-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
What Research Is Needed to Stop TB? Introducing the
article has not abstract
Published in the journal: What Research Is Needed to Stop TB? Introducing the. PLoS Med 8(11): e32767. doi:10.1371/journal.pmed.1001135
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001135Summary
article has not abstract
Summary Points
-
Current tuberculosis (TB) control tools are insufficient to confront the global burden of TB. Novel tools and interventions are highly needed.
-
The Stop TB Partnership and the WHO Stop TB Department have launched the TB Research Movement, with the aim of boosting TB research and accelerating progress in TB control towards international targets.
-
In this paper, we describe the development of the Research Movement strategic plan, highlighting progress in its two key components: (1) the analysis of the global funding landscape for TB research, and (2) the development of a global TB research agenda.
-
Through this strategic plan, the TB research movement is creating a framework for concrete actions to harmonize and synergize TB research efforts globally, so that the poor and vulnerable populations burdened by TB will reap the dividend of less TB through more research and innovation.
Introduction
With 9.4 million new cases and 1.7 million deaths worldwide in 2009, tuberculosis (TB) constitutes an unacceptable burden of human suffering and loss [1]. The tools available for TB control are old, lack effectiveness, and are not readily accessible in many settings: the diagnosis of pulmonary TB still relies in most high-burden countries on sputum microscopy, a century old technology; treatment of tuberculosis is based on drugs that are over 40 years old and requires direct supervision to ensure full treatment adherence and prevent drug resistance; and the only TB vaccine (BCG), first used in 1922, has a variable protective efficacy in adults. Novel tools are needed for better TB care and control worldwide [2].
Research has a key role to play in meeting health and development goals. Based on the World Health Organization's (WHO) Stop TB Strategy, the Stop TB Partnership has developed the Global Plan to Stop TB 2011–2015, which lays out the activities to be achieved by 2015 towards elimination of TB (defined as ≤1 TB case per million population per year) by 2050 [3]. The plan sets out a roadmap for halving TB prevalence and deaths globally by 2015, compared with 1990 levels. However, while it is estimated that the incidence rate of TB has been falling globally since 2004, the present rate of decline (less than 1% per year) is insufficient to reach the elimination goal by 2050 [1]. Any realistic prospect of achieving this goal depends both on the better and wider use of existing technologies and the development of revolutionary new technologies for TB control. This would be possible only through an acceleration of research across the continuum, from basic to implementation [4].
Recognizing this, the Stop TB Partnership and the WHO Stop TB Department have launched the TB Research Movement, with the aim of boosting TB research and accelerating progress in TB control towards international targets [5],[6]. We describe here the strategy developed to address the objectives (Box 1) of the Research Movement and the progress made over the last 2 years.
Box 1. The TB Research Movement Objectives
Objectives of the Research Movement:
-
To provide leadership and advocacy to mobilize increased resources in support of a coherent and comprehensive global TB research agenda to meet the Stop TB goals and targets; and
-
To provide a forum for funders and implementers of TB research to coordinate plans and actions, with the result of ensuring that research needs are addressed, opportunities identified, and gaps filled.
The Research Movement Strategic Plan
The TB Research Movement is based at the Stop TB Partnership secretariat, housed by the WHO in Geneva, and works in close collaboration with the WHO Stop TB Department and with the Working Groups of the Stop TB Partnership. It operates as an umbrella for research-related issues at the Partnership, and receives advice from the Partnership Coordinating Board and the WHO.
The strategy plan developed to address the main objectives of the Research Movement has two major components: (1) the analysis of global TB research funding (aimed at estimating the funding gap); and (2) the development of a global TB research roadmap (representing a consensus on global needs across the TB research spectrum). To this end, the Research Movement has mobilized a broad alliance of stakeholders involved in TB research and development, including scientists involved in basic, applied, and operational research, TB control managers and public health officers, donors and aid agencies, and patients and community representatives.
Analysis of Global TB Research Funding
Mapping the research funding environment involves answering the questions “who is funding TB research and development (R&D)?”, “what is being funded?”, and “how much is being granted?”. For this, the Research Movement has joined with the Treatment Action Group's (TAG) efforts in evaluating the global landscape of funding in TB R&D, through a worldwide survey of funders and donors. This survey has been carried out regularly since 2005 and brings elements of responses to the questions above and helps monitor the trends in funding TB R&D internationally [7] (Figure 1).
Fig. 1. Investment in TB R&D by research category: 2005–2009 (from TAG report 2010). 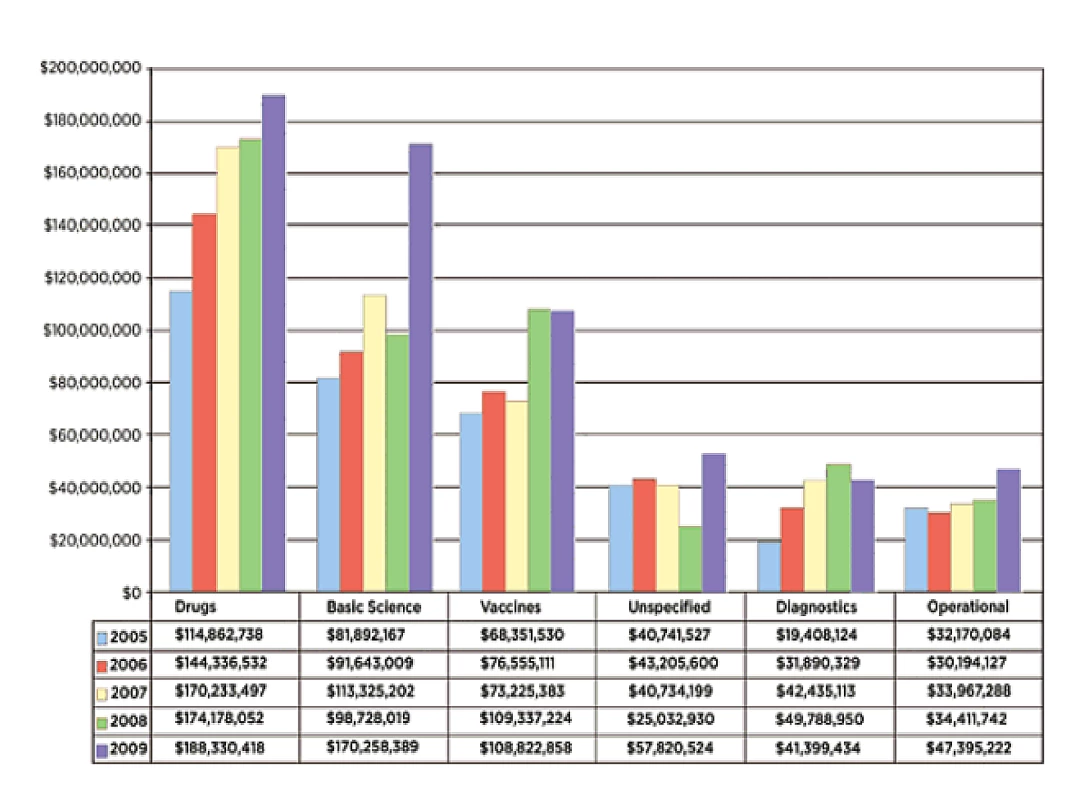
Reproduced with permission. The revised Global Plan to Stop TB 2011–2015 estimates that at least US$9.8 billion are needed in TB R&D over the next 5 years to reach the targets of 50% reduction in TB prevalence and mortality by 2015, more than twice those estimated in the initial Global Plan to Stop TB 2006–2015 (Table 1) [8]. Importantly, this updated Global Plan includes target investments for fundamental and operationalresearch, on top of the R&D for new drugs, diagnostics, and vaccines.
Tab. 1. Global Plan to Stop TB 2011–2015: total needs (US$ billion). 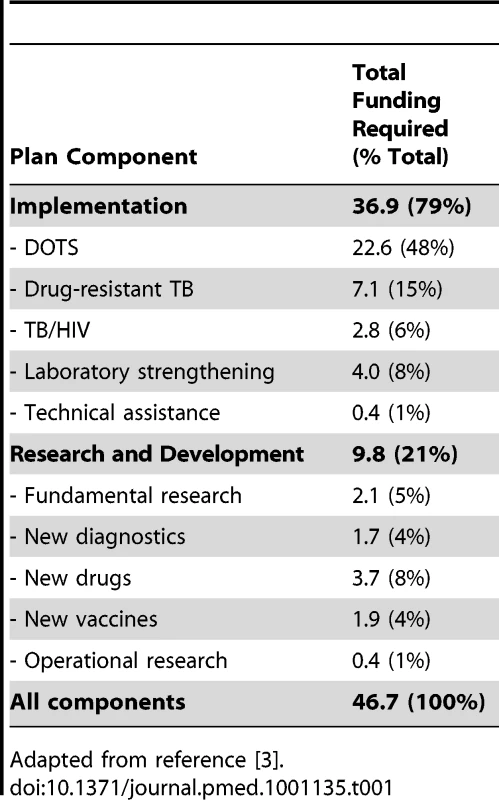
Adapted from reference [3]. From the above, the research funding gap can be estimated by comparing the results of the R&D funding survey to the research needs outlined in the Global Plan to Stop TB 2011–2015. Based on TAG Report 2010 [7], assuming that 2009 funding estimates are maintained throughout 2011–2015, and adjusting for inflation, the total funding gap for the next 5 years (2011–2015) is estimated at US$6.4 billion (64%) (Figure 2). The biggest gap in absolute terms is in R&D of new drugs, but the largest in relative terms (as a percentage of total funding required for that component) is R&D of new diagnostics. Remarkably, despite a significant boost in funding R&D for new tools in the past few years, TB research globally remains grossly underfunded, with a funding gap that is disproportionately greater for TB research (60%) than for implementation (35%) (Table 2).
Fig. 2. Funding required and available by research component 2011–2015. 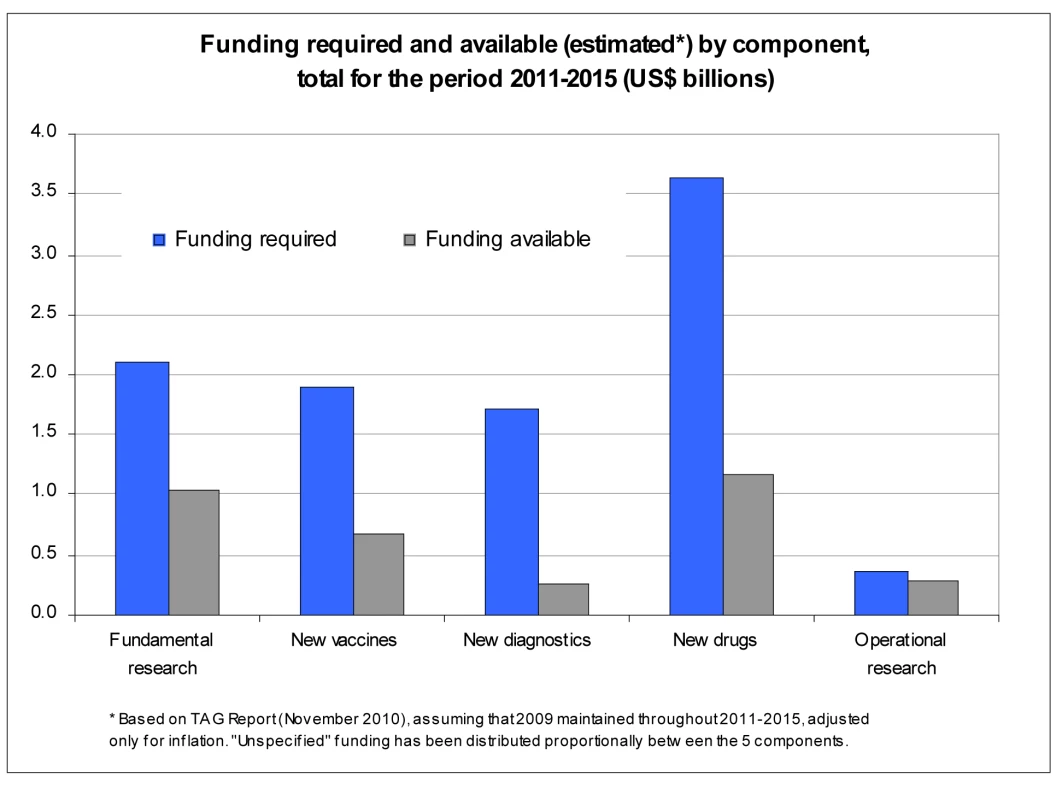
Tab. 2. Funding required and funding available under two possible scenarios and likely funding gaps (US$ billions). 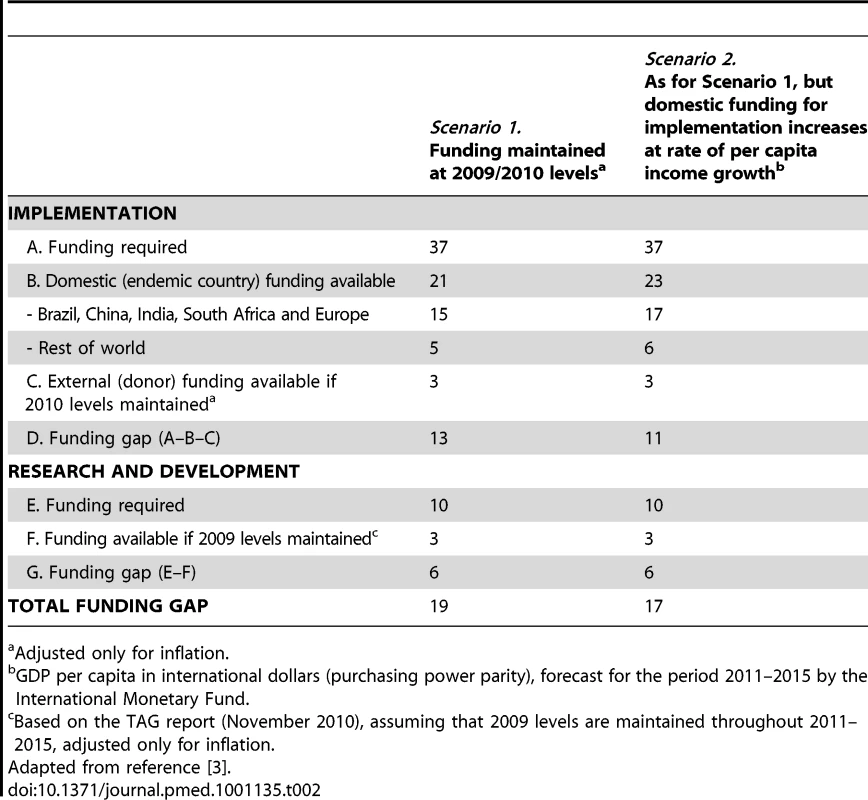
Adjusted only for inflation. The Global TB Research Roadmap
In compiling a global TB research agenda, the fundamental question is “what research is required to stop TB?” This involves answering the questions of “what are we researching in TB?” and “are we trying to answer the right questions?”, i.e., “are we doing studies in areas where evidence is lacking?”. In addressing these questions, we needed to identify critical gaps in research that represent bottlenecks for development of new tools. This allowed the development of a coherent and comprehensive global TB research roadmap towards TB elimination that encompasses all aspects of research, from basic science for discovery, to development of new tools, and their optimal uptake for better TB control.
The steps in developing the global TB research roadmap include a series of consecutive activities that are described below: (1) an inventory of the research agendas; (2) the development of key research questions; and (3) the prioritization of research questions.
Inventory of TB research agendas
Over the past decade, a variety of research agendas has been developed by various groups. A systematic review of these TB research agendas was carried out to evaluate the main research questions and themes, assess the methods used to select priorities, and identify any consistent message emerging from these agendas [9].
The review identified 33 papers. The priority areas for research were: drug development (28 articles), diagnosis (27), epidemiology (20), health services research (16), basic research (13), and vaccine development (13) (Table 3). Research questions were usually quite broad in scope. The most focused questions were on treatment and prevention of multidrug-resistant TB and TB/HIV co-infection, reflecting the inefficiencies of sputum-smear microscopy and the limits of the currently recommended short-course chemotherapy, which is inefficient against drug-resistant forms of the disease and is difficult to combine with standard antiretroviral therapy. The importance of epidemiology and health system research probably reflects the need for studies to optimize the availability and cost-effectiveness of interventions for TB control. The relatively low priority assigned to basic research may indicate the difficulty of establishing an agenda in a field that is mostly investigator driven.
Tab. 3. Number of studies identifying priority topics for TB research in a systematic review of 33 articles with TB research priorities. 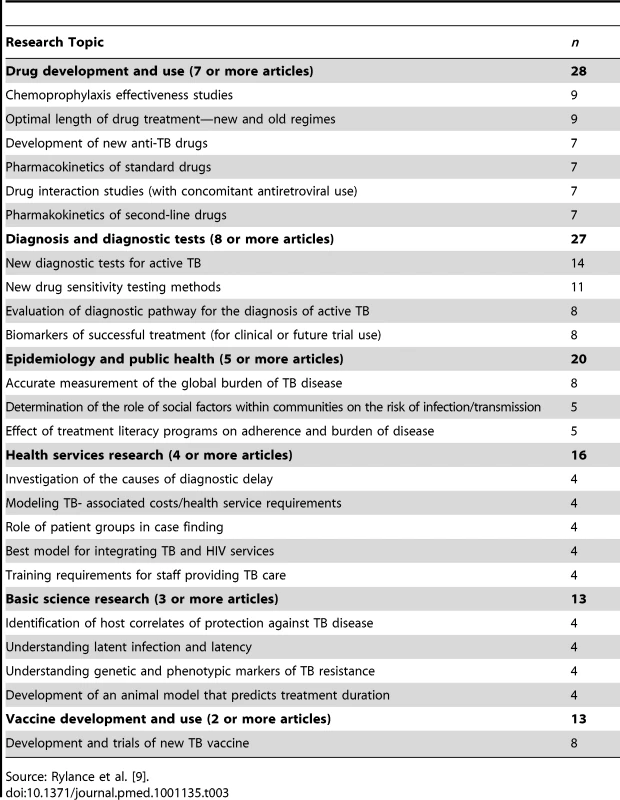
Source: Rylance et al. [9]. The methods used to identify priorities in these various agendas varied greatly. Most of these relied on expert meetings with consensus seeking, but few used objectively measurable criteria to select research priorities. Increased recourse to systematic reviews and use of clearly described and reproducible criteria to assess the importance of the research questions would greatly help in the establishment of research priorities.
Workshops to identify key research questions
Four workshops were organized in 2009 and 2010 to map out the landscape of TB research and identify gaps and priorities across the research continuum:
-
Two workshops were organized on new diagnostics, drugs, and vaccines. These assembled scientists, program managers, public–private partnerships, representatives from civil society, donors, and members of the Stop TB Partnership Working Groups. The objectives were to review the progress achieved since 2006 in the Global Plan to Stop TB 2006–2015, and update the research activities needed for the development of new diagnostics, drugs, and vaccines to meet the targets of the Global Plan by 2015.
-
A workshop on basic research for TB was organized in Bethesda, Maryland, United States, in March 2010, with the support of the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) and TAG. The objective was to define the critical priority questions that need to be addressed in the fundamental research area to underpin the development of new drugs, diagnostics and vaccines. This workshop was a major step in reiterating the importance of fundamental science as the driver of innovation for improved TB control [10].
-
A workshop was organized in May 2010 with the support of the Global Fund to Fight AIDS, Tuberculosis and Malaria to identify the operational (i.e., implementation, programmatic) research priorities to improve TB care and control. Five areas were identified: (i) access to, screening for, and diagnosis of drug-susceptible and drug-resistant TB; (ii) development of sustainable collaboration with all practitioners for TB care and control; (iii) prevention and treatment of TB in HIV-infected TB patients; (iv) optimal access to and delivery of treatment for drug-susceptible and drug-resistant TB; and (v) capacity building. Participants developed a list of operational research questions that were subsequently circulated to the Working Groups of the Stop TB Partnership for comments and suggestions [11]. One of the main outcomes of the workshop was the development of a document that lists the research priorities in the five key areas and provides, for each of these, a synopsis of the relevant methods and designs to address these research questions [12].
The prioritization of research questions
More than 250 research questions were identified within these workshops in the areas of fundamental science, R&D of new diagnostics, drugs, and vaccines, and operational and public health research. We set up a prioritization process to rank these questions using clearly defined and objectively measurable indicators, adapted from the Child Health Nutrition Research Initiative [13]. Prioritization was primarily based on the value that a scientific question is adding to the research area, how critical it is for the development of new tools and how it provides guidance for the implementation of these tools, to ultimately reduce morbidity and mortality due to TB. The priority ranking process was carried out independently by a group of 50 multi-disciplinary stakeholders representing a wide scope of research areas, public health, clinical care, and program management aspects.
Development of a roadmap for international TB research towards elimination
A coherent list of key research questions to be addressed for better TB control was established. We then requested experts to estimate the timeline under which these key research questions will be addressed in a chronological sequence, and their feasibility, so as to assess how the responses to these questions will fill the knowledge gaps and be conducive to further questions. The roadmap for international TB research is thus a living document that will be updated according to progress made. It is expected that the roadmap will provide a common framework for scientific disciplines to work concurrently and collaboratively towards better TB control, and will serve to promote TB research worldwide, including in high-burden countries.
The roadmap was presented and discussed at an international strategic meeting held in March 2011 in Bellagio, Italy, with the co-sponsorship of the Rockefeller Foundation. This meeting assembled major stakeholders, including key scientific thought leaders, representatives from nongovernmental organizations (NGOs), the vice ministers of health of Brazil and South Africa, and the top worldwide investors in TB research. The aim was to discuss the roadmap's vision of harmonized, synergistic, global TB research efforts through coordination of actions and funding. A summary of the main conclusions is provided in Box 2. In short, the Bellagio participants agreed that the Research Roadmap was a key vehicle to speed-up and coordinate TB research worldwide and that it be formally endorsed by the Coordinating Board of the Stop TB Partnership. It is expected that the roadmap will serve as a reference for activities carried out in the described research areas in support of the research objectives described in the Global Plan 2011–2015 and beyond.
Box 2. Conclusions of the Bellagio Meeting (16 March 2011)
The participants at the Bellagio meeting encouraged the Stop TB Partnership to endorse the Roadmap for International Research to Eliminate TB, publish it promptly as an independent document, and facilitate the execution of the following Action Plan:
-
Elaborate key areas of emphasis from the research roadmap to define an action plan for global TB research (including research advocacy);
-
Initiate consultations with countries, especially BRICS countries, researchers, policy makers, the private sector, and civil society to explore the key areas of emphasis/action plan and build ownership;
-
Match existing funded research with areas of emphasis to avoid unnecessary duplication, leverage existing resources and infrastructure to catalyse more effective collaborations;
-
Funders will establish a harmonization and coordination mechanism for research support.
Discussion
Devising a creative research response to the global TB epidemic is a pressing health research issue. In the context of health and human development, research to accelerate progress in TB control will have a direct impact not only on decreasing suffering and saving lives, but also on alleviating poverty and promoting social and economic development.
Much progress has been made over the last decade in the development of new tools for better TB control after decades of neglect. The TB diagnostics pipeline has rapidly expanded, and a major breakthrough was the recent introduction of Xpert MTB/RIF, a molecular assay capable of diagnosing TB and the presence of rifampicin resistance in 100 minutes [14]. The pipeline of new TB drugs has substantially expanded with more than 15 compounds in preclinical and clinical development, including nine novel candidates currently in phase I and II trials [15]. Ten vaccine candidates have entered clinical trials, four of which are presently in phase II trials [16]. Through the commitment of public–private partnerships and the engagement of major public and private research donors, there is a potential for efficient new tools to be available before 2015. This is, however, insufficient. As suggested by a recent mathematical model, the possibility to effectively control and eliminate TB by 2050 would rely on the combined and synergistic implementation of several novel strategies, including improved diagnosis of drug-susceptible and drug-resistant TB, shorter treatment of overt TB cases (≤2 months), scaled-up treatment of latently infected persons (especially in high-risk populations), and mass vaccinations campaigns using a more effective vaccine [17]. This will happen only through synergistic efforts in all areas of R&D.
This formidable challenge will not be met without increased investment in fundamental research for a better understanding of the natural history of TB in humans [3]. Further progress is needed to develop a point of care diagnostic tool that would diagnose all forms of TB in all settings, including latent TB infection [18]. While novel drugs are reaching the late development phase, we still need to identify suitable combinations to treat optimally all forms of TB in all populations and risk groups, as well as latent TB infection [19]. Lastly, the search for highly effective vaccines for the prevention of TB in all populations needs to be continued and amplified [16]. For each of these tools, the development pathway has a high attrition rate, and the chances of a successful product emerging from the end of the development pipeline depend in large part on the number of potential products entering the pipeline [19],[20].
Research is also needed downstream to identify means to improve TB control with existing tools, and guide the uptake and scale-up of innovations within reinforced health systems in endemic countries [21]. In this, the WHO plays a crucial normative role in assessing the evidence to endorse (or not) the new tool(s) or intervention(s) and providing guidance on their implementation through policy recommendations and technical support. This process has been conducted recently for new molecular diagnostics [20], and needs to be pursued for the new anti-TB drugs that will soon become available [19]. For this to happen smoothly, global coordination of efforts is essential to ensure rapid transfer of products and innovations to endemic countries.
There are, however, major challenges to be overcome. In view of the current global economic situation and the likelihood that available support by current major donors may stagnate or even decline in the near future, there is a need to increase the number and diversity of donors, and make the most rational use of any dollar spent on research. Countries with a high TB burden, especially Brazil, Russia, India, China, and South Africa (BRICS), could play a major role in TB R&D through increased contribution, as exemplified by the recent declaration from the first BRICS Health Ministers Summit in Beijing, China, in July 2011 [22],[23], which recognized the “need to establish priorities in research and development” and called for “increased innovation” in TB, and for support of “transfer of technologies and innovation in a sustainable way”.
Further discussions are needed so that key donors in TB research develop a consensus on funding a harmonized global TB research agenda for the years to come, and to enable the much needed acceleration in the development of new tools for control and their rapid uptake in policy and practice. This can take the form of coordinated cross-disciplinary projects to expedite research in specifically identified key strategic areas that will lead to concrete public health outcomes. It is expected that the International Roadmap for Tuberculosis Research will serve as a framework for concrete actions to synergize TB research efforts globally and catalyze the development of new research collaborations to address difficult and yet unanswered questions in TB. The Research Movement can play a crucial role in leveraging existing resources and infrastructure to accelerate research for much needed progress in TB control towards achievement of international targets [2],[3].
Zdroje
1. WHO 2009 Global tuberculosis control. WHO report 2009 (WHO/HTM/TB/2009) Geneva WHO
2. MaraisBJRaviglioneMCDonaldPRHarriesADKritskiAL 2010 Scale-up of services and research priorities for diagnosis, management and control of tuberculosis: a call to action. Lancet 375 9732 2179 2191
3. Stop TB Part and WHO 2010 Global plan to stop TB, 2011–2015: transforming the fight towards elimination of tuberculosis Geneva WHO
4. ChaissonREHarringtonM 2009 How research can help control tuberculosis? Int J Tuberc Lung Dis 13 5 558 568
5. World Health Organization 2006 Engaging for health. 11th General Programme of Work 2006–2015. A global health agenda Geneva WHO
6. RaviglioneMCUplekarM 2006 WHO's new StopTB Strategy. Lancet 367 952 955
7. Treatment Action Group 2010 2010 report on tuberculosis research funding trends, 2005–2009 New York Treatment Action Group Available: http://www.treatmentactiongroup.org/tbrd2010. Accessed 3 November 2011
8. Stop TB Part and WHO 2006 The global plan to stop TB, 2006–2015. WHO/HTM/STB/2006.35 Geneva WHO
9. RylanceJPaiMLienhardtCGarnerP 2010 Priorities for tuberculosis research: a systematic review. Lancet Inf Dis 10 886 892
10. Stop TB Partnership 2010 Workshop on fundamental research for tuberculosis. Draft workshop report; 18–19 March 2010; Bethesda, Maryland, United States Geneva World Health Organization Available: http://www.stoptb.org/assets/documents/research/report%20fundamental%20research%20workshop.pdf. Accessed 20 October 2011
11. Stop TB Partnership 2010 Promotion and rationalization of operational research activities in TB control. Draft workshop report; 11–12 May 2010; Geneva, Switzerland Geneva World Health Organization Available: http://www.stoptb.org/assets/documents/research/Draft%20Report%20OR%20Workshop%20v4.pdf. Accessed 20 October 2011
12. Stop TB Partnership, Global Fund Against AIDS, Tuberculosis and Malaria, World Health Organization 2011 Priorities in operational research to improve tuberculosis care and control Geneva WHO
13. RudanIEl ArifeenSBlackRECampbellH 2007 Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis 7 56 61
14. BoehmeCCNabetaPHillemannDNicolMPShenaiS 2010 Rapid molecular detection of tuberculosis and rifampicin resistance. N Engl J Med 363 11 1005 1015
15. MaZLienhardtCMcIlleronHNunnAJWangX 2010 Global tuberculosis drug development pipeline: the need and the reality. Lancet 375 9731 2100 2109
16. KaufmannSHEHusseyGLambertP-H 2010 New vaccines for tuberculosis. Lancet 375 9731 2110 2119
17. Abu-RaddadLJSabatelliLAchterbergJTSugimotoJDLonginiIMJr 2009 Epidemiological benefits of more effective tuberculosis vaccines, drugs and diagnostics. Proc Natl Acad Sci U S A 106 33 13980 13985
18. WallisRSPaiMMenziesDDohertyTMWalzlG 2010 Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375 9729 1920 1937
19. LienhardtCVernonARaviglioneMC 2010 New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Curr Opin Pulm Med 16 3 186 193
20. PaiMMinionJSteingartKRamsayA 2010 New and improved tuberculosis diagnostics: evidence, policy, practice, and impact. Curr Opin Pulm Med 16 3 271 284
21. LienhardtCCobelensF 2011 Operational research for improved TB control: the scope, the needs and the way forward. Int J Tuberc Lung Dis 15 1 6 13
22. BRICS Health Ministers 11 July 2011 BRICS Health Ministers' Meeting–Beijing Declaration. Available: http://keionline.org/node/1183. Accessed 20 October 2011
23. UNAIDS 11 July 2011 First meeting of BRICS health ministers brings new leadership to global health [press release]. Available: http://www.unaids.org/en/resources/presscentre/featurestories/2011/july/20110711bchinabrics/. Accessed 20 October 2011
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Voluntary Medical Male Circumcision: Strategies for Meeting the Human Resource Needs of Scale-Up in Southern and Eastern Africa
- Voluntary Medical Male Circumcision: Translating Research into the Rapid Expansion of Services in Kenya, 2008–2011
- Quality of Maternal Health Care: A Call for Papers for a Maternal Health Task Force–PLoS Collection
- Voluntary Medical Male Circumcision: Matching Demand and Supply with Quality and Efficiency in a High-Volume Campaign in Iringa Region, Tanzania
- Priorities for Research on Equity and Health: Towards an Equity-Focused Health Research Agenda
- What Research Is Needed to Stop TB? Introducing the
- Responsible Governance for Mental Health Research in Low Resource Countries
- Voluntary Medical Male Circumcision: Modeling the Impact and Cost of Expanding Male Circumcision for HIV Prevention in Eastern and Southern Africa
- Effect of Supplementation with Zinc and Other Micronutrients on Malaria in Tanzanian Children: A Randomised Trial
- HIV, Gender, Race, Sexual Orientation, and Sex Work: A Qualitative Study of Intersectional Stigma Experienced by HIV-Positive Women in Ontario, Canada
- Voluntary Medical Male Circumcision: A Framework Analysis of Policy and Program Implementation in Eastern and Southern Africa
- Voluntary Medical Male Circumcision: Logistics, Commodities, and Waste Management Requirements for Scale-Up of Services
- Optimal Uses of Antiretrovirals for Prevention in HIV-1 Serodiscordant Heterosexual Couples in South Africa: A Modelling Study
- Managing the Demand for Global Health Education
- Post-neonatal Mortality, Morbidity, and Developmental Outcome after Ultrasound-Dated Preterm Birth in Rural Malawi: A Community-Based Cohort Study
- Voluntary Medical Male Circumcision: An Introduction to the Cost, Impact, and Challenges of Accelerated Scaling Up
- On the Futility of Screening for Genes That Make You Fat
- Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis
- Physical Activity Attenuates the Influence of Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children
- A Head-to-Head Comparison of Four Artemisinin-Based Combinations for Treating Uncomplicated Malaria in African Children: A Randomized Trial
- Evidence-Based Guidelines for Mental, Neurological, and Substance Use Disorders in Low- and Middle-Income Countries: Summary of WHO Recommendations
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Evidence-Based Guidelines for Mental, Neurological, and Substance Use Disorders in Low- and Middle-Income Countries: Summary of WHO Recommendations
- Voluntary Medical Male Circumcision: Strategies for Meeting the Human Resource Needs of Scale-Up in Southern and Eastern Africa
- Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis
- Physical Activity Attenuates the Influence of Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



