-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Reporting and Methods in Clinical Prediction Research: A Systematic Review
Background:
We investigated the reporting and methods of prediction studies, focusing on aims, designs, participant selection, outcomes, predictors, statistical power, statistical methods, and predictive performance measures.Methods and Findings:
We used a full hand search to identify all prediction studies published in 2008 in six high impact general medical journals. We developed a comprehensive item list to systematically score conduct and reporting of the studies, based on recent recommendations for prediction research. Two reviewers independently scored the studies. We retrieved 71 papers for full text review: 51 were predictor finding studies, 14 were prediction model development studies, three addressed an external validation of a previously developed model, and three reported on a model's impact on participant outcome. Study design was unclear in 15% of studies, and a prospective cohort was used in most studies (60%). Descriptions of the participants and definitions of predictor and outcome were generally good. Despite many recommendations against doing so, continuous predictors were often dichotomized (32% of studies). The number of events per predictor as a measure of statistical power could not be determined in 67% of the studies; of the remainder, 53% had fewer than the commonly recommended value of ten events per predictor. Methods for a priori selection of candidate predictors were described in most studies (68%). A substantial number of studies relied on a p-value cut-off of p<0.05 to select predictors in the multivariable analyses (29%). Predictive model performance measures, i.e., calibration and discrimination, were reported in 12% and 27% of studies, respectively.Conclusions:
The majority of prediction studies in high impact journals do not follow current methodological recommendations, limiting their reliability and applicability.
: Please see later in the article for the Editors' Summary
Published in the journal: Reporting and Methods in Clinical Prediction Research: A Systematic Review. PLoS Med 9(5): e32767. doi:10.1371/journal.pmed.1001221
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001221Summary
Background:
We investigated the reporting and methods of prediction studies, focusing on aims, designs, participant selection, outcomes, predictors, statistical power, statistical methods, and predictive performance measures.Methods and Findings:
We used a full hand search to identify all prediction studies published in 2008 in six high impact general medical journals. We developed a comprehensive item list to systematically score conduct and reporting of the studies, based on recent recommendations for prediction research. Two reviewers independently scored the studies. We retrieved 71 papers for full text review: 51 were predictor finding studies, 14 were prediction model development studies, three addressed an external validation of a previously developed model, and three reported on a model's impact on participant outcome. Study design was unclear in 15% of studies, and a prospective cohort was used in most studies (60%). Descriptions of the participants and definitions of predictor and outcome were generally good. Despite many recommendations against doing so, continuous predictors were often dichotomized (32% of studies). The number of events per predictor as a measure of statistical power could not be determined in 67% of the studies; of the remainder, 53% had fewer than the commonly recommended value of ten events per predictor. Methods for a priori selection of candidate predictors were described in most studies (68%). A substantial number of studies relied on a p-value cut-off of p<0.05 to select predictors in the multivariable analyses (29%). Predictive model performance measures, i.e., calibration and discrimination, were reported in 12% and 27% of studies, respectively.Conclusions:
The majority of prediction studies in high impact journals do not follow current methodological recommendations, limiting their reliability and applicability.
: Please see later in the article for the Editors' SummaryIntroduction
In recent years there has been an increasing interest in the methodology of prediction research [1]–[16]. Prediction research includes both diagnostic prediction studies studying the ability of variables or test results to predict the presence or absence of a certain diagnosis, and prognostic studies studying predictors of the future occurrence of outcomes [6],[11],[15]. Both types of prediction research may include single variable (or predictor or test) studies, multivariable studies aimed at finding the independently contributing predictors among multiple candidate predictors, or the development, validation, or impact assessment of multivariable prediction models. Many have stressed the importance of pre-defining the key aspects of a study, including aims, study design, study population, clinically relevant outcomes, candidate predictors, sample size considerations, and statistical analysis. Use of poor methods may lead to biased results [2],[7],[10],[12],[13],[15],[17]–[32].
We performed a comprehensive literature review of articles published in high impact general medical journals to assess whether prediction research in the recent literature was conducted according to methodological recommendations. We considered all types of clinical prediction studies and all methodological issues that are considered to be important in prediction research, rather than on specific types of outcomes [33], specific methodological issues [34], or specific disease areas [20],[21],[35]–[37]. We focus on the reporting of aim, design, study sample, definition and measurement of outcomes and candidate predictors, statistical power and analyses, model validation, and results, including predictive performance measures.
Methods
Literature Search
We fully hand searched the six highest impact (based on Web of Knowledge impact factors) general medicine journals for the year 2008 (Figure 1). We excluded all studies that were not original research (e.g., editorials, letters) or had no abstract. One reviewer (W. B.) examined titles and abstracts of citations to identify prediction studies. The full text of all thus selected studies was obtained, and two authors (W. B. and N. P. A. Z.) independently assessed eligibility; in case of doubt a third independent reader was involved (K. G. M. M. or Y. V.).
Fig. 1. Flowchart of included studies. 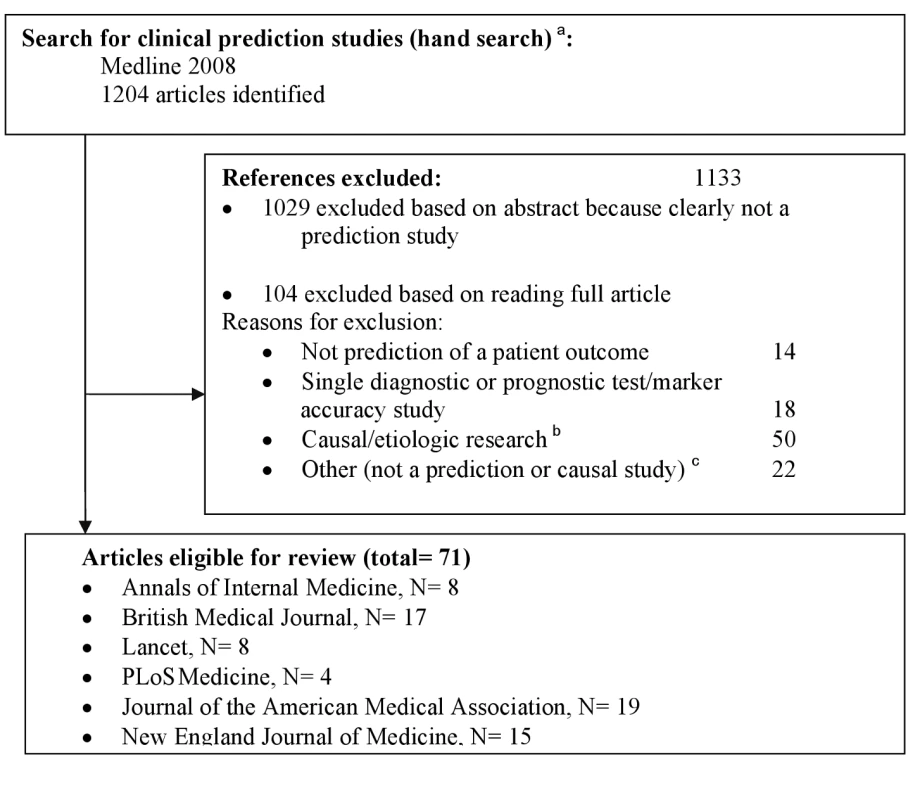
aThe hand search included only studies with an abstract, published in 2008 in The New England Journal of Medicine, The Lancet, JAMA: the Journal of the American Medical Association, Annals of Internal Medicine, BMJ, and PLoS Medicine. The following publication types were excluded beforehand: editorials, bibliographies, biographies, comments, dictionaries, directories, festschrifts, interviews, letters, news, and periodical indexes. bStudies, generally conducted in a yet healthy population, aimed at quantifying a causal relationship between a particular determinant or risk factor and an outcome, adjusting for other risk factors (i.e., confounders). cFor example, see [72]. Inclusion Criteria
We focused on multivariable prediction studies that were defined as descriptive studies where the aim was to predict an outcome by two or more independent variables, i.e., a causal relationship between independent variable(s) and outcome was not necessarily assumed [6],[11]. We included both diagnostic and prognostic multivariable prediction studies. We excluded studies that investigated a single predictor, test, or marker (such as single diagnostic test accuracy or single prognostic marker studies), studies that investigated only causality between one or more variables and an outcome, and studies that could not contribute to patient care, e.g., predictor finding studies to predict citation counts.
Development of Item List
We developed a comprehensive item list based on the existing methodological recommendations for conducting and reporting prediction research, and on extensive discussions among the co-authors. To this aim we studied existing reporting statements and checklists (e.g., CONSORT, REMARK, STARD, and STROBE) and quality assessment tools from other domains (e.g., QUADAS) for those aspects that also pertain to multivariable prediction studies, e.g., study aims, design, and participant selection [4],[38]–[41]. Further, to identify additional aspects relevant for good conducting and reporting of multivariable prediction research, we consulted published recommendations for prediction research and the references of these studies [1]–[10],[12]–[16],[20],[21],[27],[28],[42]–[44].
Data Extraction
Data were extracted to investigate both the reporting of and use of methods known to influence the quality of multivariable prediction studies. The main items that we extracted are summarised in Box 1. For investigation of statistical power in prediction studies, we considered the individual multivariable models within studies, because power differed among models within a single study.
Box 1. Overview of Items Addressed in This Review
Study design
Type of prediction study (e.g., model development); participant sampling or selection method (e.g., cohort, case-control approach)
Participants
Participant recruitment; follow-up; inclusion and exclusion criteria; setting (e.g., primary or secondary care or general population)
Candidate predictors
Clear definition to ensure reproducibility; coding of predictor values; assessment blinded for outcome
Outcome
Clear definition to ensure reproducibility; type of outcome; assessment blinded for predictors
Statistical power
Effective sample size (e.g., number of outcome events compared to number of candidate predictors)
Selection of predictors
Selection of predictors prior to statistical analysis and within statistical analysis; use of variable selection strategies (e.g., backward selection); criterion for predictor inclusion (e.g., p<0.05)
Handling of missing values
Reporting of missing values per predictor, or number or percentage of participants with missing values; reporting of procedures for dealing with missing values
Presentation of results
Reporting of univariable and multivariable predictor–outcome effects; reporting of full or final model
Model performance measures and validation
Type of predictive performance measures reported (e.g., C-statistic and calibration); type of validation (e.g., internal or external)
Items were scored as present, absent, not applicable, or unclear. If an item concerned a numeric value (e.g., the number of participants) we extracted this value. If a description was unclear, we classified it as not described or separately reported it in our tables. If studies referred to other papers for detailed descriptions, the corresponding items were checked in those references.
Two authors (W. B., N. P. A. Z.) independently extracted the data. In case of doubt, items were discussed with a third and fourth reviewer (K. G. M. M., Y. V.). The inter-reviewer agreement on the data extraction was assessed by calculating the percentage of overall agreement between the two reviewers.
Analysis
We distinguished five types of multivariable prediction research.
Predictor finding studies
These studies aim to explore which predictors out of a number of candidate predictors independently contribute to the prediction of, i.e., are associated with, a diagnostic or prognostic outcome [3],[6],[28].
Model development studies without external validation
These studies aim to develop a multivariable prediction model, e.g., for use in practice to guide patient management. Such studies aim to identify the important predictors, assign the (mutually adjusted) weights per predictor in some kind of multivariable analysis, and develop a final multivariable prediction model [6],[7]. These studies might include internal validation studies, such as random split-sample methods, cross-validation, or bootstrapping [43].
Model development studies with external validation
These studies have the same aim as the previous type but also test the performance of the developed model in a so-called external dataset from, e.g., another time period (temporal validation) or another hospital, country, or setting (geographical validation). Explicit withholding of the data from some study centres for validation was also considered as (geographical) external validation.
External validation studies without or with model updating
These studies aimed to assess the performance of a previously reported prediction model using new participant data that were not used in the development process, and in some cases adjusted or updated the model based on the validation data when there was poor validation [8]–[10],[45]–[47].
Model impact studies
These studies aim to quantify the effect or impact of using a prognostic or diagnostic prediction model on patient or physician behaviour and management, patient health outcomes, or cost-effectiveness of care, relative to not using the model [9],[45],[48].
We grouped results by type of multivariable prediction research, medical specialty (oncology, cardiovascular diseases, other), and whether the prediction analysis was a primary or secondary study aim.
Ethics Statement
An ethics statement was not required for this work.
Results
We identified 1,204 articles by hand searching, of which 71 met the inclusion criteria (Figure 1 and Text S2). Most studies were excluded based on title or abstract. It was difficult to distinguish, from study abstracts alone, between descriptive predictor finding studies and articles studying causality of a factor. We thus read 104 full text papers, excluding 50 studies deemed causal (Figure 1). The PRISMA checklist is provided as Text S1.
Data Extraction and Reviewer Agreement
The two reviewers agreed on a median of 92% (interquartile range 75%–100%) of the items extracted. Most discrepancies related to specific participant sampling strategies or participant sources and were resolved after discussion with a third reviewer.
The main challenge was to distinguish between predictor finding and model development studies. Authors in general did not explicitly state their aim, so we used full text interpretations to classify studies as predictor finding or model development studies.
Study Aims
Most multivariable prediction studies were published in the field of cardiovascular diseases (n = 24) (Table 1). The aim was usually to identify independently contributing predictors of an outcome (n = 51/71). Of the prediction modelling studies (n = 20), the vast majority were model development studies, without (n = 11) or with (n = 3) external validation. Pure external validation (n = 3) and impact studies (n = 3) were rare. There were few multivariable diagnostic studies (n = 5). In the 71 publications, 135 models or sets of predictors were studied. For example, in predictor finding studies a search for independently contributing predictors might be applied across different participant subgroups (e.g., males versus females) for multiple outcomes, and in prediction modelling studies, more than one model might be developed or validated (e.g., for different outcomes or presenting a basic and extended model).
Tab. 1. Aim of the included multivariable prediction studies, subdivided by clinical domains. 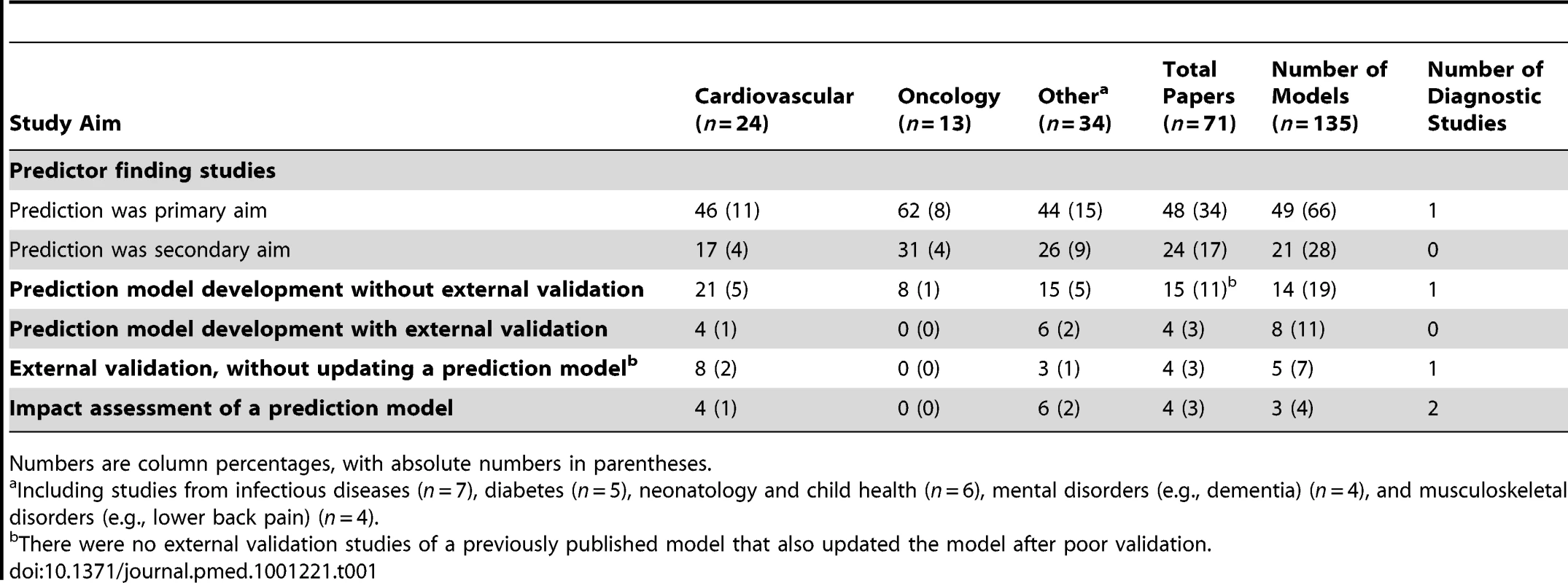
Numbers are column percentages, with absolute numbers in parentheses. Design of Participant Sampling
A cohort, nested case-control, or case-cohort design is commonly recommended for prognostic and diagnostic model development and validation [6]. A prospective cohort is preferable, because it enables optimal measurement of predictors and outcome. A retrospective cohort may allow for a longer follow-up period but usually at the expense of poorer data [6]. Randomized trial data have advantages similar to those of prospective cohort data, unless restrictive eligibility criteria make the study sample unrepresentative. Further, treatments proven to be effective in the trial should be included or adjusted for in the modelling. Cohort, nested case-control and case-cohort datasets each allow for calculation of absolute outcome risk [6],[18],[49]. A non-nested case-control design may, however, be sufficient for predictor finding studies, since these studies generally do not aim to calculate absolute risks.
We found that case-control designs were indeed used only by predictor finding studies (Table 2). Prospective cohort data, either observational or randomized trial data, were most frequently used (n = 44). Quantifying the impact of a prediction model on participant outcome requires a comparative study design [9],[48]. A randomized trial was used by two of the three impact studies; the third used an observational before-after (prospective) cohort design, comparing participant outcomes before and after the introduction of a prediction model.
Tab. 2. Study design in relation to study aim. 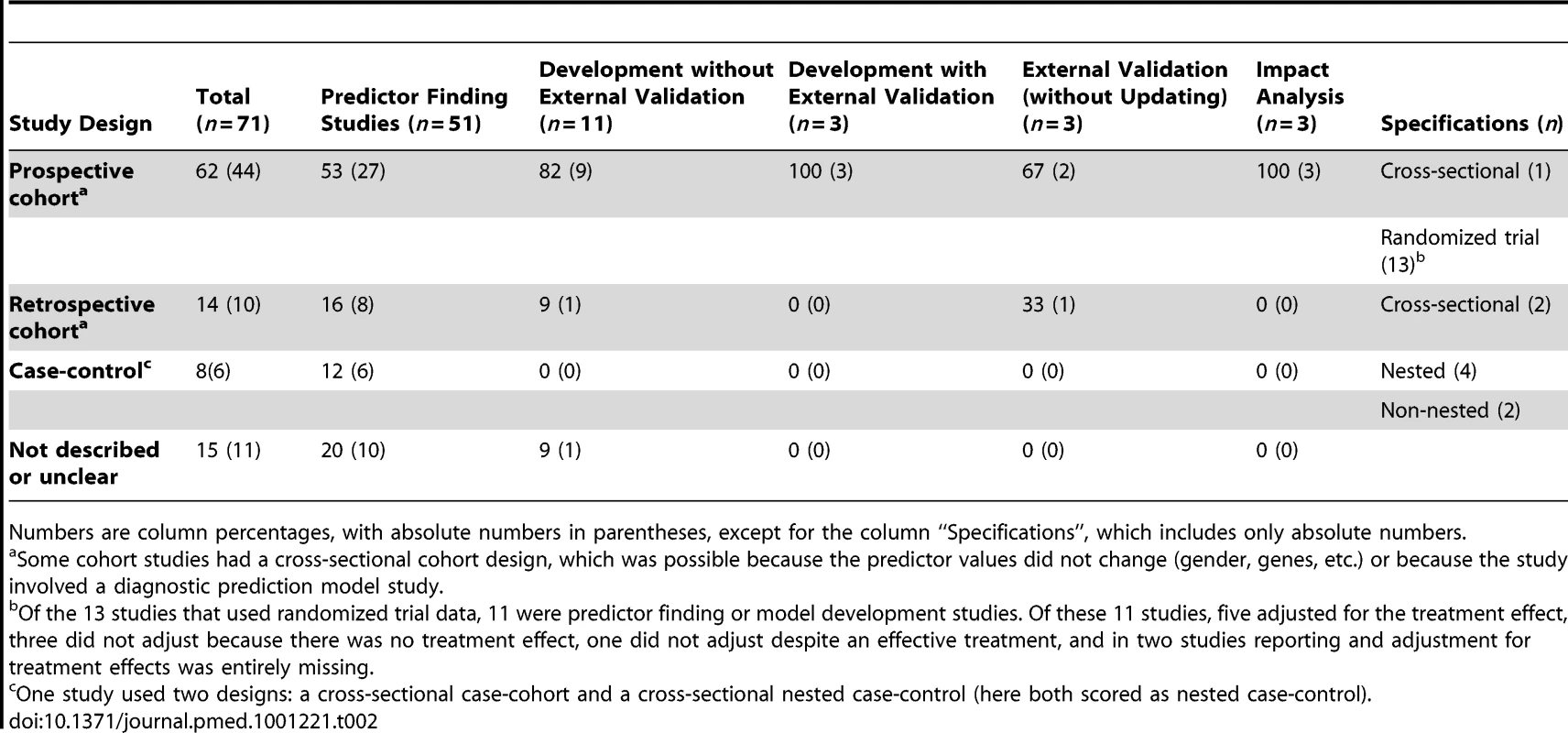
Numbers are column percentages, with absolute numbers in parentheses, except for the column “Specifications”, which includes only absolute numbers. Participant Recruitment, Follow-Up, and Setting
Participant recruitment was in general well described. Inclusion criteria were reported in 64/71 studies. Description of the cohort characteristics was clear in 68/69 of the relevant prediction studies (not applicable for two non-nested case-control studies). Study recruitment dates were reported in 88% of the studies. Length of follow-up was not reported in nine studies, leaving readers unable to know the time period for the predicted risks of the models. Whether (all) consecutive participants were included or how many participants refused to participate was rarely reported and so could not be evaluated. The majority of studies involved participants from a hospital setting (38%) or the general (healthy) population (27%). Clinical setting was not reported in 4% of the studies.
Outcome
In outcome reporting, we expected differences between studies with prediction as a primary versus a secondary aim, but this was not observed. Outcomes were well defined in 62/68 studies (Table 3). However, only 12 studies reported that they blinded the outcome measurement for predictor values. Knowledge of the predictors might influence outcome assessment, resulting in a biased estimation of the predictor effects for the outcome [6],[17],[39]. 11/68 studies had all cause mortality as the outcome, where such bias would not be a factor.
Tab. 3. Reporting of outcomes. 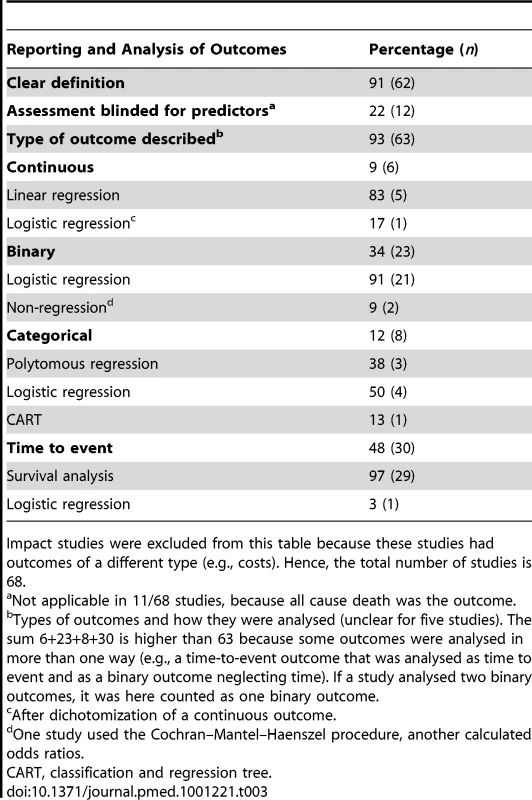
Impact studies were excluded from this table because these studies had outcomes of a different type (e.g., costs). Hence, the total number of studies is 68. Most studied outcomes were binary or time-to-event outcomes. Some outcomes are binary per se, but in some studies, continuous, categorical, and time-to-event data were analyzed as binary outcomes, a practice that is not recommended because less accurate predictions are likely to result, as with dichotomizing predictor variables [50].
Prediction of more than one outcome was very common in predictor finding studies, apparently because of their exploratory aim (Table S1). However, selective reporting of outcomes (and predictors) is often a risk [51]. Unfortunately, study registration is not mandated for prediction research, so it is generally impossible to assess whether some outcomes were analysed but not reported.
A number of studies predicted a combined endpoint (14/71). The use of a combined endpoint will give problems if the predictor effect is in opposite directions for different outcomes included in the composite endpoint [52],[53].
Candidate Predictors
Description of the candidate predictor variables was in general clear (59/68) (Table 4). In 51 of the 68 studies, predictor measurement was blinded for the outcome: in 44, simply because of the prospective design; in seven non-prospective studies, predictor measurement was explicitly blinded for the outcome. One study also assessed the predictors independently, i.e., the predictors that were studied for whether they added value to an existing model were assessed without knowledge of the predictors in that model. Predictor interaction (non-additivity) was tested in 25 of the 51 predictor finding studies and in 11 of the 14 model development studies (total n = 36). Dichotomization of continuous predictors is still common practice (21/64), despite being discouraged for decades [3],[50].
Tab. 4. Reporting of candidate predictors. 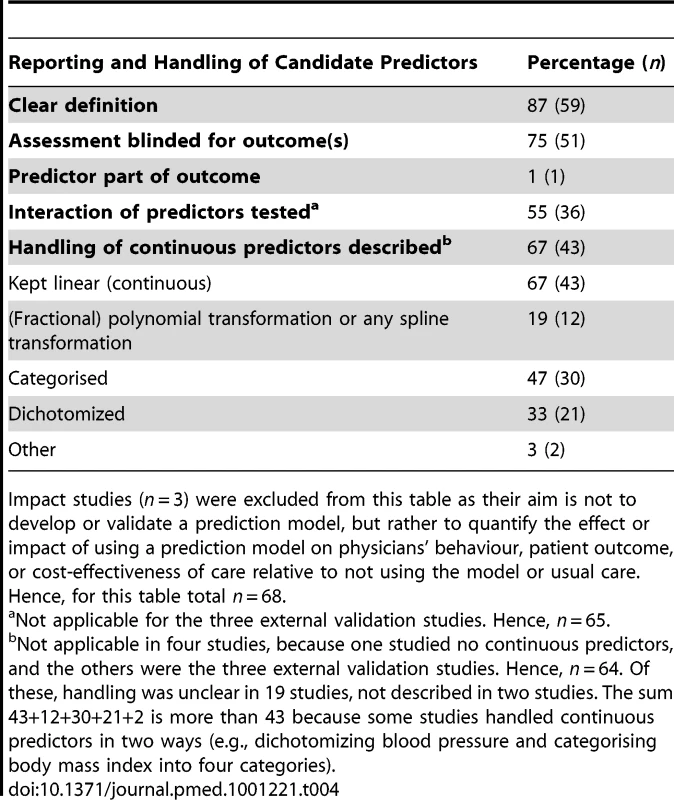
Impact studies (n = 3) were excluded from this table as their aim is not to develop or validate a prediction model, but rather to quantify the effect or impact of using a prediction model on physicians' behaviour, patient outcome, or cost-effectiveness of care relative to not using the model or usual care. Hence, for this table total n = 68. Statistical Power
For assessment of statistical power in studies estimating predictor effects for binary or categorical event outcomes, the number of participants in the smallest group determines the effective sample size. A frequently mentioned rule of thumb is “10 events needed per candidate predictor” [12],[25],[26],[30],[54]. For time-to-event outcomes, the effective sample size is also highly related to the number of participants who experience the event [12]. For continuous outcomes, the effective sample size is determined by the number of participants included in the linear regression analysis.
The number of candidate predictors should include all variables initially considered in the study as potential predictors, and not only those considered or included in the multivariable analysis. The candidate predictors also include the number of predictor interactions tested and the number of dummy variables used to include a categorical predictor in a model.
For predictor finding and model development studies, we calculated the statistical power of the fitted models based on (1) the number of predictors eventually included in the final model and (2) the number of candidate predictors (Table 5). Based on the former, as expected, the statistical power was indeed >10 events per variable in 84 (11+60+1+13) of the 124 (96+28) models fitted in these studies. However, there was insufficient reporting of the number of candidate predictors in the vast majority of these studies, such that a proper estimation of the statistical power could not be made. In the studies that clearly described the number of candidate predictors, the effective sample size was <10 events per variable in 50% (n = 21) of the presented models.
Tab. 5. Effective sample size of the included studies (reflecting statistical power). 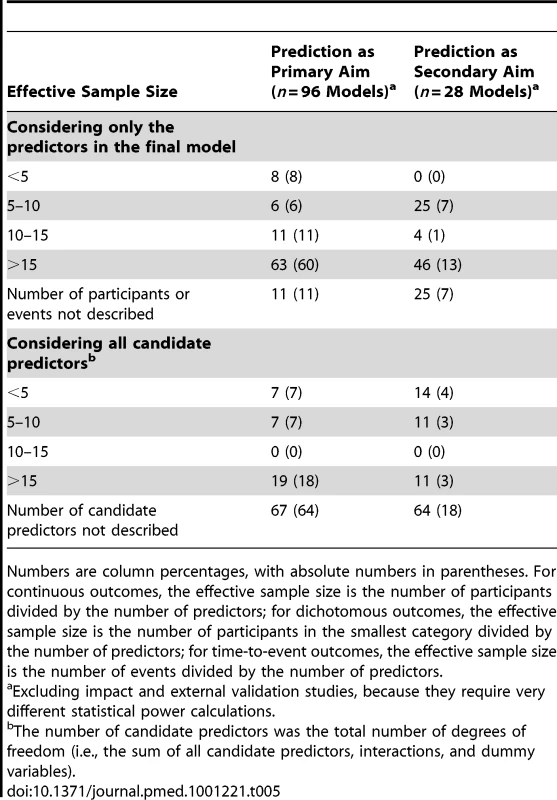
Numbers are column percentages, with absolute numbers in parentheses. For continuous outcomes, the effective sample size is the number of participants divided by the number of predictors; for dichotomous outcomes, the effective sample size is the number of participants in the smallest category divided by the number of predictors; for time-to-event outcomes, the effective sample size is the number of events divided by the number of predictors. To externally validate a prediction model, a minimum effective sample size of 100 participants with and 100 without the event has been recommended [55]. Given this, effective sample size was sufficient in the majority of the external validation studies (9/13 models).
Across all 71 included prediction studies, only 12 gave an explicit sample size calculation.
Selection of Candidate and Final Predictors
Adequate reporting of predictor selection methods used is important, because the number of candidate predictors and how they were selected at various stages of the study can both influence the specific predictors included in the final multivariable model, and thus affect the interpretation of the results 12,13,43,56. This issue is not specific to prediction studies but also arises in causal research, although here variables to be included in the multivariable modelling are usually referred to as confounders. Ideally, candidate predictors are selected based on theoretical or clinical understanding. There is no clear cut method that is widely recommended to select independent variables from candidate variables. However, many methodological reports have shown that selection based on (significant) univariable predictor–outcome associations is not recommended, as this method increases the chance of biased results in terms of spurious predictors and overfitted and unstable models [12],[13],[43],[56]. In multivariable analyses, predictors are most often selected based on backward or forward selection, typically using a significance level of 0.05. However, the use of multivariable selection methods can also lead to overfitting and unstable models, especially when there are relatively few outcome events and many predictors analysed [10],[12],[13].
We found that selection of candidate predictors was described for 36 (75%) of the studies where prediction was the primary aim and for eight (47%) of the studies where prediction was a secondary aim (Table 6). In studies with prediction as the primary aim, the majority (71%) selected their candidate predictors based on existing literature, whereas this was less often the case (29%) in studies with prediction as a secondary aim.
Tab. 6. Method of predictor selection, stratified by whether prediction was the primary or secondary study aim. 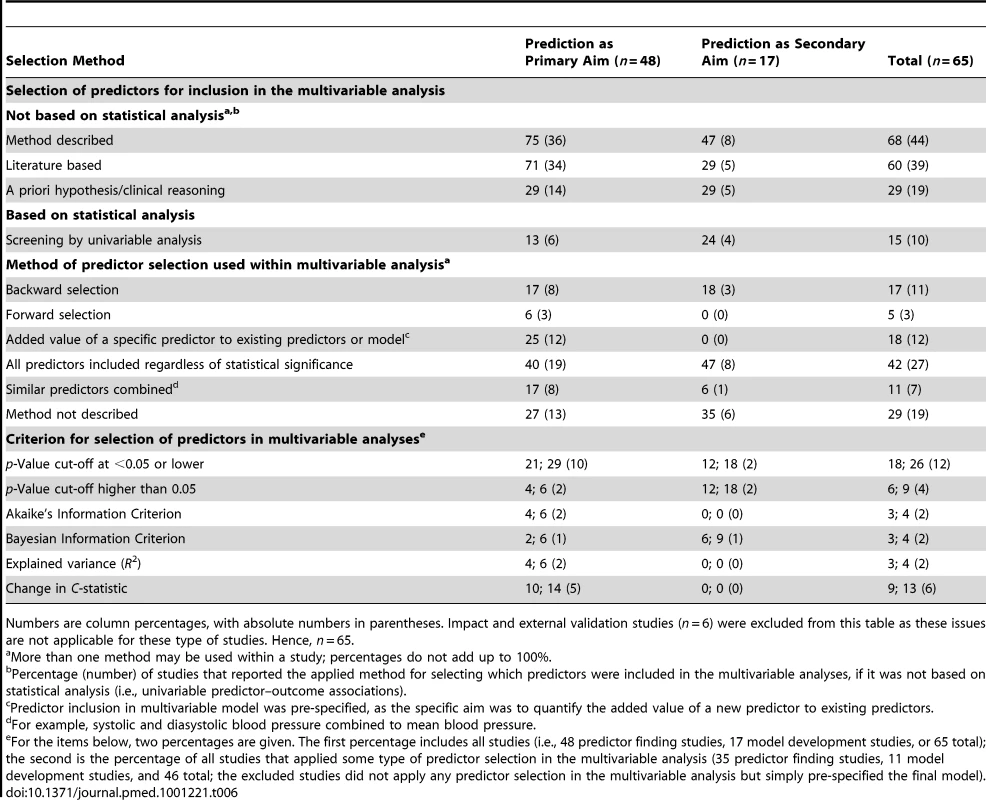
Numbers are column percentages, with absolute numbers in parentheses. Impact and external validation studies (n = 6) were excluded from this table as these issues are not applicable for these type of studies. Hence, n = 65. Pre-selection of candidate predictors for inclusion in the multivariable analyses based on univariable predictor–outcome associations was used in 13% of the primary-aim and in 24% of the secondary-aim prediction studies.
The method of selection of predictors within multivariable models was not described in 19% of the studies. Studies reported using backward selection in 17% of the primary-aim and 18% of the secondary-aim studies, whereas forward selection was reported in 6% and 0%, respectively. 18% of all studies investigated the added value of specific predictors, and 42% included predictors regardless of statistical significance.
The most commonly reported criterion for predictor selection in multivariable models was a p-value of <0.05, used in 18% of all studies (and in 26% of the studies that indeed applied predictor selection in multivariable analyses). Other criteria, such as Akaike's Information Criterion or R2, were used much less frequently.
Missing Values
Missing values in clinical study data rarely occur completely at random. Commonly missing values are related to observed participant or disease characteristics. Exclusion of participants with missing values will therefore not only lead to loss of statistical power, but often also to biased results 10,12,13,23,24,57,58. Imputation, notably multiple imputation, of missing values is often advocated to preserve power and obtain less biased results, on the assumption that the reason for the missing data is not entirely due to non-observed information (i.e., data are not “missing not at random”). When there are few missing observations, for example <5% of the individual values in the data, sometimes simple methods are advocated such as single imputation or imputation of the mean [13],.
Occurrence and handling of missing values was not described or was unclear in 38% of all studies (Table 7). If reported, it was mostly reported by “missing values per predictor” (58%). Loss to follow-up was reported in 46% of the studies where this was applicable.
Tab. 7. Handling of missing values, stratified by whether prediction was the primary or secondary study aim. 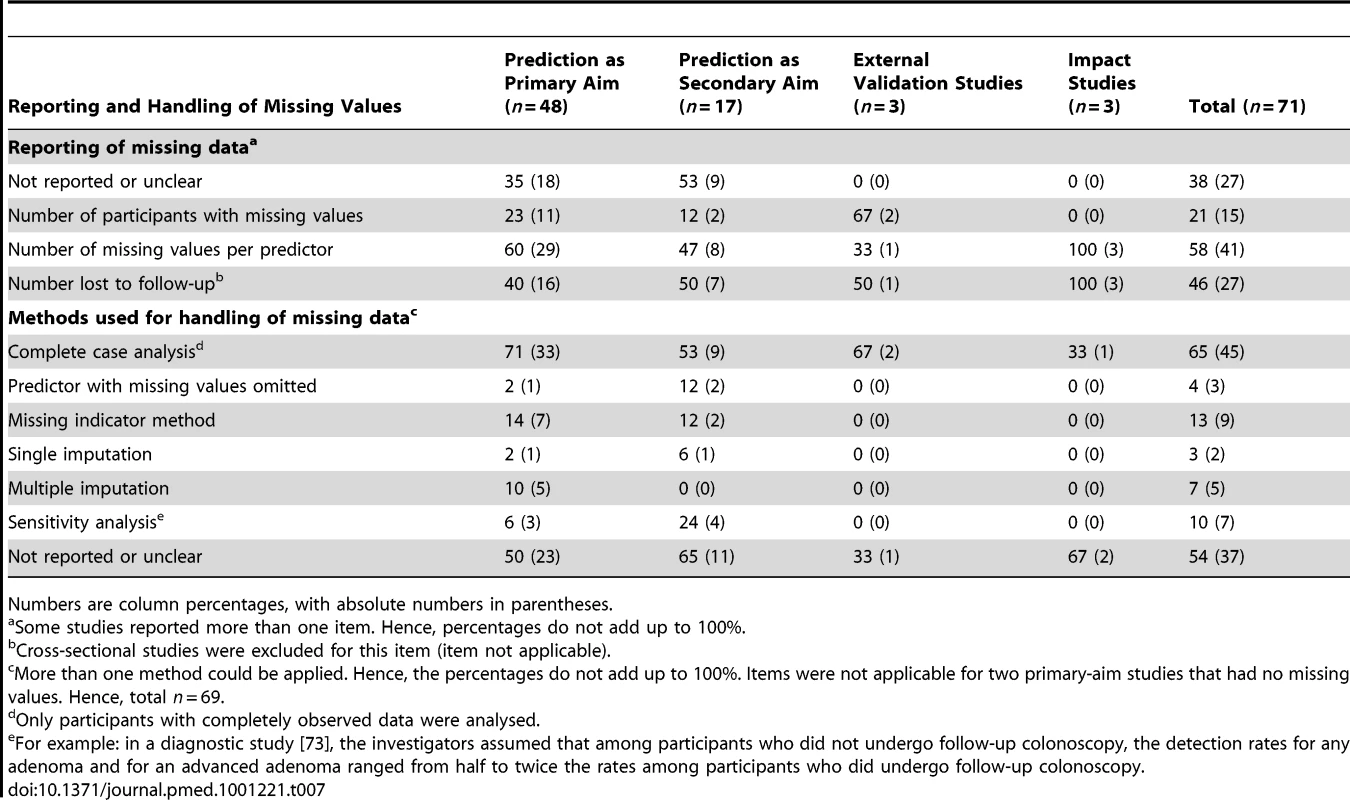
Numbers are column percentages, with absolute numbers in parentheses. Analysis of participants with complete data (i.e., complete case analysis) was performed in the vast majority of studies. It is likely that the studies that did not or unclearly reported the method of handling missing values applied a complete case analysis as well. By comparison, multiple imputation, the most rigorous strategy for dealing with missing values, was used in only 8% of all studies. With the missing indicator method, a dummy or indicator (0/1) variable is created for every predictor with missing values, with 1 indicating a missing value for the original predictor and 0 indicating an observed value. This predictor is then included as a separate predictor in the multivariable analysis. Even though this method is known to lead to biased results in almost all observational studies [13],[23],[60]–[62], it was still used in 13% of the studies investigated here.
Presentation of Results
Most guidelines, such as the STROBE guidelines for the reporting of observational studies or the REMARK guidelines for tumour marker prognostic studies, specifically advise investigators to report both unadjusted results (i.e., from univariable analysis, yielding the association of each candidate predictor with the outcome) and adjusted results (i.e., from a multivariable analysis) [4],[38],[63]. Presenting results from both analyses allows readers insight in the predictor selection strategies and allows them to determine the influence of the adjustment for other predictors. For prediction studies that apply predictor selection methods in the multivariable analyses, the presentation of a “full” model, a model that includes all predictors considered, may therefore be valuable.
Few studies reported adjusted (20%) or unadjusted (18%) results of the full model with all candidate predictors considered (Table 8). The majority, 65% of the predictor finding and 79% of the model development studies, reported the predictor coefficients or effect estimates of the model after predictor selection (the final model).
Tab. 8. Presentation of the results, stratified by type of prediction study. 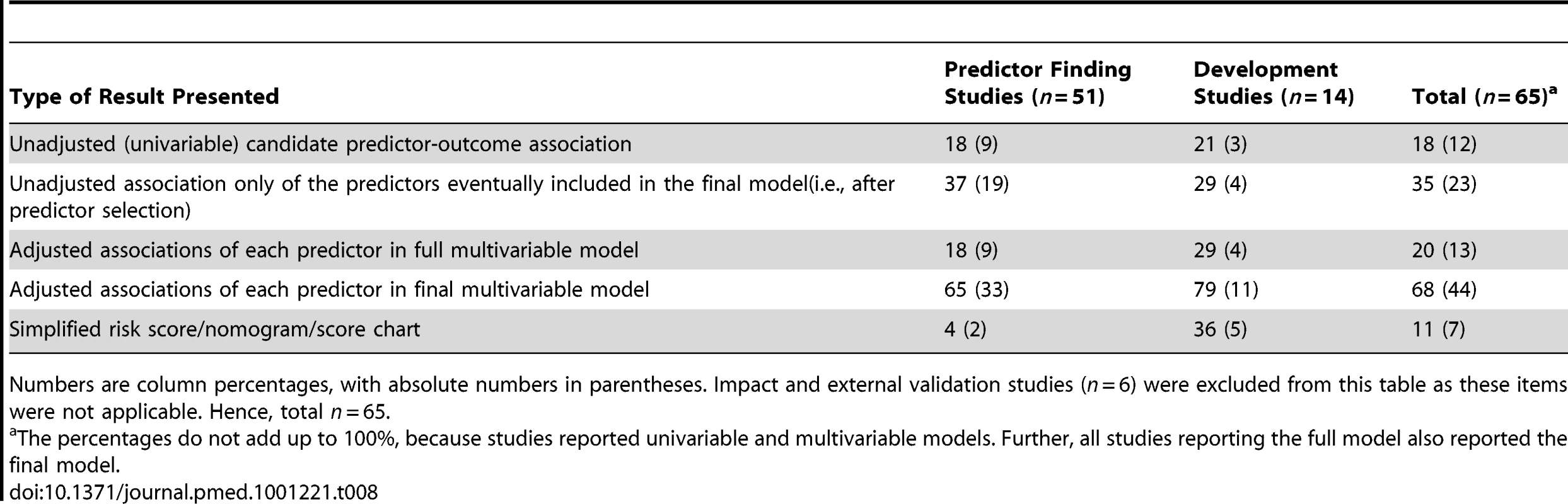
Numbers are column percentages, with absolute numbers in parentheses. Impact and external validation studies (n = 6) were excluded from this table as these items were not applicable. Hence, total n = 65. Model Performance and Internal and External Validity
The assessment of the predictive performance of a prediction model is important for understanding how predictions from the model correspond to the observed outcomes. Predictive performance of a model can be assessed on the same data that was used to generate the results (referred to as the apparent performance in the development dataset), or in random (cross-validated) subsamples of the development dataset, or using resampling techniques (like bootstrapping), all referred to as internal validation of the performance of the prediction model [2],[8],[10],[48],[64],[65]. Quantifying or validating a model's predictive performance in new subject data (i.e., subjects other than those used for the model development or internal validation) is the most rigorous form of model validity assessment and is referred to as external validation [8],[10],[32],[64],[66].
In prediction research, two main types of prediction performance measures are usually distinguished: calibration, which is the agreement between predicted outcome and observed outcome, and discrimination, which is the ability to separate participants with and without the outcome of interest [13],[67]. In addition, overall measures for discrimination and calibration (e.g., the R2 and Brier scores) may also be reported.
Calibration was reported in only a few studies (Table 9). If done, the Hosmer-Lemeshow statistic was the most often reported calibration measure. Discrimination was assessed with the C-statistic or area under the receiver operating characteristic (ROC) curve in 12% of the predictor finding and, as expected, 80% of the model development and external validation studies. R2 and Brier score were reported in very few studies. Internal validation was performed in 33% (n = 5) of the 14 model development studies, and external validation in only four studies.
Tab. 9. Model performance measures, stratified by type of prediction study. 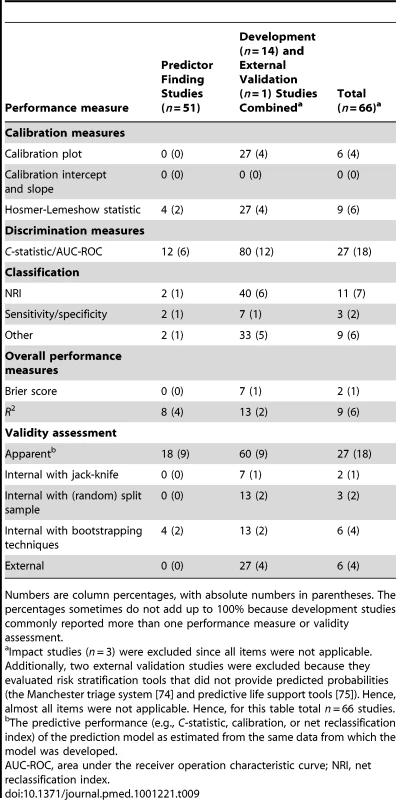
Numbers are column percentages, with absolute numbers in parentheses. The percentages sometimes do not add up to 100% because development studies commonly reported more than one performance measure or validity assessment. Discussion
We have described the state of current prediction research, and highlighted aspects that clearly need improvement. We assessed the reporting and methods in all clinical prediction studies published in six high-impact general medical journals in 2008. Our investigation found that among the 71 prediction studies identified, the vast majority were predictor finding studies (n = 51), followed by model development studies (n = 14). External validation and model impact studies were rare (n = 6). Study design, participant selection, definitions of outcomes and predictors, and predictor selection were generally well reported. However, improvements are clearly needed, both in conduct and in reporting of the following: how predictors and outcomes are assessed (with a focus on mutual blinding); the handling of continuous predictors; whether predictor interactions are studied; statistical power and effective sample size considerations; occurrence and handling of missing data; the presentation of the results in both the univariable and multivariable analysis; and the methods used to quantify and notably validate the predictive performance of prediction models.
We found that 14 studies developed new prediction models (of which three included an external validation). Three studies externally validated models, and three investigated the impact of an existing prediction model. Various reports have indicated that in prediction modelling research there is an unfortunate practice of developing new models instead of externally validating or updating existing models [8]–[10],[15],[29],[36],[48]. However, we found a similar number of model development studies (n = 14), and studies that aimed to evaluate an existing model (n = 6+3).
We do acknowledge that various basic items are well described and reported in prediction studies, for example aim, participant selection, inclusion and exclusion criteria, and design. These items have been identified as important in several well-known guidelines for reporting of clinical research [4],[38]–[41]. Journals systematically, and apparently effectively, refer to these guidelines in their “instructions for authors”. However, sample size considerations, applied statistical methods, and procedures for dealing with missing data were poorly reported despite being highlighted in several reporting guidelines. Poor reporting of sample size rationale has also been observed by others [3],[21],[68]. Further, we could not assess statistical power or effective sample size for many studies because of inadequate reporting of the number of candidate predictors.
In descriptions of participant selection, it often remained unclear whether participants were included in an unbiased way, notably with respect to refusals and whether all consecutive eligible participants were included. In contrast to randomized therapeutic trials, flow diagrams were hardly ever presented in prediction studies, which may reflect the difficulties of using these in prediction modelling studies because of the use of multiple analyses. The REMARK guidelines for prognostic tumour marker studies recommend using a REMARK profile table instead of a flow diagram [69].
Good reporting of how candidate predictors were pre-selected in our review compares favourably with other reviews [15],[20],[21],[29],[33],[35],[36],[48]. However, the methods used for further predictor selection during the statistical analyses were poorly reported. Univariable pre-selection and predictor selection in multivariable analyses based solely on p-values (with a <0.05 cut-off) was often used in predictor finding and model development studies. This approach may notably be problematic with low numbers of events and many candidate predictors. As the exact number of events per candidate predictor could almost never be assessed, it was not possible to determine whether reported results were indeed subject to overfitting or optimistic predictive performances. Several studies, however, did not rely exclusively on these methods for predictor selection, but rather also, as is recommended, included established predictors in their model regardless of statistical significance in their dataset.
Most studies reported the occurrence of missing data but did not report sufficient detail. Complete case analysis was by far the most commonly used approach to handle missing values, despite many methodological recommendations to do otherwise [10],[12],[13],[23],[24],[57],[58]. Recommended methods, such as multiple imputation, were applied and reported in very few studies, although this may be due to the fact that consensus in recommending these methods was arrived at only recently. As the reasons for missing values were insufficiently described in most studies that applied a complete case analysis, it was impossible to judge whether imputation methods would indeed have been appropriate.
Most studies correctly reported the (adjusted) predictor effects derived from the final multivariable analyses. Only a few studies also reported results of the univariable analyses, which is often a useful comparator. As noted in the REMARK guidelines [4], a comprehensive reporting of both univariable and multivariable analyses would allow readers to evaluate the adjustment for other predictors.
We observed much variation in the reporting of predictive performance measures. C-statistics or area under the ROC curves were the most frequently reported discrimination statistics, whereas measures of calibration (such as calibration slope) and overall measures of fit were rarely reported. Calibration measures are essential in model validation studies, to judge whether the predicted probabilities indeed match observed frequencies of the outcome under study.
The majority of model development studies reported predictive performance in the development data only. This apparent model performance, however, is generally too optimistic, as the model has been tailored to the dataset at hand. Overfitting is even more likely when the number of candidate predictors is large relative to the effective sample size [10],[12],[70]. The extent of this optimism may be estimated with so-called internal validation techniques [10],[12],[43],[70], but use of these techniques was rare. Similarly, only a very few model development studies reported an external validation of the model in the same paper. Accordingly, the generalisability of the performance of these reported models, especially in studies where prediction was the primary aim, is difficult to evaluate.
To further frame our results, a few issues need to be addressed. We examined prediction studies published in six high impact journals, likely representing higher quality studies. Reporting may have improved since 2008, although this is unlikely since no major reporting guidelines for this type of research have been produced recently. The recently published GRIPS statement and existing guidelines such as the REMARK guidelines, though focussed on specific types of studies, may improve reporting of future prediction research [4],[71]. We note that our work assessed researchers' reporting and statistical methods in modelling, and not necessarily the appropriateness of the design and conduct of the studies. Conduct of prediction research may be better than reported in the papers, since journals impose limits on the lengths of papers. It is important to note that a methodologically weak or statistically underpowered study is still a poor quality study, whether or not it is well reported. However, if it is poorly reported, then the reader will be unable to gauge its relevance and reliability.
To conclude, we identified poor reporting and poor methods in many published prediction studies, which limits the reliability and applicability of the published findings. However, encouraging findings included the frequent use of prospective studies, and adequate description of participant selection, predictor and outcome definitions, and the process for (pre)selection of candidate predictors. Improvement is clearly needed in blinding of assessment of outcomes for predictor information, many aspects of data analysis, the presentation of results of multivariable analyses, and the methods used to quantify and validate the predictive performance of a developed prediction model. Only a very small minority of the papers involved the most useful approaches in predicting participant clinical outcomes, namely, external validations or impact assessments of a previously developed prediction model.
Supporting Information
Zdroje
1. AltmanDGRileyRD 2005 Primer: an evidence-based approach to prognostic markers. Nat Clin Pract Oncol 2 466 472
2. AltmanDG 2007 Prognostic models: a methodological framework and review of models for breast cancer. LymanGHBursteinHJ Breast cancer. Translational therapeutic strategies New York New York Informa Healthcare 11 26
3. AltmanDGLymanGH 1998 Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat 52 289 303
4. McShaneLMAltmanDGSauerbreiWTaubeSEGionM 2005 Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97 1180 1184
5. RothwellPM 2008 Prognostic models. Pract Neurol 8 242 253
6. MoonsKGRoystonPVergouweYGrobbeeDEAltmanDG 2009 Prognosis and prognostic research: what, why, and how? BMJ 338 b375
7. RoystonPMoonsKGAltmanDGVergouweY 2009 Prognosis and prognostic research: developing a prognostic model. BMJ 338 b604
8. AltmanDGVergouweYRoystonPMoonsKG 2009 Prognosis and prognostic research: validating a prognostic model. BMJ 338 b605
9. MoonsKGAltmanDGVergouweYRoystonP 2009 Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 338 b606
10. SteyerbergEW 2009 Clinical prediction models: a practical approach to development, validation, and updating New York Springer
11. GrobbeeDEHoesAW 2007 Clinical epidemiology: principles, methods, and applications for clinical research Sudbury (Massachusetts) Jones and Bartlett Publishers
12. HarrellFE 2001 Regression modeling strategies with applications to linear models, logistic regression and survival analysis New York Springer Verlag
13. HarrellFEJrLeeKLMarkDB 1996 Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15 361 387
14. GrobbeeDE 2004 Epidemiology in the right direction: the importance of descriptive research. Eur J Epidemiol 19 741 744
15. LaupacisASekarNStiellIG 1997 Clinical prediction rules. a review and suggested modifications of methodological standards. JAMA 277 488 494
16. McGinnTGGuyattGHWyerPCNaylorCDStiellIG 2000 Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA 284 79 84
17. LijmerJGMolBWHeisterkampSBonselGJPrinsMH 1999 Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 282 1061 1066
18. RutjesAWReitsmaJBVandenbrouckeJPGlasASBossuytPM 2005 Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 51 1335 1341
19. RutjesAWReitsmaJBDiNMSmidtNvan RijnJC 2006 Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174 469 476
20. MallettSRoystonPWatersRDuttonSAltmanDG 2010 Reporting performance of prognostic models in cancer: a review. BMC Med 8 21
21. MallettSRoystonPDuttonSWatersRAltmanDG 2010 Reporting methods in studies developing prognostic models in cancer: a review. BMC Med 8 20
22. ConcatoJFeinsteinARHolfordTR 1993 The risk of determining risk with multivariable models. Ann Intern Med 118 201 210
23. DondersARvan der HeijdenGJStijnenTMoonsKG 2006 Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 59 1087 1091
24. GreenlandSFinkleWD 1995 A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol 142 1255 1264
25. PeduzziPConcatoJKemperEHolfordTRFeinsteinAR 1996 A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49 1373 1379
26. PeduzziPConcatoJFeinsteinARHolfordTR 1995 Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48 1503 1510
27. HaydenJACotePBombardierC 2006 Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144 427 437
28. HaydenJACotePSteenstraIABombardierC 2008 Identifying phases of investigation helps planning, appraising, and applying the results of explanatory prognosis studies. J Clin Epidemiol 61 552 560
29. WassonJHSoxHCNeffRKGoldmanL 1985 Clinical prediction rules. Applications and methodological standards. N Engl J Med 313 793 799
30. SteyerbergEWEijkemansMJHabbemaJD 1999 Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol 52 935 942
31. SteyerbergEWBleekerSEMollHAGrobbeeDEMoonsKG 2003 Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J Clin Epidemiol 56 441 447
32. BleekerSEMollHASteyerbergEWDondersARDerksen-LubsenG 2003 External validation is necessary in prediction research: a clinical example. J Clin Epidemiol 56 826 832
33. OttenbacherKJOttenbacherHRToothLOstirGV 2004 A review of two journals found that articles using multivariable logistic regression frequently did not report commonly recommended assumptions. J Clin Epidemiol 57 1147 1152
34. MackinnonA 2010 The use and reporting of multiple imputation in medical research—a review. J Intern Med 268 586 593
35. MushkudianiNAHukkelhovenCWHernandezAVMurrayGDChoiSC 2008 A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes. J Clin Epidemiol 61 331 343
36. PerelPEdwardsPWentzRRobertsI 2006 Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak 6 38
37. LeushuisEvan der SteegJWSteuresPBossuytPMEijkemansMJ 2009 Prediction models in reproductive medicine: a critical appraisal. Hum Reprod Update 15 537 552
38. Von ElmEAltmanDGEggerMPocockSJGotzschePC 2007 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370 1453 1457
39. BossuytPMReitsmaJBBrunsDEGatsonisCAGlasziouPP 2003 Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med 138 40 44
40. WhitingPRutjesAWReitsmaJBBossuytPMKleijnenJ 2003 The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3 25
41. MoherDHopewellSSchulzKFMontoriVGotzschePC 2010 CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340 c869
42. SteyerbergEWVergouweYKeizerHJHabbemaJD 2001 Residual mass histology in testicular cancer: development and validation of a clinical prediction rule. Stat Med 20 3847 3859
43. SteyerbergEWHarrellFEJrBorsboomGJEijkemansMJVergouweY 2001 Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54 774 781
44. StiellIGWellsGA 1999 Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med 33 437 447
45. TollDBJanssenKJVergouweYMoonsKG 2008 Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol 61 1085 1094
46. SteyerbergEWBorsboomGJvan HouwelingenHCEijkemansMJHabbemaJD 2004 Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med 23 2567 2586
47. JanssenKJMoonsKGKalkmanCJGrobbeeDEVergouweY 2008 Updating methods improved the performance of a clinical prediction model in new patients. J Clin Epidemiol 61 76 86
48. ReillyBMEvansAT 2006 Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Ann Intern Med 144 201 209
49. BiesheuvelCJVergouweYOudegaRHoesAWGrobbeeDE 2008 Advantages of the nested case-control design in diagnostic research. BMC Med Res Methodol 8 48
50. RoystonPAltmanDGSauerbreiW 2006 Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 25 127 141
51. KirkhamJJDwanKMAltmanDGGambleCDoddS 2010 The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 340 c365
52. TomlinsonGDetskyAS 2010 Composite end points in randomized trials: there is no free lunch. JAMA 303 267 268
53. LimEBrownAHelmyAMussaSAltmanDG 2008 Composite outcomes in cardiovascular research: a survey of randomized trials. Ann Intern Med 149 612 617
54. VittinghoffEMcCullochCE 2007 Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165 710 718
55. VergouweYSteyerbergEWEijkemansMJHabbemaJD 2005 Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol 58 475 483
56. SunGWShookTLKayGL 1996 Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol 49 907 916
57. BurtonAAltmanDG 2004 Missing covariate data within cancer prognostic studies: a review of current reporting and proposed guidelines. Br J Cancer 91 4 8
58. GorelickMH 2006 Bias arising from missing data in predictive models. J Clin Epidemiol 59 1115 1123
59. MarshallAAltmanDGHolderRL 2010 Comparison of imputation methods for handling missing covariate data when fitting a Cox proportional hazards model: a resampling study. BMC Med Res Methodol 10 112
60. KnolMJJanssenKJDondersAREgbertsACHeerdinkER 2010 Unpredictable bias when using the missing indicator method or complete case analysis for missing confounder values: an empirical example. J Clin Epidemiol 63 728 736
61. WhiteIRThompsonSG 2005 Adjusting for partially missing baseline measurements in randomized trials. Stat Med 24 993 1007
62. MiettinenOS 1985 Theoretical epidemiology. Principles of occurrence research in medicine New York Wiley
63. VandenbrouckeJPvon ElmEAltmanDGGotzschePCMulrowCD 2007 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4 e297 doi:10.1371/journal.pmed.0040297
64. AltmanDGRoystonP 2000 What do we mean by validating a prognostic model? Stat Med 19 453 473
65. JusticeACCovinskyKEBerlinJA 1999 Assessing the generalizability of prognostic information. Ann Intern Med 130 515 524
66. SauerbreiW 1999 The use of resampling methods to simplify regression models in medical statistics. Appl Stat 48 313 329
67. VergouweYSteyerbergEWEijkemansMJHabbemaJD 2002 Validity of prognostic models: when is a model clinically useful? Semin Urol Oncol 20 96 107
68. BachmannLMPuhanMAter RietGBossuytPM 2006 Sample sizes of studies on diagnostic accuracy: literature survey. BMJ 332 1127 1129
69. MallettSTimmerASauerbreiWAltmanDG 2010 Reporting of prognostic studies of tumour markers: a review of published articles in relation to REMARK guidelines. Br J Cancer 102 173 180
70. Van HouwelingenJCLe CessieS 1990 Predictive value of statistical models. Stat Med 9 1303 1325
71. JanssensACIoannidisJPvan DuijnCMLittleJKhouryMJ 2011 Strengthening the reporting of Genetic Risk Prediction Studies: the GRIPS Statement. PLoS Med 8 e1000420 doi:10.1371/journal.pmed.1000420
72. PletcherMJBibbins-DomingoKLewisCEWeiGSSidneyS 2008 Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med 149 91 99
73. ImperialeTFGlowinskiEALin-CooperCLarkinGNRoggeJD 2008 Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med 359 1218 1224
74. Van VeenMSteyerbergEWRuigeMvan MeursAHRoukemaJ 2008 Manchester triage system in paediatric emergency care: prospective observational study. BMJ 337 a1501
75. SassonCHeggAJMacyMParkAKellermannA 2008 Prehospital termination of resuscitation in cases of refractory out-of-hospital cardiac arrest. JAMA 300 1432 1438
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2012 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Nech brouka žít… Ať žije astma!
- Intermitentní hladovění v prevenci a léčbě chorob
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Innovation and Access to Medicines for Neglected Populations: Could a Treaty Address a Broken Pharmaceutical R&D System?
- Pregnancy and Infant Outcomes among HIV-Infected Women Taking Long-Term ART with and without Tenofovir in the DART Trial
- Prevalence and Risk of Violence and the Physical, Mental, and Sexual Health Problems Associated with Human Trafficking: Systematic Review
- Putting Evidence into Practice: The Series on Global Mental Health Practice
- Does Development Assistance for Health Really Displace Government Health Spending? Reassessing the Evidence
- Stepped Care for Maternal Mental Health: A Case Study of the Perinatal Mental Health Project in South Africa
- The Midwives Service Scheme in Nigeria
- The Effect of Elevated Body Mass Index on Ischemic Heart Disease Risk: Causal Estimates from a Mendelian Randomisation Approach
- Improving Access to Mental Health Care and Psychosocial Support within a Fragile Context: A Case Study from Afghanistan
- Reporting and Methods in Clinical Prediction Research: A Systematic Review
- Criminal Justice Reform as HIV and TB Prevention in African Prisons
- A New Deal for Global Health R&D? The Recommendations of the Consultative Expert Working Group on Research and Development (CEWG)
- Six-Year Follow-Up of Impact of Co-proxamol Withdrawal in England and Wales on Prescribing and Deaths: Time-Series Study
- Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration
- A Prescription for Improving Drug Formulary Decision Making
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Prescription for Improving Drug Formulary Decision Making
- Does Development Assistance for Health Really Displace Government Health Spending? Reassessing the Evidence
- Pregnancy and Infant Outcomes among HIV-Infected Women Taking Long-Term ART with and without Tenofovir in the DART Trial
- Prevalence and Risk of Violence and the Physical, Mental, and Sexual Health Problems Associated with Human Trafficking: Systematic Review
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



