety profile in particular because viral DNA does not integrate into the genome of transfected cells. However, AAV repeated administration triggers anti-viral immune response and its packaging capacity is too limited to hold the DNAH5 gene (13.9 kb). High-capacity episomal vectors offer the possibility to deliver not only a large gene but also the whole genomic locus including regulatory regions which ensures a physiological expression with tissue and time specificity. However, delivery of extrachromosomal DNA with non viral or viral strategies is so far limited to cell lines. This system, tough, is appropriate for long-term treatment as in PCD because chromosome-based episomal systems contain elements enabling the cell to process episome as an additional chromosome with a mitotic stability >99% in cell lines [38].
Material and Methods
Patient Data
The diagnosis of a male patient affected by PCD was based on clinical signs (recurrent upper and lower respiratory tract infections since early in life, nasal polyps and complete situs inversus – Kartagener syndrome). In addition, he was sterile. Electron microscopy of the cilia showed no ODA. Ex vivo culture of epithelial airway cells according to Jorissen's method demonstrated that his cilia were 6 µm-long and totally immotile. Analysis of his DNA demonstrated that he harbored heterozygous compound DNAI1 mutations: c.48+2_48+3insT and c.1543G>A [20]. This patient signed an informed consent allowing us to experiment on nasal biopsies which consisted in removing the most anterior part of his middle nasal turbinate on both side. This study complies with the rules of the local ethical committee.
Human Airway Epithelial Cell Culture
Human airway epithelial cells (HAECs) from normal subjects were obtained from nasal turbinates which were removed and discarded in the process giving access to the ethmoidal sinus. Patients were operated for tumours located in the ethmoidal region and had no respiratory disease.
Cells from control subjects and the patient were grown using the immerged cell culture previously described by Jorissen et al. [27]. Briefly, ciliated cells were isolated and cultured the day following the biopsy (J+1) in collagen-coated flasks to de-differentiate in non-ciliated cells. When they reached 80–90% confluence, collagen was digested (J', 7–10 days post-seeding) and cells were suspended in flasks with rotation to re-differentiate in the form of ciliated vesicles.
Cells were infected at J+1 or J+3. Non-ciliated cells were harvested at J+7 to 10 (J') and re-ciliated cells were fully re-differentiated at J'+28.
Cloning of DNAI1 cDNA
To generate DNAI1 cDNA (AF091619), we extracted total RNA from ciliated HAEC and synthesized the cDNA. A pair of primers was designed to amplify the full-length cDNA. Six supplementary sets of specific primers were used to sequence DNAI1 cDNA. The full-length DNAI1 cDNA PCR product was cloned.
Addition of enzyme restriction sites in 5′ and 3′ DNAI1 cDNA sequence was performed using two sets of primers. The BAMHIDNAI1_for and NCOIDNAI1_for primers (forward) added BamH I and Nco I restriction sites upstream DNAI1 cDNA sequence, respectively. The XHOIDNAI1_rev primer (reverse) added a Xho I restriction site downstream DNAI1 cDNA sequence.
Addition of hemagglutinin (HA) tag downstream DNAI1 cDNA sequence was performed by PCR using lentiviral vectors containing DNAI1 cDNA (pK+DNAI1 or pK-DNAI1) as template and upDNAI1_for (forward)/lowHA_rev (reverse) primers. For more information see Text S1.
Lentiviral Vectors
The lentiviral vector system used in this study was derived from SIV vectors described by Negre et al. [25],[26]. Five different lentiviral vector constructs were used all under the transcriptional control of the human Elongation Factor-1 promoter (EFS): (1) pR4SA-EFS-GFP-W (pGFP), (2) pK-DNAI1, (3) pK+DNAI1, (4) pK-HA, (5) pK+HA. The pGFP vector contains the eGFP cDNA sequence. The pK-DNAI1 and pK+DNAI1 vector constructs are essentially the same as the pGFP vector but with the DNAI1 cDNA sequence replacing the eGFP gene sequence, using BamH I/Xho I and Nco I/Xho I sites, respectively (Figure 2A). The pK-DNAI1 vector has a modified Kozak sequence with an intact DNAI1 cDNA sequence whereas pK+DNAI1 has an intact Kozak sequence with a modification at position 4 of the DNAI1 cDNA sequence, resulting in DNAI1 second amino acid modification: isoleucine>valine. The pK-HA and pK+HA vector constructs correspond to pK-DNAI1 and pK+DNAI1 vectors, respectively, with a 3′ DNAI1 cDNA hemagglutinin (HA) tag addition, using Blp I/Xho I restriction sites (Figure 2B).
Lentiviral Transduction
Three sets of experiments were carried out.
In the first set, normal HAECs were transduced the day of seeding (J+1) on collagen-coated flasks or at J+3, and the cells were incubated for 24 hours with the lentivirus at MOI (Multiplicity Of Infection) of 7, 35 and 75 in the presence or absence of 6 µg/mL Polybrene (1,5-dimethyl-1,5-diazaundecamethylene polymethobromide, hexadimethrine bromide). MOI is the ratio of infectious agents (e. g. lentivirus) to infection targets (e. g. cells). Then, medium was completely changed in order to remove debris and inactive lentiviruses. Two days post-infection, reporter gene expression was analyzed by FACS.
In the second set of experiments, normal HAECs were transduced or not the day of seeding (J+1) at MOI75 with Polybrene with pK-HA or pK+HA. RNA was extracted before or after re-ciliation.
In the third set of experiments, transduction was performed on DNAI1-mutated HAECs at J+1, at MOI 75, with the use of Polybrene and cells were cultured to generate ciliated vesicles.
RT–PCR Analysis
Tagged DNAI1 gene expression was revealed by reverse transcription PCR (RT-PCR) on infected HAEC. Non-ciliated cells were collected the day of collagen digestion (J') and ciliated cells were collected when they were fully covered by cilia (J'+28).
Poly(A)+ mRNA was isolated by the Dynabeads Oligo(dT)25 purification kit, according to the manufacturer's protocol (Dynal Biotech, Norway).
Western Blotting Analysis
Transient transfection of 293Bosc cells was performed with each four different plasmids: pK-DNAI1, pK+DNAI1, pK-HA or pK+HA. Western blot analysis was carried out according to standard techniques (Text S1).
Video Analysis and Ciliary Beat Frequency (CBF)
In order to assess the functional activity of the ciliated DNAI1-mutated cells after lentiviral transduction, video recordings were performed with a ×40 objective lens in the light path at different steps of the culture in flasks, by using an Olympus IX50 inverted phase-contrast microscope. The control eGFP fluorescence was observed with a magnifying digital camera SCION CFW 1308M (Scion Corporation, Frederick, MD) and the recovery of ciliary beating was recorded with high speed digital video camera pco.1200 hs (PCO, Germany). The digital image-sampling rate was software-controllable using CamWare and for all experiments the sampling rate was set at 500 frames per second (fps).
For measurements of ciliary beat frequency (CBF), the video images of active ciliated cells were captured with a ×100 oil-immerged objective lens, using Leica DMRXA microscope and pco.1200 hs camera. A time-motion representation was obtained and analyzed using ImageJ software (National Institutes of Health, USA). Briefly, a line cutting cilia close to their tip was drawn. The “reslice” function of ImageJ was then used to obtain the video signal along this line (y axis) during 400 msec (x axis). Finally, the data were expressed as mean±SD from at least three regions of interest. Values for normal HAECs were used as controls.
Transmission Electron Microscopy (TEM) and Statistical Analysis
Transmission electron microscopic analyses were processed on ciliated vesicles obtained after HAECs culture, as described by Jorissen et al. [27]. Amounts of ODA were expressed as mean±SD from n axonemes (n = 17 for pGFP, n = 21 for pK-DNAI1, n = 11 for pK-HA). Values of HAEC infected with pGFP, pK-DNAI1 or pK-HA were statistically analysed by Student t test and the significance was calculated for a two-tailed test.
See Text S1 for more details.
Supporting Information
Zdroje
1. AfzeliusBA
1976 A human syndrome caused by immotile cilia. Science 193 317 319
2. BlouinJL
MeeksM
RadhakrishnaU
SainsburyA
GehringC
2000 Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. Eur J Hum Genet 8 109 118
3. El ZeinL
OmranH
BouvagnetP
2003 Lateralization defects and ciliary dyskinesia: lessons from algae. Trends Genet 19 162 167
4. NonakaS
TanakaY
OkadaY
TakedaS
HaradaA
1998 Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95 829 837
5. KartagenerM
StuckiP
1962 Bronchiectasis with situs inversus. Arch Pediatr 79 193 207
6. PedersenH
RebbeH
1975 Absence of arms in the axoneme of immobile human spermatozoa. Biol Reprod 12 541 544
7. DouekE
BannisterLH
DodsonHC
1975 Recent advances in the pathology of olfaction. Proc R Soc Med 68 467 470
8. Ibanez-TallonI
GorokhovaS
HeintzN
2002 Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum Mol Genet 11 715 721
9. KennedyMP
OmranH
LeighMW
DellS
MorganL
2007 Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 115 2814 2821
10. MooreA
EscudierE
RogerG
TamaletA
PelosseB
2006 RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet 43 326 333
11. NarayanD
KrishnanSN
UpenderM
RavikumarTS
MahoneyMJ
1994 Unusual inheritance of primary ciliary dyskinesia (Kartagener's syndrome). J Med Genet 31 493 496
12. BartoloniL
BlouinJL
PanY
GehrigC
MaitiAK
2002 Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A 99 10282 10286
13. BudnyB
ChenW
OmranH
FliegaufM
TzschachA
2006 A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet 120 171 178
14. DuriezB
DuquesnoyP
EscudierE
BridouxAM
EscalierD
2007 A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A 104 3336 3341
15. LogesNT
OlbrichH
FenskeL
MussaffiH
HorvathJ
2008 DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet 83 547 558
16. OlbrichH
HaffnerK
KispertA
VolkelA
VolzA
2002 Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet 30 143 144
17. OmranH
KobayashiD
OlbrichH
TsukaharaT
LogesNT
2008 Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456 611 616
18. PennarunG
EscudierE
ChapelinC
BridouxAM
CacheuxV
1999 Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet 65 1508 1519
19. SchwabeGC
HoffmannK
LogesNT
BirkerD
RossierC
2008 Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat 29 289 298
20. GuichardC
HarricaneMC
LafitteJJ
GodardP
ZaegelM
2001 Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). Am J Hum Genet 68 1030 1035
21. ZariwalaMA
LeighMW
CeppaF
KennedyMP
NoonePG
2006 Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med 174 858 866
22. NaldiniL
BlomerU
GallayP
OryD
MulliganR
1996 In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272 263 267
23. HarveyBG
LeopoldPL
HackettNR
GrassoTM
WilliamsPM
1999 Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Invest 104 1245 1255
24. LimberisM
AnsonDS
FullerM
ParsonsDW
2002 Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther 13 1961 1970
25. NegreD
DuisitG
MangeotPE
MoullierP
DarlixJL
2002 Lentiviral vectors derived from simian immunodeficiency virus. Curr Top Microbiol Immunol 261 53 74
26. NegreD
MangeotPE
DuisitG
BlanchardS
VidalainPO
2000 Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther 7 1613 1623
27. JorissenM
WillemsT
Van der SchuerenB
VerbekenE
De BoeckK
2000 Ultrastructural expression of primary ciliary dyskinesia after ciliogenesis in culture. Acta Otorhinolaryngol Belg 54 343 356
28. WangG
DavidsonBL
MelchertP
SlepushkinVA
van EsHH
1998 Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol 72 9818 9826
29. WangG
SlepushkinV
ZabnerJ
KeshavjeeS
JohnstonJC
1999 Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 104 R55 62
30. JefferyPK
LiD
1997 Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J 10 1655 1662
31. ChilversMA
McKeanM
RutmanA
MyintBS
SilvermanM
2001 The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J 18 965 970
32. IbricevicA
PekoszA
WalterMJ
NewbyC
BattaileJT
2006 Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol 80 7469 7480
33. SzecsiJ
BosonB
JohnssonP
Dupeyrot-LacasP
MatrosovichM
2006 Induction of neutralising antibodies by virus-like particles harbouring surface proteins from highly pathogenic H5N1 and H7N1 influenza viruses. Virol J 3 70
34. EvansMJ
JohnsonLV
StephensRJ
FreemanG
1976 Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 35 246 257
35. ParkKS
WellsJM
ZornAM
WertSE
LaubachVE
2006 Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 34 151 157
36. HajjR
BaranekT
Le NaourR
LesimpleP
PuchelleE
2007 Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 25 139 148
37. SzecsiJ
DruryR
JosserandV
GrangeMP
BosonB
2006 Targeted retroviral vectors displaying a cleavage site-engineered hemagglutinin (HA) through HA-protease interactions. Mol Ther 14 735 744
38. LufinoMM
EdserPA
Wade-MartinsR
2008 Advances in high-capacity extrachromosomal vector technology: episomal maintenance, vector delivery, and transgene expression. Mol Ther 16 1525 1538
Štítky
Genetika Reprodukčná medicína
Článek Neocentromeres Come of Age
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2009 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Neocentromeres Come of Age
- Mitotic Recombination: Why? When? How? Where?
- Life, Death, Differentiation, and the Multicellularity of Bacteria
- Capturing the Spectrum of Interaction Effects in Genetic Association Studies by Simulated Evaporative Cooling Network Analysis
- Measures of Autozygosity in Decline: Globalization, Urbanization, and Its Implications for Medical Genetics
- Ciliary Beating Recovery in Deficient Human Airway Epithelial Cells after Lentivirus Gene Therapy
- Parallel Germline Infiltration of a Lentivirus in Two Malagasy Lemurs
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Neocentromeres Come of Age
- Capturing the Spectrum of Interaction Effects in Genetic Association Studies by Simulated Evaporative Cooling Network Analysis
- Mitotic Recombination: Why? When? How? Where?
- Life, Death, Differentiation, and the Multicellularity of Bacteria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



