The influence of hyaluronan addition on thickness, weight, uniformity of mass and water content of mucoadhesive films
Vliv přídavku hyaluronanu na tloušťku, hmotnost, hmotnostní stejnoměrnost a obsah vody mukoadhezivních filmů
Experimentální studie sledovala vlastnosti bukálních mukoadhezivních filmů (tloušťka, hmotnost, hmotnostní stejnoměrnost aobsah vlhkosti ve filmech) připravených metodou odpařování rozpouštědla. Filmy obsahovaly buď jeden mukoadhezivní polymer (hyaluronát sodný odvou různých molekulových hmotnostech nebo sodná sůl karboxymethylcelulosy), případně kombinaci uvedených polymerů. Jako nejdůležitější parametr hodnocení filmů se zvolil obsah vody v bukálních mukoadhezivních filmech. Obsah vody byl ovlivněn jak molekulovou hmotností hyaluronátu sodného, tak také poměrem jednotlivých mukoadhezivních polymerů ve složení bukálních filmů. Složení filmů ovlivňuje také ostatní kvalitativní parametry filmů.
Klíčová slova:
bukální mukoadhezivní filmy • metoda odpařování rozpouštědla • hyaluronát sodný • sodná sůl karboxymethylcelulosy • obsah vody
Authors:
Veronika Walicová; Jan Gajdziok; Markéta Gajdošová; David Vetchý
Authors place of work:
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno
Published in the journal:
Čes. slov. Farm., 2016; 65, 94-98
Category:
Original Articles
Summary
Characteristics of the buccal mucoadhesive films (film thickness, film weight, uniformity of mass and moisture content) prepared by solvent casting method were tested in this experimental study. The formulations consisted either of one mucoadhesive polymer (sodium hyaluronate of two different molecular weights and sodium carboxymethylcellulose) or combinations thereof. On the basis of the aforementioned tests, it was determined that water content was influenced by the molecular weight of sodium hyaluronate as well as by the ratio of mucoadhesive polymers in the composition. The composition of the films influences also other tested parameters.
Key words:
buccal mucoadhesive films • solvent casting method • sodium hyaluronate • sodium carboxymethylcellulose • water content
Introduction
Hyaluronic acid is a linear polysaccharide from the group of glycosaminoglycans, composed of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units. Due to its anionic character, it is mainly presented in the form of sodium salt (sodium hyaluronate; generally called hyaluronan), which plays an important role in the composition of extracellular matrix, synovial fluids and intracellular space of skin1, 2). Traditionally, it was extracted from cocks’ combs, but nowadays it is usually obtained by means of fermentation mediated by Streptococcus sp.3). Hyaluronan is able to change dermal volume and skin compressibility by immobilization of water in tissues. It can also influence cell proliferation, differentiation and tissue repairing processes2). Hyaluronan and its degradation products affect beneficially several aspects of wound healing and angiogenesis4). The mucoadhesive properties of hyaluronic acid (and its derivatives) have been attributed to its ability of hydrogen bonds forming and electronic interactions with mucin. However, recent studies indicate that interpenetration and physical interlocking with mucin chains could present the most important mechanism of mucoadhesive behaviour of this polymer5).
Modern drug formulations based on mucoadhesion process have recently come to the foreground of the therapeutic interest6). The most promising mucoadhesive dosage form for buccal administration represent flexible buccal mucoadhesive films that could help to protect the wound surface and prolong residence time of the drug on the specific site of action7). Mucoadhesive films are promising candidates for the oral administration of many drugs in order to ensure their systemic effect or local action in the oral cavity6).
To date, the most used mucoadhesive polymers for the development of mucoadhesive films represents hydrophilic polymers forming hydrogels, e.g. carbomer, polycarbophil, xanthan gum, sodium alginate, chitosan and widely used cellulose derivatives8, 9). The most commonly used cellulose derivatives in formulation of mucoadhesive films are carboxymethylcellulose, hydroxypropylmethylcellulose, methylcellulose10), etc. Stronger mucoadhesive properties exhibit thiolated polymers simulating binding of glycoproteins to sialic and sulfonic acids on the mucosal surfaces8). Buccal mucoadhesive films with sodium hyaluronate (hydrogel forming polymer) have not been extensively studied yet, although they could contribute to effective moist healing of oral mucosa lesions.
In the presented experiment the effect of two types of sodium hyaluronate addition (varying in Mw) on the properties (thickness, weight, uniformity of mass and water content) of buccal mucoadhesive films prepared by solvent casting method was evaluated.
Experimental part
Materials
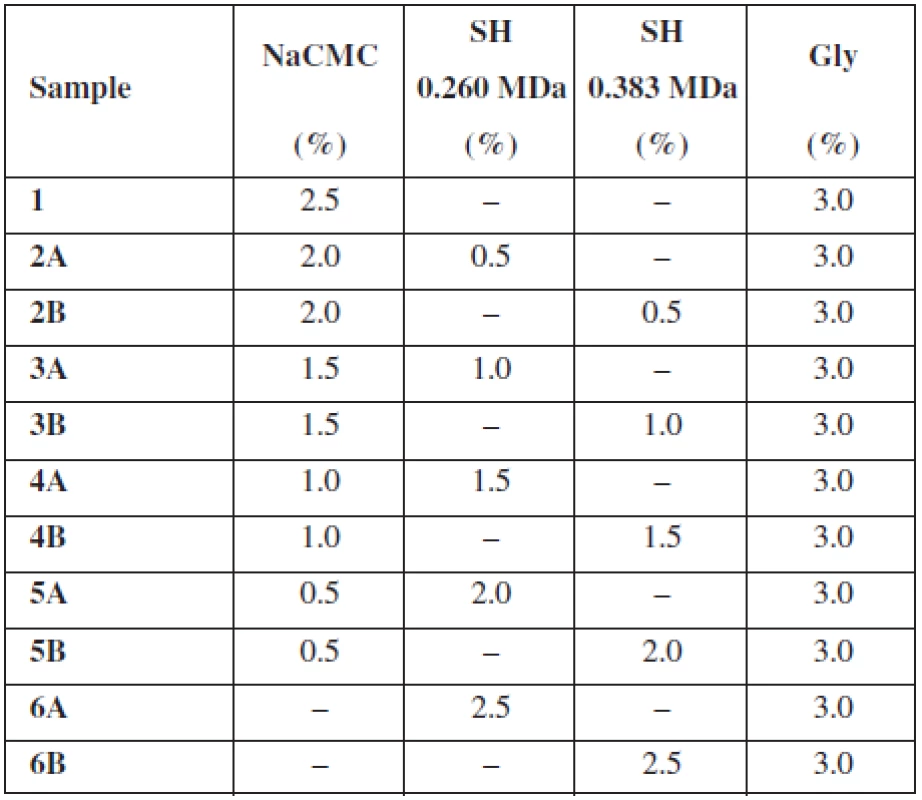
Two different types of sodium hyaluronate (SH-HySilk, Contipro Pharma, CZ) with Mw 0.260 MDa and 0.383 MDa were used as the mucoadhesive and film forming polymers with expected positive effect on mucosa lesions healing. A semi-synthetic cellulose derivative – sodium carboxymethylcellulose (NaCMC-Blanose type 7LF-Ph, Ashland Aqualon Functional Ingredients, USA) with Mw 0.091 MDa was used as the second mucoadhesive and film forming polymer. In all samples, glycerol (Gly, Dr. Kulich Pharma, CZ) was incorporated in concentration of 3% (w/w) as plasticizer. Distilled water of analytical grade was used as solvent. Percentage (w/w) of excipients in casting dispersion is summarized in Table 1.
Preparation of buccal mucoadhesive films
Monolayer hydrogel films were prepared by the solvent casting method. Each casting dispersion (Table 1) was stirred for 30 min (300 rpm) using overhead stirrer (RZR 2021, Heidolph Instruments, D) and subsequently let swell for 48 h. Finally, dispersions were stirred for 2 min (11 000 rpm) with Ultra-turrax® (T25 basic, IKA®-Werke, D). Using an automatic pipette Transferpette® S (Brand, UK), 18 ml of the casting dispersion was cast into a round plastic mold (63 mm in diameter). Solvent was left to evaporate at room temperature for approximately 48 h. After partial evaporation of water, another 9 ml of dispersion was added to the same molds and dried at room temperature11). From the final films, samples of sizes suitable for each evaluation test were punched.
Evaluation of prepared films
Film thickness
Film thickness was measured using the optical microscope (SMZ 1500, Nikon, JPN). The square sample (25 ⋅ 25 mm) of the film was vertically fixed in a holder. The edge of the film was shot by colour digital camera and sample thickness was measured by software NIS-Elements (Nikon, JPN) at 5 different places of the film, repeated 3 times11). Results are presented as average values and SD of each sample.
Film weight and uniformity of mass
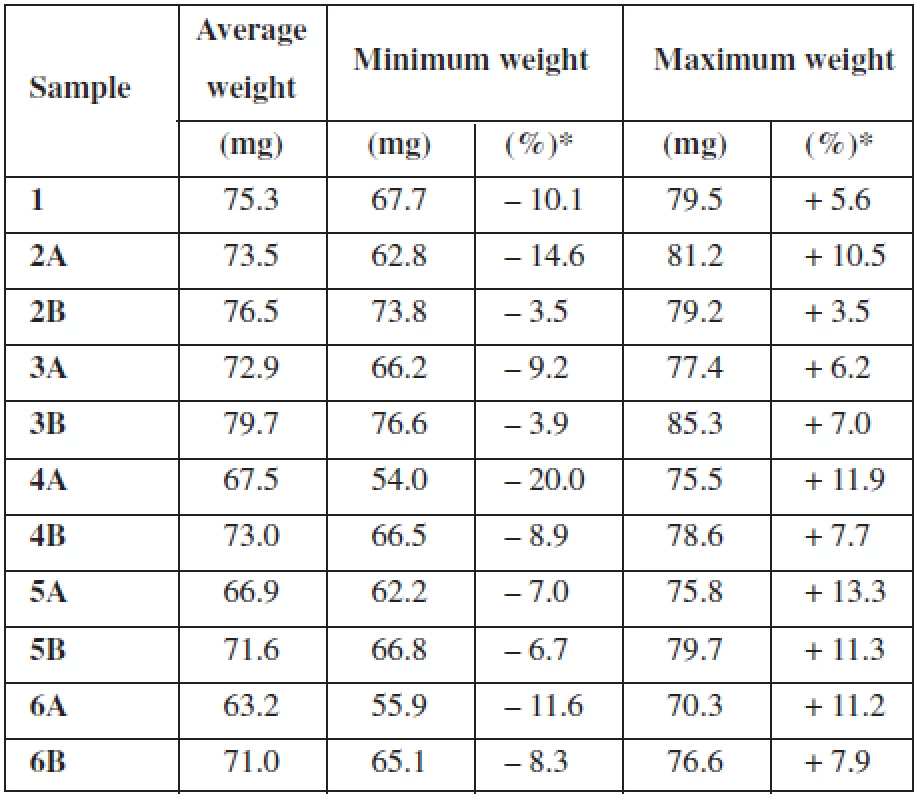
Uniformity of mass of mucoadhesive films is not specified in the European Pharmacopoeia3). Therefore, test for uniformity of mass of single-dose preparations was used. 20 round samples (15 mm in diameter) of the dosage form were individually weighted and the average mass and SD was determined. Limits for uncoated and film-coated tablets with average mass 80 mg or less were applied. Not more than 2 of the individual masses could deviate from the average mass by more than 10% and none by more than twice that percentage3). Results are presented as average values and SD of each sample (film weight) and also as average values with minimum and maximum value and their percentage deviation from average (uniformity of mass).
Moisture content
Halogen moisture analyzer (Excellence Plus HX 204, Mettler Toledo, CH) was used for determination of moisture content of square sample (25 ⋅ 25 mm) of each batch. Measurement was carried out on the basis of thermogravimetric principle, i.e. the preweighed sample was heated to 105 °C until it attains constant weight. The drying process was terminated if the loss in weight fell below 1 mg over 50 s. The difference in weight (at the beginning and end of the measuring process) gives the amount of moisture presented in the film12). The measurement was repeated 3 times, and results are presented as average values and SD of each sample.
Results and discussion
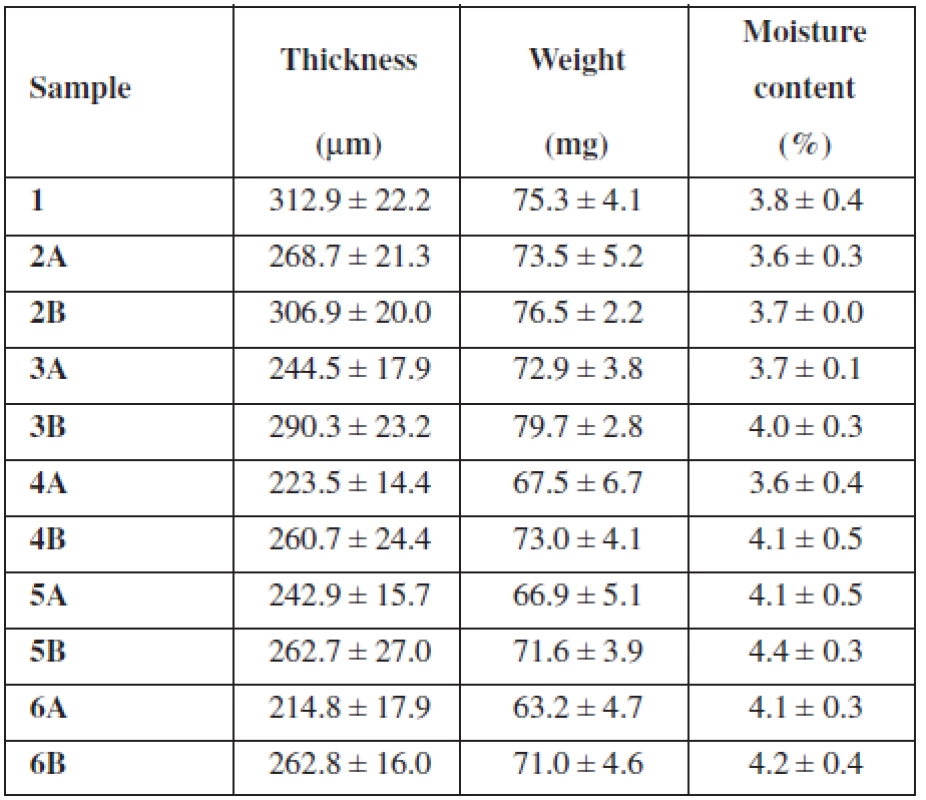
The purpose of this study was to evaluate the effect of SH on the thickness, uniformity of mass and water content of buccal mucoadhesive films based on two different mucoadhesive polymers – NaCMC, SH (two types varying in Mw) and their combination. As shows Table 1, the overall concentration of the mucoadhesive polymers in each casting dispersion was 2.5% (w/w) with an increase in concentration of SH by half a percent gradually in samples 2–6. Samples identified as A contained SH with Mw 0.260 MDa and samples B included SH with higher Mw 0.383 MDa. Properties of films prepared using solvent casting method are summarized in Table 2.
Film thickness
Samples 2A–6A showed a resulting film thickness from 214.8 μm ± 17.9 μm to 268.7 μm ± 21.3 μm (Table 2), which represents values suitable for their buccal application (thickness between 50 and 1000 μm)12). Samples 2B–6B showed thickness from 260.7 μm ± 24.4 μm to 306.9 μm ± 20.0 μm and are also convenient for buccal administration (Table 2). Figure 1 reveals decreasing of the films’ thickness with an increase in concentration of SH (Mw 0.260 MDa). Sample 5A with thickness 242.9 μm ± 15.7 μm represents an exception, which could be caused by inaccuracies in film structure. Figure 1 also shows decreasing of the films’ thickness with an increase in concentration of SH (Mw 0.383 MDa) until the concentration of SH reaches 1.5%. With further increase in SH (Mw 0.383 MDa) concentration, the thickness remained similar. Sample 1 containing only NaCMC as mucoadhesive polymer was slightly thicker than all samples with SH (Table 2). Films’ thickness is probably influenced by the Mw of the polymer. There must be presented more NaCMC macromolecules in the film sample 1 structure due to lower Mw of NaCMC (0.091 MDa) in comparison to SH (0.260 MDa and 0.383 MDa). Greater thickness of samples containing SH with higher Mw (0.383 MDa) than with lower Mw (0.260 MDa) could be influenced by higher water binding capacity of SH with higher Mw, due to longer polymeric chains and greater content of hydrophilic groups. There could be expected extensive interaction of water with SH, due to its polar structure caused by H-bonds with –OH groups and also electrostatic interactions with carboxylic sites. Despite this fact, water retention of SH usually remains small until relative humidity does not exceed 75%13).

Film weight and uniformity of mass
The weight of prepared film round samples (15 mm in diameter) 2A–6A ranged from 63.2 mg ± 4.7 mg to 73.5 mg ± 5.2 mg (Table 2) with a downtrend with SH concentration increase (Fig. 2). The weight of prepared film samples 2B–6B ranged from 71.0 mg ± 4.6 mg to 79.7 mg ± 2.8 mg (Table 2). Figure 2 reveals increasing of films’ weight with an increase in concentration of SH (Mw 0.383 MDa) until the concentration of SH (Mw 0.383 MDa) reaches 1%. With further increase in concentration of SH (Mw 0.383 MDa) film’s weight decreased. All samples B with SH of higher Mw showed higher weight than equivalent samples containing SH of lower Mw (Table 2).

The comparison of results obtained from film thickness evaluation and weight measurement showed that the weight of majority of film samples remained with the increase in concentration of SH almost unchanged or was slightly increased (Fig. 2), whereas the thickness of films was decreasing more significantly. This dependence could also indicate an ability of SH to bind water. Furthermore, with increasing concentration of SH were prepared films more sticky and plasticized (visual observation). This could be also regarded to higher water binding capacity of SH than of NaCMC, because water molecules might interpose in the polymer chains functioning as a plasticizer14).
For the uniformity of mass testing, deviations from average weight for each individual measurement were calculated. For a better overview, Table 3 shows only minimum and maximum deviation from the average weight of film expressed in mg and %. All the samples met the test conditions except sample 4A3). Sample 4A deviated from the average mass by more than 10% in 3 individual cases and therefore exceeded the test limits.
Moisture content
The previously presented observations were confirmed by the moisture content measurement using halogen moisture analyzer (Table 2). The moisture content of sample 1 containing NaCMC as the only mucoadhesive polymer was 3.8% ± 0.4%. The moisture content of samples with low SH concentration showed values 3.6% ± 0.3% (sample 2A) and 3.7% ± 0.0% (sample 2B). The moisture content of samples with SH as the only mucoadhesive polymer showed values 4.1% ± 0.3% (sample 6 A) and 4.2% ± 0.4% (sample 6B). Water binding capacity in the films influences also the addition of a plasticizer; e.g. films containing plasticizer glycerol have higher water uptake and water binding properties than films with PEG 40015). Due to the incorporation of glycerol into each sample in the same concentration it can be assumed that water binding capacity of the films is influenced only by the type and the ratio of used polymers. With an increase in concentration of SH raised water retention, expressed as the moisture content (Fig. 3). Moisture content of films containing SH with higher Mw was higher than the moisture content of films containing SH of lower Mw (Fig. 3), due to longer polymeric chains of SH molecule with higher content of hydrophilic groups (hydroxyl, acetamide, carboxylic group). Comparison of sample 1 with sample 6A and 6B showed higher water binding capacity for SH films than NaCMC films.

Conclusions
The results of this study showed that monolayer buccal mucoadhesive films from NaCMC, SH and their combination could be succesfully prepared by solvent casting method. With one exception, all samples met the test limits for uniformity of mass. Evaluation techniques used in this study provided an insight into the amount of water which could be bounded in prepared films and could also influence film thickness and weight. Water content was affected both by Mw and concentration of SH. Water content is an important indicator of quality of buccal mucoadhesive films with an impact on the final properties of the dosage form.
Acknowledgement
The authors thank Contipro Pharma, CZ as the kindly donator of sodium hyaluronate samples.
This work was supported by project IGA VFU Brno No. 308/2015/FaF.
Conflict of interest: none.
Received 13 Januar 2016
Accepted 20 Februar 2016
Assoc. prof. Dr. Jan Gajdziok, Ph.D. • V. Walicová • M. Gajdošová • D. Vetchý
Department of Pharmaceutics, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences
Palackeho tr. 1946/1, 612 42 Brno, Czech Republic
e-mail: gajdziokj@vfu.cz
Zdroje
Schiller J., Volpi N., Hrabárová E., Šoltés L. Hyaluronic acid: a natural biopolymer. In: Kalia, S., Averous, L. eds. Biopolymers: Biomedical and environmental applications. Hoboken: John Wiley & Sons, Inc. 2011.
2. Kogan G., Šoltés L., Stern R., Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007; 29, 17–25.
3. European Directorate for the Quality of Medicines & Health Care. The European Pharmacopoeia 8th edition 2014 (8.1.. http://online6.edqm.eu/ep801/ (19.8.2015).
4. Price R. D., Berry M. G., Navsaria H. A. Hyaluronic acid: the scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007; 60, 1110–1119.
5. Sosnik A., das Neves J., Sarmento B. Mucoadhesive polymers in the design of nano–drug delivery systems for administration by non–parenteral routes: a review. Prog. Polym. Sci. 2014; 39, 2030–2075.
6. Gajdziok, J., Holešová, S., Štembírek, J., Pazdziora, E., Landová, H., Doležel, P., Vetchý, D. Carmellose mucoadhesive oral films containing vermiculite/chlorhexidine nanocomposites as innovative biomaterials for treatment of oral infections. BioMed Res. Int. 2015; 2015, 1–15.
7. Salamat-Miller N., Chittchang M., Johnston T. P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005; 57, 1666–1691.
8. Gajdziok, J., Vetchý, D. Mukoadhezivní polymery v lékových formách. Chem. Listy 2012; 106, 632–638.
9. Bajerová, M., Gajdziok, J., Dvořáčková, K., Masteiková, R., Kollár, P. Polosyntetické deriváty celulosy jako základ hydrofilních gelových systémů. Čes. slov. Farm. 2008; 57, 63–69.
10. Landová, H., Daněk, Z., Gajdziok, J., Vetchý, D., Štembírek, J. Mucoadhesive films as perspective oral dosage form. Čes. slov. Farm. 2013; 62, 4–11.
11. Vetchý D., Landová H., Gajdziok J., Doležel P., Daněk Z., Štembírek J. Determination of dependencies among in vitro and in vivo properties of prepared mucoadhesive buccal films using multivariate data analysis. Eur. J. Pharm. Biopharm. 2014; 86, 498–506.
12. Nair A. B., Kumria R., Harsha S., Attimarad M., Al-Dhubiab B. E., Alhaider I. A. In vitro techniques to evaluate buccal films. J. Control Release. 2013; 166, 10–21.
13. Jouon N., Rinaudo M., Milas M., Desbrières J. Hydration of hyaluronic acid as a function of the counterion type and relative humidity. Carbohydr. Polym. 1995; 26, 69–73.
14. Borges A. F., Silva C., Coelho J. F., Simões S. Oral films: current status and future perspectives: I – Galenical development and quality attributes. J. Control Release. 2015; 206, 1–19.
15. Bajdik, J., Marciello, M., Caramella, C., Domján, A., Süvegh, K., Marek, T., Pintye-Hódi, K. Evaluation of surface and microstructure of differently plasticized chitosan films. J. Pharm. Biomed. Anal. 2009; 49, 655–659.
Štítky
Pharmacy Clinical pharmacologyČlánok vyšiel v časopise
Czech and Slovak Pharmacy

2016 Číslo 3
Najčítanejšie v tomto čísle
- Rhodiola rosea and its neuropsychotropic effects
- Ageing and Alzheimer disease – system dynamics model prediction
- Preparation and evaluation of indomethacin loaded alginate microspheres
- The influence of hyaluronan addition on thickness, weight, uniformity of mass and water content of mucoadhesive films
