-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Mitotic Recombination: Why? When? How? Where?
article has not abstract
Published in the journal: Mitotic Recombination: Why? When? How? Where?. PLoS Genet 5(3): e32767. doi:10.1371/journal.pgen.1000411
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000411Summary
article has not abstract
DNA damage repair, loss of heterozygosity, and chromosome rearrangement are important aspects of genome stability, and all are tied to mitotic recombination. Despite the importance of mitotic recombination, the most basic questions about this process remain poorly understood. This is in part because mitotic recombination, in contrast to meiotic recombination, is rare on a per cell division basis [1]. A number of systems have been devised to detect or select for mitotic recombination. In this issue of PLoS Genetics, Lee et al. [2] describe a novel system that represents a major step forward in the study of spontaneous mitotic recombination events. Their studies have given us new insights into the why, when, how, and where of mitotic recombination.
Mitotic recombination was first described by Stern in his classic Drosophila experiments [3]. For Stern, “recombination” referred only to reciprocal crossovers (RCOs) (Figure 1A). A severe limitation of most RCO assays is that only one of the two reciprocal products can be recovered. Barbera and Petes [4] devised a clever method to recover both products of RCOs in Saccharomyces cerevisiae. They used this method to measure rates of spontaneous and induced mitotic recombination. Lee et al. have brought increased power to this assay by performing it in diploids with ∼0.5% heterology between the sequences of homologous chromosomes. This design allowed mapping of RCOs at high resolution, and also allowed study of another aspect of recombination—gene conversion (Figure 1C and 1D). Their analysis led to several key findings that provide unique and sometimes surprising insights into questions about mitotic recombination.
Fig. 1. Reciprocal crossovers and gene conversion. 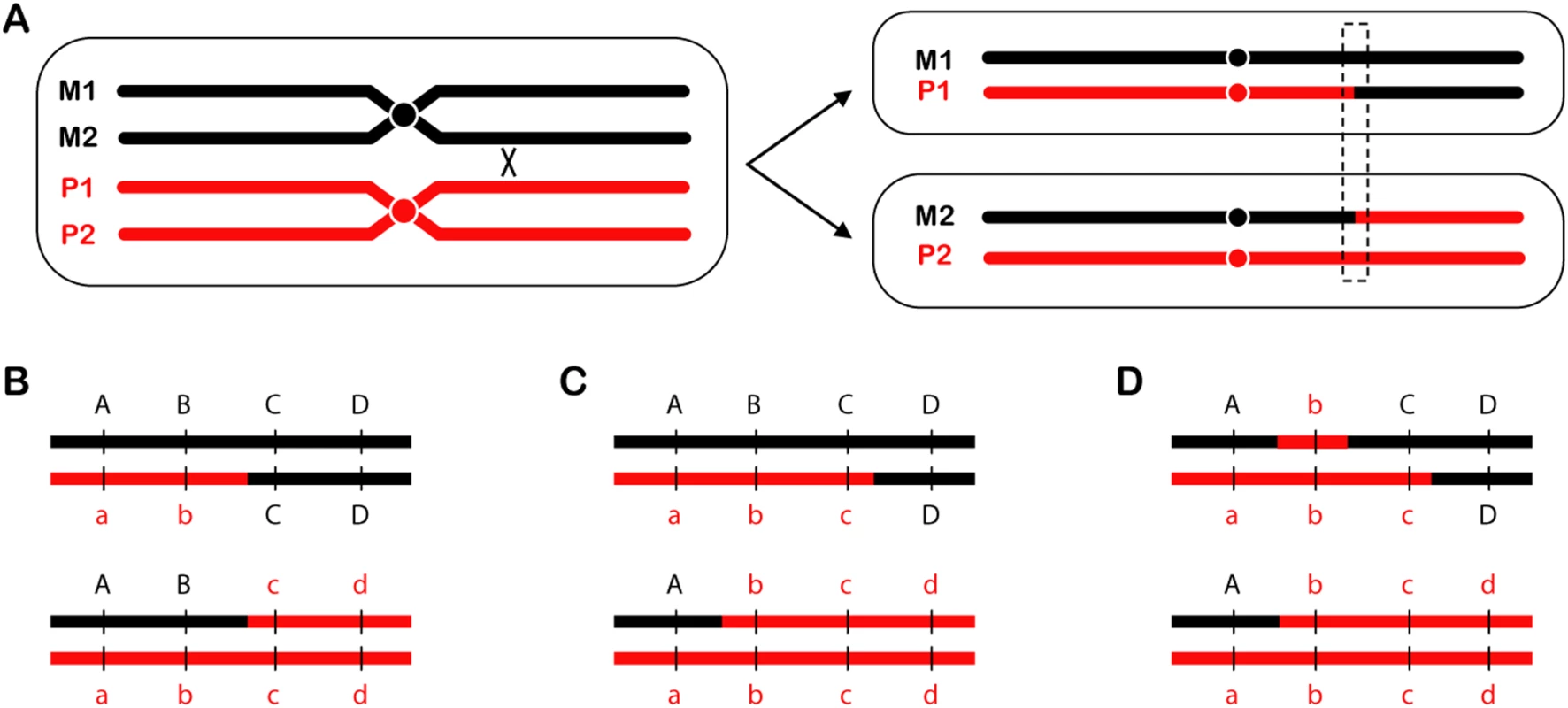
(A) An RCO is depicted between chromatids of two homologous chromosomes. One segregation pattern results in daughter cells that have become homozygous for the sequence distal to the crossover site. (B–D) A close-up view of the region outlined by the dotted box, showing different gene conversion tract configurations detectable using markers a through d. (B) No conversion tract, either because there was no gene conversion or the tract was too small to be detected with the markers available. All markers are still present in a 2∶2 ratio. (C) A typical gene conversion event produces a tract that alters some of the markers (b and c) to a 3∶1 ratio. Note that conversion tracts can only be detected if both reciprocal products (i.e., both daughter cells) are recovered and analyzed, as done by Lee et al. (D) Lee et al. observed some tracts that were wholly or partially 4∶0. In the example shown here, marker b has segregated 4∶0, but marker c has segregated 3∶1; this is therefore a 4∶0/3∶1 hybrid gene conversion tract. Why?
Why does mitotic recombination, which can be detrimental, occur? Answering this question begins with asking what initiates the process. Lee et al. suggest that most spontaneous RCOs are initiated by DNA double-strand breaks (DSBs). Recombinational repair of a DSB requires a template; when the homologous chromosome serves that role, it provides the opportunity for an RCO. Since there is also evidence that single-stranded nicks and gaps are recombinogenic [5], it is likely that several types of DNA lesions may be important for spontaneous mitotic recombination events. In addition, some recombinogenic agents (such as ultraviolet radiation) are thought to produce nicks that result in DSBs when the nicked DNA is replicated [6]. Thus, the question of why becomes tied up with the question of when.
When?
At what point in the cell cycle does mitotic recombination occur? Whereas meiotic recombination occurs during meiosis, most mitotic recombination probably does not occur during mitosis, but during interphase. Analysis of gene conversion tracts associated with RCOs provides clues about when during interphase mitotic recombination takes place. Gene conversion is a nonreciprocal exchange of genetic information. Normal gene conversion between homologous chromosomes produces a 3∶1 ratio of alleles (Figure 1C); however, Lee et al. also detected 4∶0 and 3∶1/4∶0 hybrid tracts (Figure 1D). Lee et al. argue that a 4∶0 tract most likely results when a break occurs prior to DNA replication, but repair takes place after replication. As depicted in Figure 8 of Lee et al., replication of a broken chromatid results in sister chromatids that are both broken at the same position. Since both are broken, the homologous chromosome must be used as a repair template. If both broken chromatids repair off the homologous chromosome, a 4∶0 or 4∶0/3∶1 hybrid is produced, depending on whether or not both tracts are identical. The high frequency of 4∶0 and 3∶1/4∶0 hybrid tracts suggests that a considerable fraction of the breaks that results in RCOs occur before replication.
How?
What is the molecular mechanism by which mitotic recombination is accomplished? The results presented by Lee et al. suggest that RCOs with different gene conversion tract lengths may be produced by different mechanisms. Short tracts may result from a DSB repair pathway involving heteroduplex formation followed by mismatch repair [1]. A heteroduplex is a region of DNA composed of strands that are derived from two different chromosomes. Polymorphisms between the two chromosomes will result in mismatches, and repair of these mismatches can result in gene conversion. Although this mechanism has been proven to be important for meiotic gene conversion, in which the conversion tracts are usually 1–2 kb long, evidence that it can produce the very long conversion tracts (average of 12 kb) observed by Lee et al. is lacking.
Lee et al. argue that the very large conversion tracts (some up to 100 kb in length) may reflect a different process, gap repair [7]. If a DSB is processed to form a gap, or if two DSBs occur on the same chromatid, the potential for extensive heteroduplex formation is eliminated. Instead, the entire gap is filled using the homologous chromosome as a template, resulting in a long patch of gene conversion. If these long tracts are indeed produced by gap repair, it raises the question of whether the RCOs associated with these tracts arise from a double-Holliday junction (DHJ) intermediate. Meiotic crossovers in S. cerevisiae involve a DHJ intermediate [8], but it is unknown whether this structure can be produced across a long gap.
Where?
Are there hotspots for mitotic recombination as there are for meiotic recombination? It is believed that some sites are hotter for DSB formation than others. Common fragile sites (CFSs), regions of the genome prone to chromosomal DSBs, are a normal feature of mammalian chromosomes, and analogous regions have been identified in yeast [9]. Most studies of CFSs have relied on the use of replication inhibitors to increase the frequency of breaks, followed by cytological detection [10]. In contrast, the approach taken by Lee et al. allows high-resolution molecular mapping of hotspots for mitotic recombination. They found that sites of RCOs, and therefore the initial sites of spontaneous damage, were nonrandomly distributed. Furthermore, the authors uncovered evidence for the existence of one region with elevated RCOs. This is exceptionally interesting because it represents a region prone to spontaneous rather than induced DSBs. The fact that such a hotspot could be detected by examining only 1% of the genome makes this discovery more intriguing still. It will be exciting to see if other such hotspots for spontaneous damage and mitotic recombination exist, and what role they play in genome stability. Taking advantage of the flexibility of the assay used by Lee et al., such as focusing on other regions of the genome or incorporating DNA repair mutants, will surely aid in future studies.
Many questions concerning mitotic recombination remain to be answered. Perhaps the most basic of these is what makes certain regions more prone to mitotic breakage and recombination than others? The approach of Lee et al. can address this question—and others—in an exciting new way by focusing on regions prone to spontaneous damage, as opposed to induced damage. These and other studies of mitotic recombination, a process both fundamental and far reaching, promise to continue to provide interesting insights into causes of genome instability.
Zdroje
1. PaquesF
HaberJE
1999 Multiple pathways of double-strand break-induced recombination in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63 349 404
2. LeePS
GreenwellPW
DominskaM
GawelM
HamiltonM
2009 A fine-structure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet 5(3) e1000410 doi:10.1371/journal.pgen.1000410
3. SternC
1936 Somatic crossing over and segregation in Drosophila melanogaster. Genetics 21 625 730
4. BarberaMA
PetesTD
2006 Selection and analysis of spontaneous reciprocal mitotic cross-overs in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 103 12819 12824
5. LettierG
FengQ
de MayoloAA
ErdenizN
ReidRJD
2006 The role of DNA double-strand breaks in spontaneous homologous recombination in S. cerevisiae. PLoS Genet 2(11) e194 doi:10.1371/journal.pgen.0020194
6. GalliA
SchiestlRH
1999 Cell division transforms mutagenic lesions into deletion-recombinogenic lesions in yeast cells. Mutat Res 429 13 26
7. SzostakJW
Orr-WeaverT
RothsteinRJ
StahlFW
1983 The double-strand break repair model for recombination. Cell 33 25 35
8. SchwachaA
KlecknerN
1995 Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83 783 791
9. LemoineFJ
DegtyarevaNP
LobachevK
PetesTD
2005 Chromosomal translocations in yeast induced by low levels of DNA polymerase: a model for chromosome fragile sites. Cell 120 587 598
10. GloverTW
BergerC
CoyleJ
EchoB
1984 DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet 67 136 142
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2009 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Neocentromeres Come of Age
- Mitotic Recombination: Why? When? How? Where?
- Life, Death, Differentiation, and the Multicellularity of Bacteria
- Capturing the Spectrum of Interaction Effects in Genetic Association Studies by Simulated Evaporative Cooling Network Analysis
- Measures of Autozygosity in Decline: Globalization, Urbanization, and Its Implications for Medical Genetics
- Ciliary Beating Recovery in Deficient Human Airway Epithelial Cells after Lentivirus Gene Therapy
- Parallel Germline Infiltration of a Lentivirus in Two Malagasy Lemurs
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Neocentromeres Come of Age
- Capturing the Spectrum of Interaction Effects in Genetic Association Studies by Simulated Evaporative Cooling Network Analysis
- Mitotic Recombination: Why? When? How? Where?
- Life, Death, Differentiation, and the Multicellularity of Bacteria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



