Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study
In a observational study, Chiara Robba and colleagues examine the association between ultrasound based non-invasive intracranial pressure and invasive intracranial pressure measurement in neurocritical care patients.
Published in the journal:
Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study. PLoS Med 14(7): e32767. doi:10.1371/journal.pmed.1002356
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pmed.1002356
Summary
In a observational study, Chiara Robba and colleagues examine the association between ultrasound based non-invasive intracranial pressure and invasive intracranial pressure measurement in neurocritical care patients.
Introduction
Intracranial hypertension is a frequent and harmful complication of brain injury; it is an important contributing factor for secondary brain injury, and its severity and duration have been correlated with a fatal outcome [1,2].
A recent trial comparing an invasive intracranial pressure (ICP) monitoring protocol with a protocol based on imaging and clinical examination found no significant differences in patient outcome [3]. However, the trial has been criticised for being underpowered and for the methodology used to measure and treat ICP. Thus, invasive monitoring and treatment of intracranial hypertension is still widely recommended in the management of severely brain-injured patients despite a paucity of randomized evidence [4].
Invasive ICP monitoring through an intraventricular catheter or intraparenchymal microtransducer continues to be the standard of care after severe traumatic brain injury, and should be performed when indications are met [5]. Because the use of invasive transducers can cause complications including infection or haemorrhage [6–8], reliable non-invasive ICP (nICP) estimation would be helpful, especially in clinical situations where the risk–benefit balance of invasive ICP monitoring is unclear or when ICP monitoring is not immediately available or is even contraindicated [4]. Several non-invasive methods based on transcranial Doppler and optic nerve sheath diameter (ONSD) ultrasound are gaining clinical popularity due to their safety, availability, and reliability [8–13]. At present, the best accuracy for a non-invasive method reported in the literature [14,15] has been demonstrated by 2-depth high-resolution transcranial Doppler insonation of the ophthalmic artery. This method does not need calibration and is based on the measurement of the balance point when the measured parameters of blood flow velocity waveforms in the intracranial segment of the ophthalmic artery (which reflect ICP) are identical to extracranial segments (which are mechanically compressed by an externally applied pressure). Other authors [16,17] have proposed different methods for continuous nICP monitoring based on the waveform analysis of cerebral blood flow velocity from the middle cerebral artery (MCA) and arterial pressure. However, despite these promising results, non-invasive techniques remain of insufficient accuracy and temporal resolution to replace invasive ICP monitoring [18,19].
The aim of this study was to compare the accuracy of different ultrasound-based methods for nICP measurement in patients with severe traumatic brain injury undergoing invasive ICP monitoring. Such methods included the ultrasound measurement of the ONSD, venous transcranial Doppler (vTCD), and derived indices obtained from the straight sinus (such as straight sinus systolic flow velocity [FVsv]), and arterial transcranial Doppler (aTCD)–derived indices such as middle cerebral artery (MCA) pulsatility index (PIa) and diastolic flow velocity (FVd).
Methods
This is a single-centre, prospective observational study conducted from 1 January 2016 to 1 November 2016. Recruited patients were admitted at the Neurosciences Critical Care Unit, Addenbrooke’s Hospital, Cambridge, UK. The protocol was approved by the research ethics boards at the University of Cambridge (REC 15/lo/1918), and written consent was obtained from all participants’ next of kin. The article is reported as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (S1 Text). Patients older than 18 years requiring sedation, mechanical ventilation, and ICP monitoring with an admission diagnosis of severe traumatic brain injury, aneurysmal subarachnoid haemorrhage, intraparenchymal haemorrhage, or stroke were considered for inclusion. Exclusion criteria were the following: the absence of an informed consent, a known history of ocular pathology or optic nerve trauma, skull base fracture with a cerebrospinal fluid (CSF) leak, inaccessible ultrasound windows (temporal for aTCD and occipital for vTCD), and clinical or radiological suspicion of cerebral venous thrombosis or vasospasm.
Patient monitoring
Patients were sedated with a continuous infusion of propofol or midazolam, fentanyl, and, when necessary, the muscle relaxant atracurium besylate. Mechanical ventilation was targeted to maintain adequate oxygenation (SaO2 > 90%) and normocapnia (PaCO2 = 35–40 mm Hg). Intravenous fluids, vasopressors, and inotropic support (norepinephrine and/or epinephrine) were administered to achieve and maintain an adequate cerebral perfusion pressure (CPP > 60 mm Hg). Clinical management was in accordance to international guidelines [20–22].
Treatment of intracranial hypertension was based on a protocol-driven strategy, which included optimisation of arterial blood pressure (ABP) and volaemia, sedation, and infusion of hyperosmolar fluids according to our institutional guidelines.
After a decision to place an ICP monitoring device (by the neurosurgical and intensive care physician in charge), patients were enrolled in the study. ICP was measured via an intraparenchymal probe (Codman & Shurtleff, Raynham, Massachusetts, US) or a catheter inserted into the brain ventricles and connected to an external pressure transducer and drainage system (Codman, Johnson & Johnson, Raynham, Massachusetts, US).
For each patient, we collected the following characteristics: admission Glasgow Coma Scale (GCS), age, sex, height, weight, comorbidities, mechanism and severity of brain injury, and discharge Glasgow Outcome Scale (GOS). The Rotterdam and Marshall scores as well as the Fisher scale were calculated using the admission computer tomography head scan reports [22].
Ultrasound measurements
Ultrasound measurement was performed by a selected group of experienced operators (TT, JP, MB) using a standardised insonation technique to reduce inter-operator variability. The operators were blinded to the patient’s admission diagnosis, demographics, baseline characteristics, and clinical and physiological background. Operators were not blinded to the actual ICP, but they were blinded to the final formulae to obtain a nICP estimation from the different measurements. Mean arterial blood pressure, end-tidal carbon dioxide partial pressure (ETCO2), MCA flow velocities (diastolic [FVd], mean [FVm], and systolic [FVs]), straight sinus flow velocities (diastolic [FVdv], mean [FVmv], and systolic [FVsv]), and ONSD were recorded twice daily from day 1 to day 5 after ICP insertion. Additional measurements were performed in case of acute increases in ICP (above 20 mm Hg). In cases where ICP mean values changed more than ±2 mm Hg during any of the 3 studies (ONSD ultrasound, vTCD, and aTCD), the measurements were excluded from the analysis.
ONSD
Ultrasound examination of the ONSD was performed using a 7.5-MHz linear ultrasound probe (11L4, Xario 200; Toshiba, Zoetermeer, The Netherlands) using the lowest possible acoustic power that could measure the ONSD. The probe was oriented perpendicularly in the vertical plane and at around 30 degrees in the horizontal plane on the closed eyelids of both eyes of individuals in supine position with head elevated to 30 degrees. Ultrasound gel was applied on the surface of each eyelid and the measurements were made in the axial and sagittal planes of the widest diameter visible 3 mm behind the retina in both eyes. The final ONSD value was calculated by averaging 4 measured values, as previously described [23,24].
Transcranial Doppler
aTCD was performed bilaterally on the MCA through the temporal window using a traditional 2-MHz transducer (5S2, Xario 20; Toshiba, Zoetermeer, The Netherlands) with head elevated to 30 degrees, as previously described [23,25]. The final values of flow velocities were calculated by averaging the 2 measured values.
vTCD was performed on the straight sinus using a 2-MHz transducer (5S2, Xario 20; Toshiba, Zoetermeer, The Netherlands) through an occipital and transforaminal bone window at a depth of 50 to 80 mm for flow directed toward the probe, as previously described [25].
Statistical analysis
On the basis of previous reports [25–27], we hypothesized that ICP is linearly associated with ONSD, systolic flow velocity on the straight sinus (FVsv), PIa, and ABP × (1 − FVd/FVm), and verified this hypothesis in 64 patients. A multivariable linear regression model was obtained from the relationship among ICP, ONSD, and FVsv to derive an nICP estimator based on the combination of ONSD and FVsv (nICPONSD+FVsv).
Deviations from the initial statistical plan (S2 Text) were based on reviewers’ requests and consisted of inclusion of linear mixed effects model analysis for the determination of the estimation formulas for nICP and exclusion of Bland–Altman analysis.
Statistical analysis of the data was conducted with RStudio software (R version 3.1.2). Initially, multiple measurement points were averaged for each patient; therefore, every patient was represented by one single value for all variables assessed. Then, the correlations between ICP and the variables of interest—ONSD, PIa, ABP × (1 − FVd/FVm), and FVsv—were verified using the Pearson correlation coefficient (R, with the level of significance set at 0.05).
To provide prediction models for ICP estimation, the relationships between ICP and the correlated variables were expressed as linear mixed effects models (R package lme4 [28]). As fixed effects, we entered ICP and the non-invasive estimators into the model. As random effects, we had intercepts and slopes for the repeated measurement points for each patient (N = 445 measurements). A mixed effects multiple regression between ICP and 2 correlated variables, ONSD and FVsv, was also performed. Chi-square (χ2) values and p-values for the comparison of the models were obtained by likelihood ratio tests of the full model with random intercepts and slopes against the null model with random intercepts only.
The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was calculated to determine the ability of the non-invasive methods to detect raised ICP (using a threshold of 20 mm Hg; N = 445 measurements). Moreover, we also performed an analysis to determine the best ONSD and FVsv cutoff values for prediction of ICP ≥ 20 mm Hg. In ROC analysis, these are the values presenting the best sensitivity and specificity for prediction of a given threshold. The predicting ability is considered reasonable when the AUC is higher than 0.7 and strong when the AUC exceeds 0.8 [29]. Statistical differences between ROC curves were verified using the DeLong’s test for 2 correlated ROC curves (R package pROC [30]).
An analysis of variance (ANOVA) was performed to verify whether any of the variables assessed were associated with mortality in the patient cohort.
Results
In all, 80 patients with intracranial pathology requiring invasive ICP monitoring were initially considered for enrolment in this study. Among these, 3 were excluded because of the absence of written consent, 2 because it was not possible to find a temporal window, 6 because the occipital window was inaccessible (cervical collar or patient position), 3 because the straight sinus could not be insonated, and 2 because of ocular lesions that precluded the assessment of ONSD.
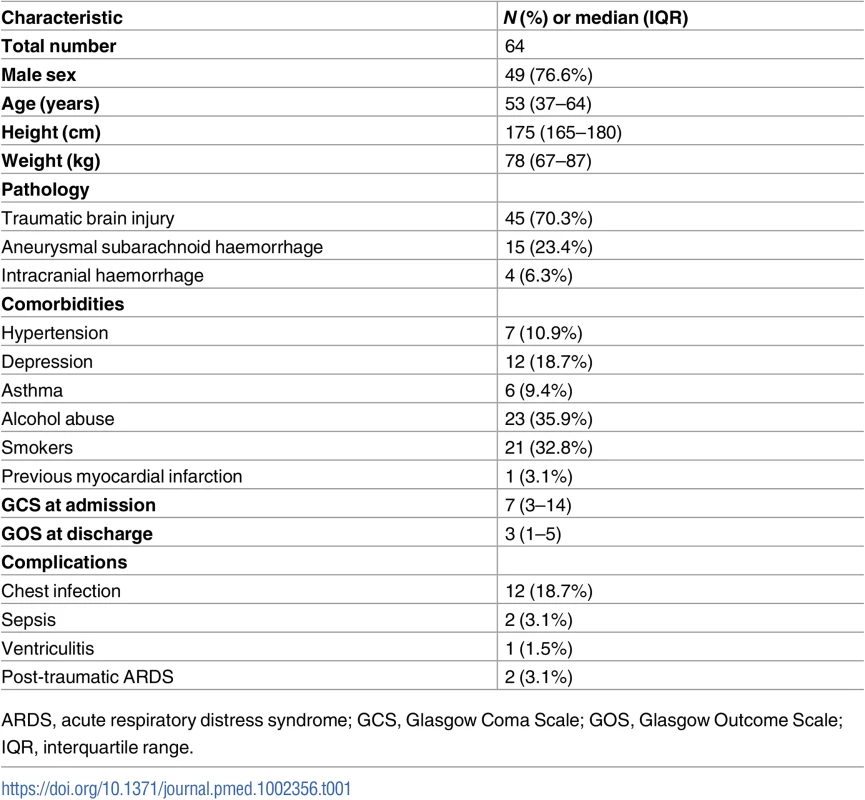
A total of 445 recordings from 64 patients (each one including ONSD ultrasound, aTCD, and vTCD) were included in the final analysis. The percentage of measurements presenting ICP ≥ 20 mm Hg was 19.3% (N = 86). The characteristics of the patients are shown in Table 1. In Table 2, we present the median (interquartile range [IQR]) values of the variables analysed.
nICP measurement
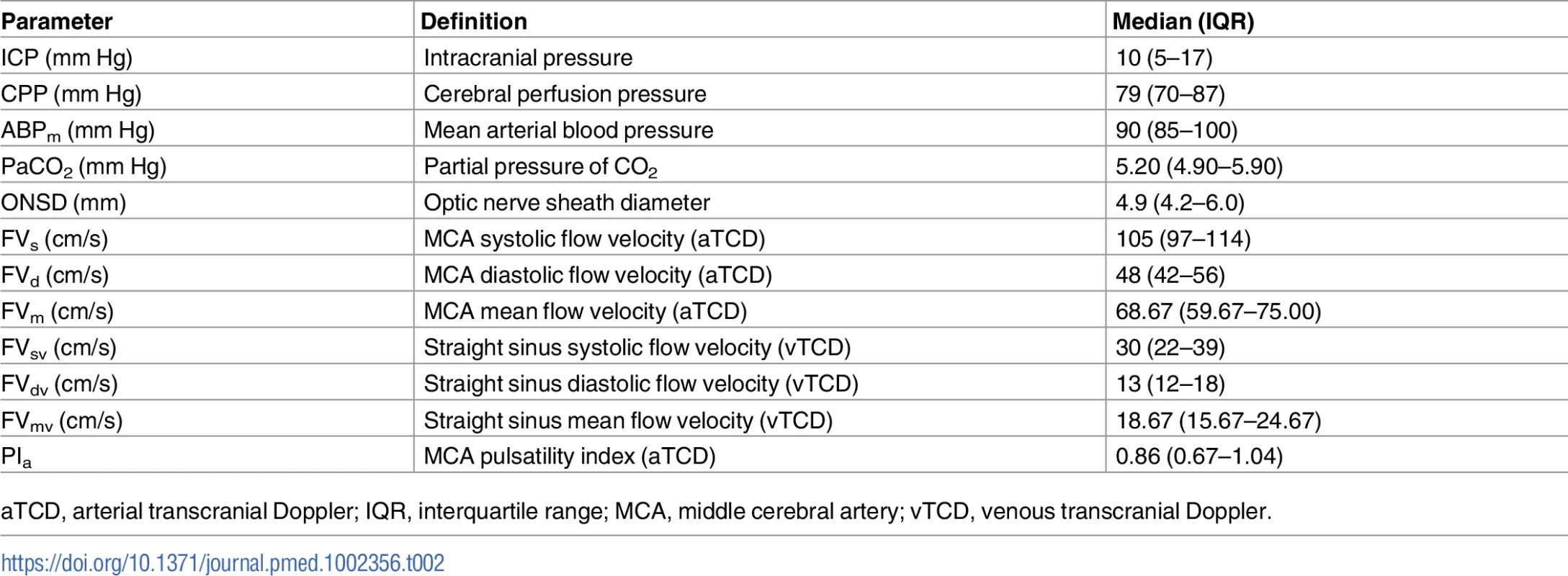
The correlation analysis between patients revealed good correlation between ICP and ONSD (R = 0.76) and between ICP and FVsv (R = 0.72), averaged per patient (N = 64)—both were statistically significant (p < 0.001) and without any influential outliers. The p-values for the correlations between ICP and PIa and ABP × (1 − FVd/FVm) were both non-significant (p = 0.63 and p = 0.36, respectively). Thus, the regression formulas adopted in this work considered only ONSD and FVsv, and the combination of both in a multiple regression model (Table 3).

Considering the variability in slopes between patients, full models allowing for random intercepts and slopes were significantly better at fitting the data than null models for a nICP estimator based on FVsv (nICPFVsv) and for nICPONSD+FVsv (χ2 = 44.19, p < 0.001, and χ2 = 40.92, p < 0.001, respectively). The inclusion of random slopes in the model describing a nICP estimator based on ONSD (nICPONSD) did not produce a significant difference in comparison to the model with random intercepts only (χ2 = 2.41, p = 0.30). The formulas of the derived models that best fitted the data are described below:
Accuracy of the nICP methods
The correlation coefficient between the ONSD method and ICP averaged per patient (N = 64) was R = 0.76; the FVsv method showed a correlation with ICP of R = 0.72. The combination of the 2 methods presented a correlation coefficient of 0.81 (Table 4; Fig 1). S1 Fig displays the regression plots between ICP and the non-invasive estimators (ONSD and FVsv) for each patient, demonstrating the slope variability between patients with multiple measurement points.
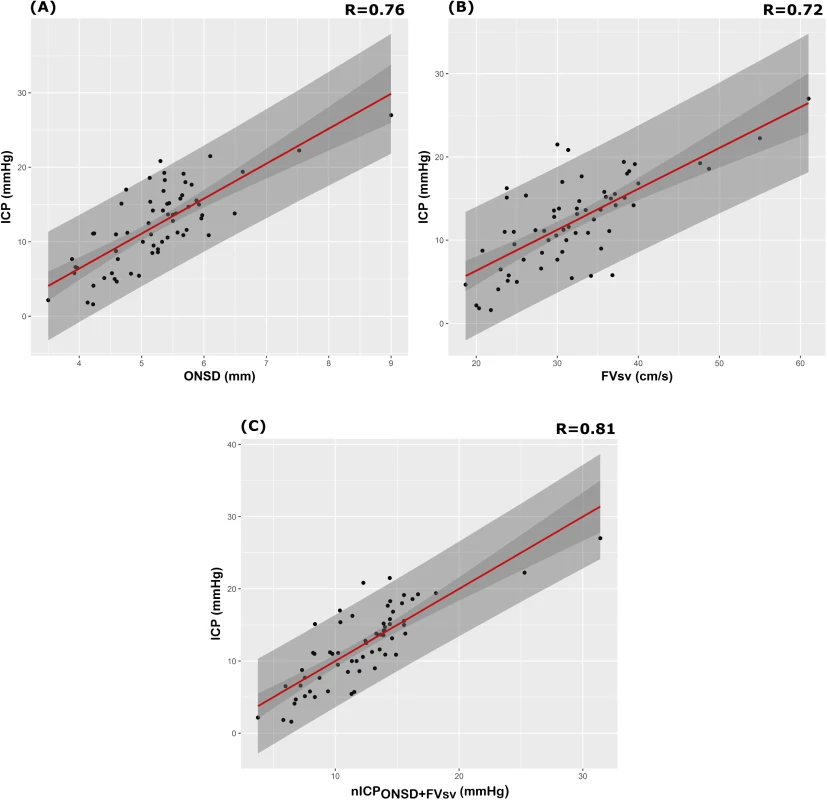
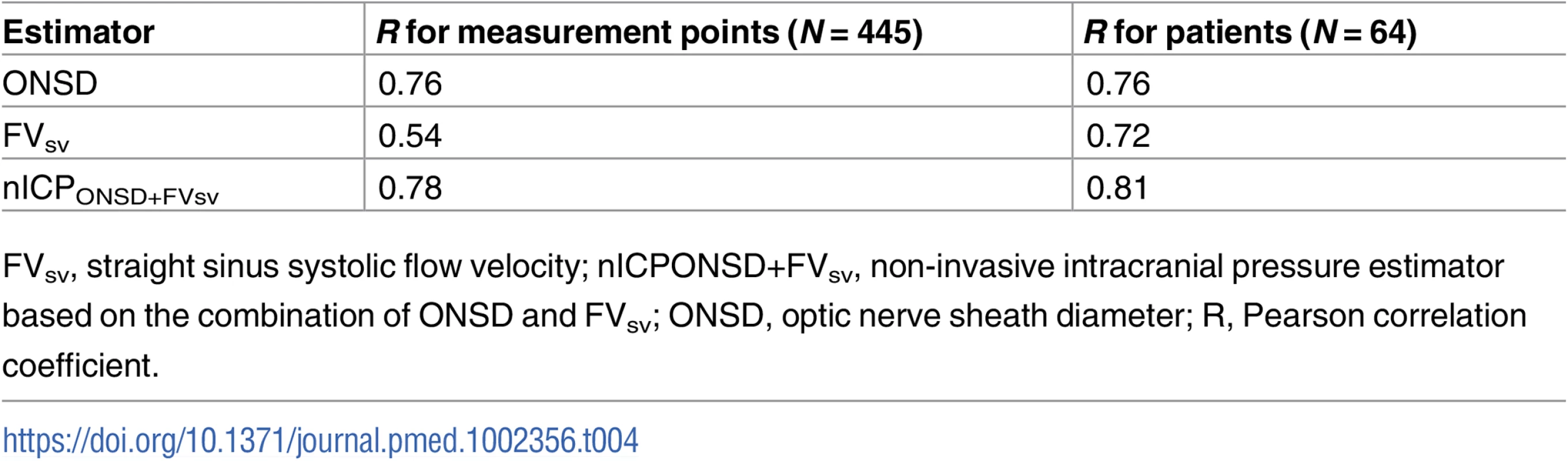
Table 5 summarises the 95% prediction and confidence intervals for the linear regressions between ICP and all non-invasive estimators. The 95% prediction interval for ONSD ranged from 5.05 ± 4.04 to 19.32 ± 4.17 mm Hg; the 95% confidence interval ranged from 11.03 ± 3.95 to 13.34 ± 4.30 mm Hg. The 95% prediction interval for FVsv ranged from 4.57 ± 3.83 to 19.79 ± 3.96 mm Hg; the 95% confidence interval ranged from 10.94 ± 3.71 to 13.43 ± 4.12 mm Hg. For the combination of the 2 methods (nICPONSD+FVsv), the 95% prediction interval ranged from 5.65 ± 4.30 to 18.72 ± 4.45 mm Hg; the 95% confidence interval ranged from 10.90 ± 4.19 to 13.47 ± 4.60 mm Hg.
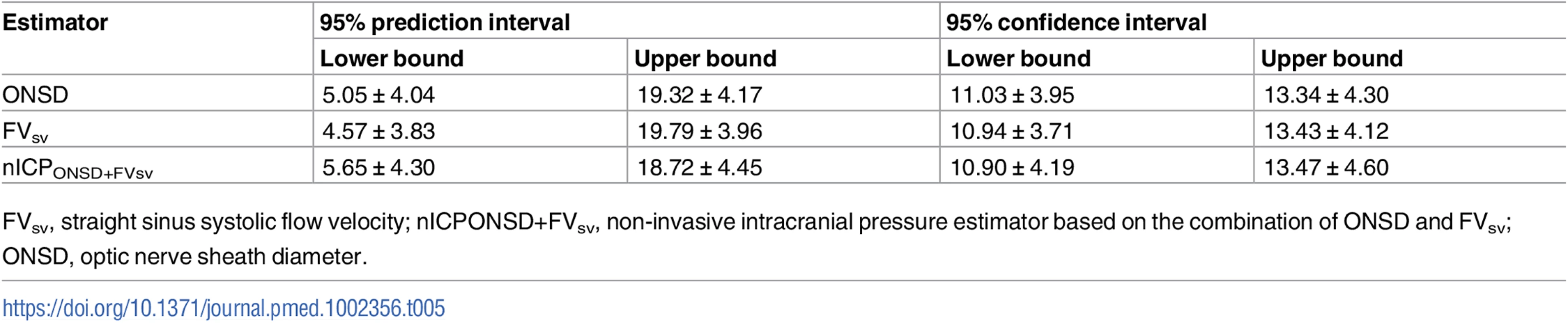
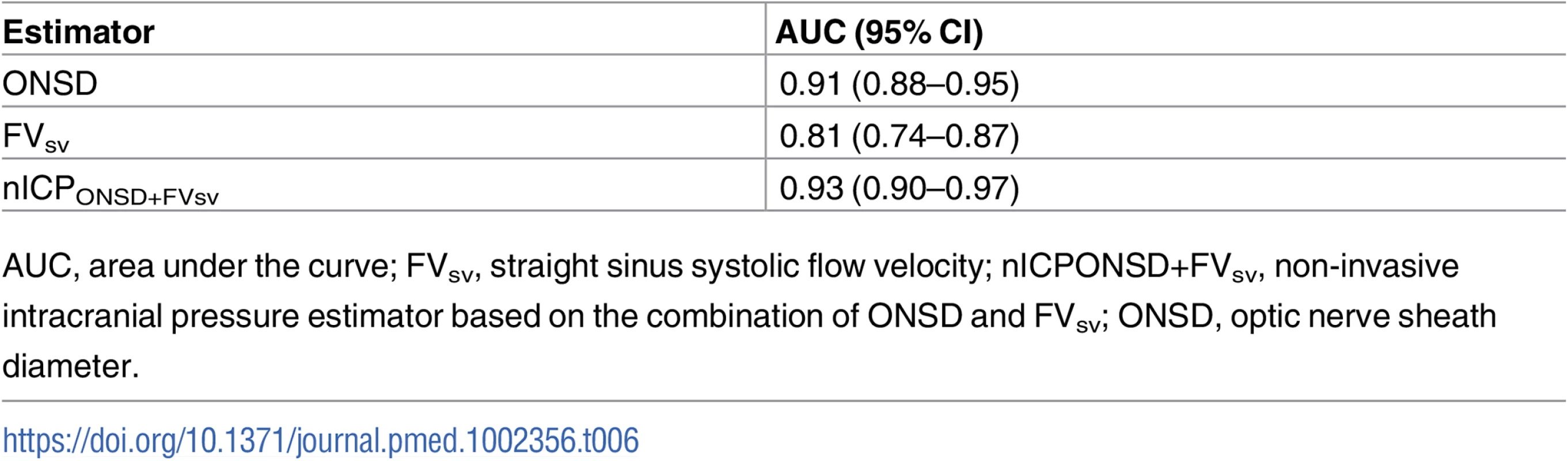
Results of ROC analysis are showed in Table 6 and Fig 2. ONSD had the best AUC among all methods for discriminating cases with intracranial hypertension (ICP ≥ 20 mm Hg) from cases without it (AUC = 0.91, 95% CI 0.88–0.95). The best ONSD and FVsv cutoff values for prediction of intracranial hypertension were 5.85 mm and 38.50 cm/s, respectively. The method based on the combination of ONSD and FVsv showed a statistically significant improvement of AUC values compared with the ONSD method alone (0.93, 95% CI 0.90–0.97, p = 0.01 [DeLong’s test]).

Mortality and nICP
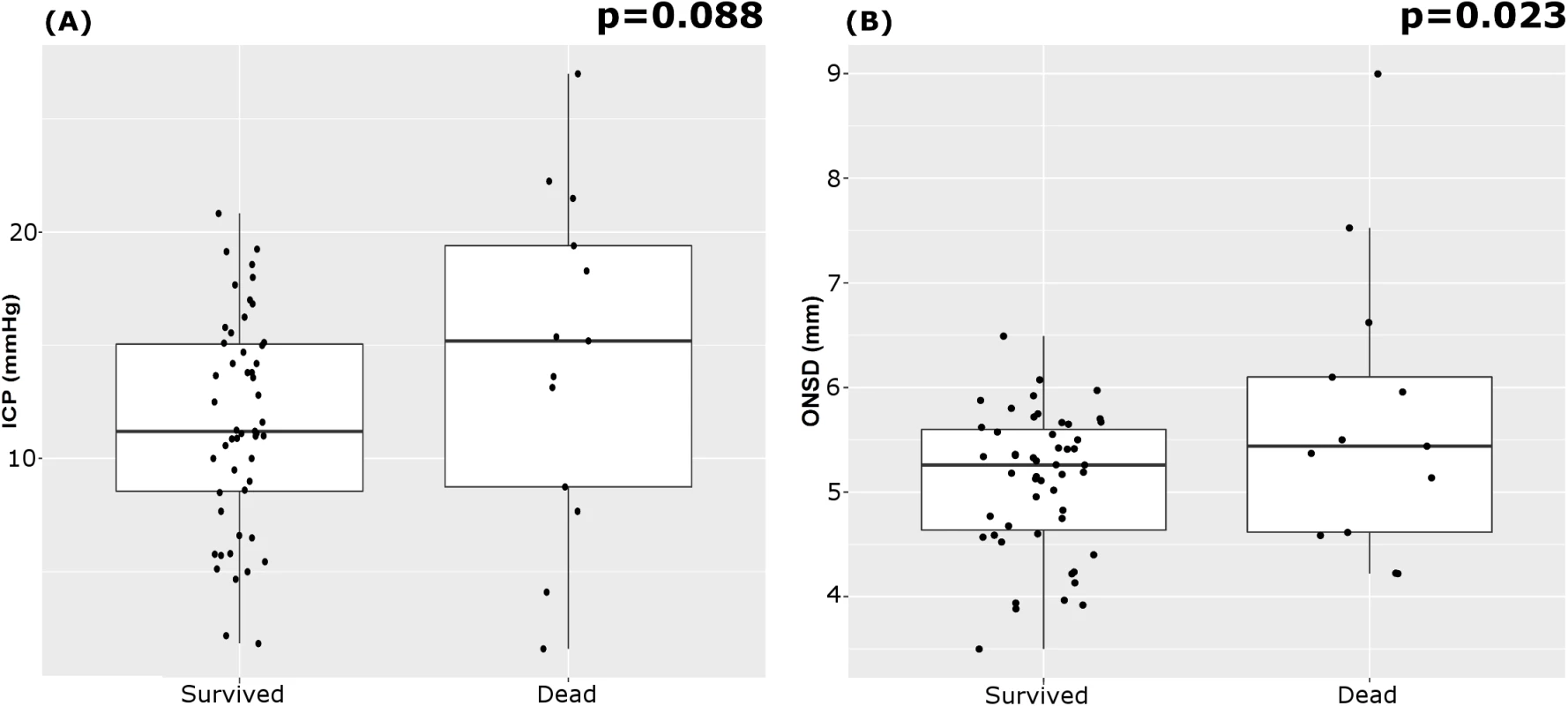
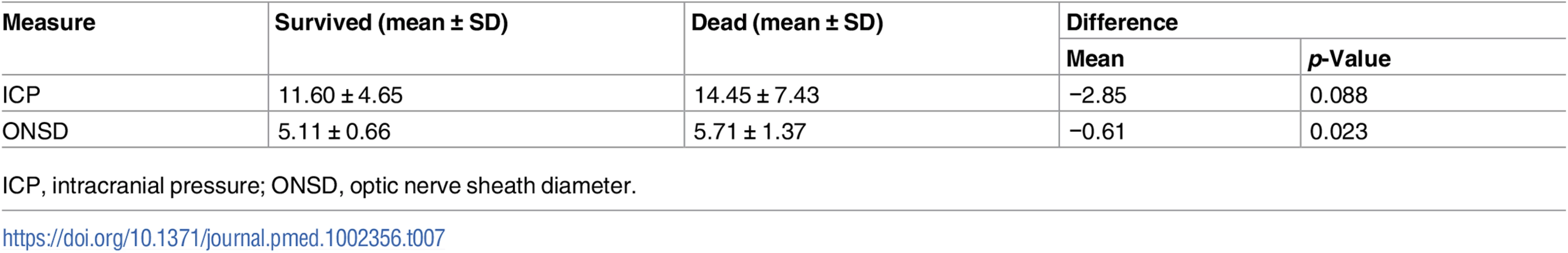
The outcome assessed at discharge revealed that 13 patients died (20%) and 51 survived. Mean ICP showed a tendency to be greater in patients who died; mean ONSD was greater in patients who died than in those who survived (Table 7; Fig 3). FVsv was not significantly different between survivors and non-survivors (p = 0.28).
Discussion
In this study, we present and compare new models for ultrasound-based non-invasive estimation of ICP, based on ONSD ultrasonography, aTCD, and vTCD. Our results show that nICP derived from ONSD has the strongest correlation with invasive ICP. Moreover, ONSD measured through ultrasound was correlated with mortality at discharge. Finally, we demonstrated that a method based on the combination of the 2 best correlated parameters in our cohort (ONSD and FVsv—nICPONSD+FVsv) performed even better across all measurement points (R = 0.78; AUC for prediction of ICP ≥ 20 mm Hg was 0.93).
Measuring ONSD and FVsv using a duplex Doppler machine is fast and does not require probe fixation or specific dedicated hardware [13]. Furthermore, ultrasonography devices are available in most emergency departments and intensive care units, and are used for many other purposes. Therefore, ultrasonography could be very useful for nICP assessment.
The optic nerve is surrounded by subarachnoid space [10,11,31]; hence, the intraorbital part of the subarachnoid space is distensible and can therefore expand if the CSF pressure increases, with the maximum ONSD fluctuations occurring in the anterior compartment. Although the diameter of the optic nerve is narrower in the anterior than in the posterior segment, increased ICP in the perioptic CSF causes a greater enlargement of the retrobulbar segment of the optic nerve sheath, 3 mm behind the globe, than of the posterior segment [32]. This is probably related to the asymmetrical distribution of the arachnoidal trabeculae and the lower density of the arachnoidal trabeculae in the retrobulbar space. ONSD has been investigated in different clinical scenarios [10,33–35], showing a good correlation with ICP measured invasively and low inter- and intra-observer variability [10,11,27,36]. Our results agree with these findings. Among the studied methods, ONSD was the most accurate in the assessment of ICP; moreover, it is a safe and quick method, as the orbital window is easily available and has no complications.
vTCD for the assessment of ICP is a poorly developed technique. It is known that increasing ICP leads to venous haemodynamic changes, as the part of the cerebral vasculature most sensitive to elevated ICP is the subarachnoid bridging veins. According to the Monro–Kellie doctrine, cerebral compliance strongly depends on the compressibility of the low-pressure venous compartment, and stasis in the pial veins occurs early as a compensatory mechanism in case of increased ICP [37,38]. Consequently, venous blood may be pooled toward larger venous vessels (straight sinus and Rosenthal vein), causing an increase in venous flow velocity. An alternative explanation may be that straight sinus can be compressed by rising ICP, and, with constant volume flow, flow velocity may increase.
Schoser et al. applied vTCD for the estimation of ICP in 30 control volunteers and 25 patients with elevated ICP and found a linear relationship, with strong correlation between mean ICP and FVsv [25]. Similarly to Schoser et al., we found that FVsv is strongly correlated with ICP, whereas other vTCD parameters (venous pulsatility index and FVdv) were not good estimators of ICP.
Although the measurement of FVsv seems promising, this technique has some limitations: it can be impossible in polytraumatic patients because of the presence of a cervical collar (6 cases in our cohort). Moreover, the insonation of the straight sinus is feasible in just 72% of cases because of anatomical variations in cerebral veins and transcranial insonation difficulties [25] (even though we had just 3 unsuccessful cases in our cohort, 3.7%).
Our method has several potential clinical applications: it could be useful when invasive monitoring is contraindicated or unavailable, or in many “borderline” situations in which the insertion of invasive monitoring is questioned but a nICP measurement could be useful [20,21]. It can also be applied in patients at risk of intracranial hypertension for causes that are not primarily neurosurgical (such as liver transplantation and intraoperative settings at risk of intracranial hypertension [23,24]) or as screening tool in the emergency department in patients where there is doubt about the need for invasive ICP monitoring.
Limitations
There are several limitations that deserve to be mentioned. First, transcranial Doppler (and ONSD) measurements were intermittent, and continuous measurements remain more feasible with invasive techniques. Second, the mixed cohort of enrolled patients, including different types of acute brain injury, may represent a bias, as the ICP and cerebral perfusion pressure thresholds for subarachnoid haemorrhage, intracerebral haemorrhage, and stroke are not as well defined as for traumatic brain injury. However, this heterogeneity increases the applicability of the study in many clinical scenarios. Other major limitations are the small number of patients included in this study, the need for specialised training to perform and interpret the ultrasound tests, and the variability in performance among different ultrasound operators.
Finally, most our measurements were obtained in patients with relatively well-controlled ICP. Although a strong correlation between nICP and invasive ICP within the range investigated supports the assumption of validity beyond the range investigated, larger validation studies will need to be performed before non-invasive techniques will be able to substitute for invasive ICP monitoring. In addition, despite our findings showing that mortality has a stronger association with ONSD than with ICP, this does not imply that it would be clinically better to monitor and manage ONSD than ICP.
Conclusion
A novel nICP monitoring method based on combined ONSD ultrasonography and vTCD was shown to have promising value for the diagnosis of intracranial hypertension, and a strong correlation with invasive ICP monitoring. This ultrasound-based method is quick, low-cost, and based on technology widely available in emergency departments and intensive care units. Whilst we still advocate the superiority of invasive ICP monitoring when this is clearly indicated, the non-invasive methodology presented here may be of potential benefit for ICP assessment in several clinical scenarios where invasive measurement is not immediately available or is contraindicated. However, this method has several limitations, and further studies are needed to confirm and validate our findings.
Supporting Information
Zdroje
1. Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care. 2006;4:8–13. doi: 10.1385/NCC:4:1:008 16498188
2. Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38:1800–9. doi: 10.1007/s00134-012-2655-4 23011528
3. Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81. doi: 10.1056/NEJMoa1207363 23234472
4. Carney N, Totten AM, OʼReilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432 27654000
5. Brain Trauma Foundation, American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury, 3rd edition. J Neurosurg. 2007;24(Suppl 1):S1-106.
6. Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24. doi: 10.3171/jns.1996.85.3.0419 8751626
7. Binz DD, Toussaint LG, Friedman JA. Hemorrhagic complications of ventriculostomy placement: a meta-analysis. Neurocrit Care. 2009;10:253–6. doi: 10.1007/s12028-009-9193-0 19224404
8. Bauer DF, Razdan SN, Bartolucci AA, Markert JM. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011;69:255–60. doi: 10.1227/NEU.0b013e31821a45ba 21471831
9. Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F, et al. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011;170:265–71. doi: 10.1016/j.jss.2011.03.009 21550065
10. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68. doi: 10.1007/s00134-011-2224-2 21505900
11. Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008;34:2062–7. doi: 10.1007/s00134-008-1149-x 18509619
12. De Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, et al. Transcranial doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17:58–66. doi: 10.1007/s12028-012-9672-6 22311229
13. Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71:853–61. doi: 10.1227/NEU.0b013e3182675b42 22791038
14. Ragauskas A, Bartusis L, Piper I, Zakelis R, Matijosaitis V, Petrikonis K, et al. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure. Neurol Res. 2014;36:607–14. doi: 10.1179/1743132813Y.0000000308 24620972
15. Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I, et al. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology. 2012;78(21):1684–91. doi: 10.1212/WNL.0b013e3182574f50 22573638
16. Schmidt B, Klingelhöfer J, Schwarze JJ, Sander D, Wittich I. Noninvasive prediction of intracranial pressure curves using transcranial Doppler ultrasonography and blood pressure curves. Stroke. 1997;28:2465–72. doi: 10.1161/01.STR.28.12.2465 9412634
17. Kashif FM, Verghese GC, Novak V, Czosnyka M, Heldt T. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med. 2012;4:129ra44. doi: 10.1126/scitranslmed.3003249 22496546
18. Robba C, Bacigaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016;134(1):4–21. doi: 10.1111/ane.12527 26515159
19. Budohoski KP, Schmidt B, Smielewski P, Kasprowicz M, Plontke R, Pickard JD, et al. Non-invasively estimated ICP pulse amplitude strongly correlates with outcome after TBI. Acta Neurochir Suppl. 2012;114:121–5. doi: 10.1007/978-3-7091-0956-4_22 22327676
20. Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112. doi: 10.1159/000346087 23406828
21. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37. doi: 10.1161/STR.0b013e3182587839 22556195
22. Maas AIR, Hukkelhoven CWPM, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–81. doi: 10.1227/01.NEU.0000186013.63046.6B 16331165
23. Robba C, Bragazzi NL, Bertuccio A, Cardim D, Donnelly J, Sekhon M, et al. Effects of Prone position and positive end-expiratory pressure on noninvasive estimators of ICP: a pilot study. J Neurosurg Anesthesiol. 2017;29(3):243–250. doi: 10.1097/ANA.0000000000000295 26998650
24. Robba C, Cardim D, Donnelly J, Bertuccio A, Bacigaluppi S, Bragazzi N, et al. Effects of pneumoperitoneum and Trendelenburg position on intracranial pressure assessed using different non-invasive methods. Br J Anaesth. 2016;117:783–91. doi: 10.1093/bja/aew356 27956677
25. Schoser BG, Riemenschneider N, Hansen HC. The impact of raised intracranial pressure on cerebral venous hemodynamics: a prospective venous transcranial Doppler ultrasonography study. J Neurosurg. 1999;91:744–9. doi: 10.3171/jns.1999.91.5.0744 10541230
26. Cardim D, Robba C, Donnelly J, Bohdanowicz M, Schmidt B, Damian M, et al. Prospective study on non-invasive assessment of ICP in head injured patients: comparison of four methods. J Neurotrauma. 2016;33(8):792–802. doi: 10.1089/neu.2015.4134 26414916
27. Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8 21769456
28. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01
29. Hosmer D, Lameshow S. Applied logistic regression. New York: John Wiley & Sons; 1989.
30. Robin AX, Turck N, Hainard A, Lisacek F, Sanchez J, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 21414208
31. Geeraerts T, Newcombe VFJ, Coles JP, Abate MG, Perkes IE, Hutchinson PJ, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12:R114. doi: 10.1186/cc7006 18786243
32. Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I. Experimental study. Pediatr Radiol. 1996;26:701–5. doi: 10.1007/BF01383383 8805599
33. Helmke K, Burdelski M, Hansen HC. Detection and monitoring of intracranial pressure dysregulation in liver failure by ultrasound. Transplantation. 2000;70:392–5. doi: 10.1097/00007890-200007270-00029 10933171
34. Komut E, Kozaci N, Sönmez BM, Yilmaz F, Komut S, Yildirim ZN, et al. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of intracranial pressure in ED. Am J Emerg Med. 2016;34:963–7. doi: 10.1016/j.ajem.2016.02.012 26944107
35. Moretti R, Pizzi B. Optic nerve ultrasound for detection of intracranial hypertension in intracranial hemorrhage patients confirmation of previous findings in a different patient population. 2009;21:16–20. doi: 10.1097/ANA.0b013e318185996a 19098619
36. Amini A, Kariman H, Arhami Dolatabadi A, Hatamabadi HR, Derakhshanfar H, Mansouri B, et al. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med. 2013;31:236–9. doi: 10.1016/j.ajem.2012.06.025 22944553
37. Valdueza JM, Schultz M, Harms L, Einhaupl KM. Venous transcranial Doppler ultrasound monitoring in acute dural sinus thrombosis. Report of two cases. Stroke. 1995;26:1196–9. 7604413
38. Wardlaw JM, Vaughan GT, Steers AJ, Sellar RJ. Transcranial Doppler ultrasound findings in cerebral venous sinus thrombosis. Case report. J Neurosurg. 1994;80:332–5. doi: 10.3171/jns.1994.80.2.0332 8283275
Štítky
Interné lekárstvoČlánok vyšiel v časopise
PLOS Medicine
2017 Číslo 7
- Statinová intolerance
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Metamizol v liečbe pooperačnej bolesti u detí do 6 rokov veku
- Co dělat při intoleranci statinů?
Najčítanejšie v tomto čísle
- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models



