Avidity of selected autoantibodies – usefulness of their determination for clinical purposes
Avidita vybraných autoprotilátek – přínos jejich stanovení pro klinické účely
Protilátky namířené proti různým autoantigenům představují heterogenní skupinu imunoglobulinů, které se liší kvalitativními i kvantitativními vlastnostmi. Důležitou kvalitativní charakteristikou protilátek je jejich afinita/avidita, která se mění v procesu jejího vyzrávání během imunitní odpovědi.
Cílem studie bylo shrnutí znalostí o aviditě vybraných autoprotilátek u určitých autoimunitních onemocnění. Avidita různých autoprotilátek se liší za různých klinických situací. V biologických tekutinách pacientů s autoimunitními onemocnění se vyskytují protilátky s nízkou, střední i s vysokou hodnotou avidity.
U autoimunitních chorob nemusí být proces vyzrávání avidity spojen s progresí od nízkých k vyšším hodnotám tak, jak je to typické pro protilátky proti exogenním antigenům. Avidita každé autoprotilátky by proto měla být posuzována individuálně. Některé studie naznačují možný přínos stanovení avidity pro upřesnění diagnózy a prognózy vybraných autoimunitních onemocnění.
Klíčová slova:
afinita – protilátky proti citrulinu – protilátky proti glomerulární bazální membráně – protilátky proti glutamátdekarboxyláze – protilátky proti inzulinu – autoprotilátky – avidita – onkoneuronální protilátky
Authors:
L. Fialová
Authors‘ workplace:
Institute of Medical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague, Czech Republic
Published in:
Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 3, s. 155-163
Category:
Review Article
Overview
Autoantibodies directed against various self-antigens comprise a heterogeneous group of immunoglobulins, which differ in their qualitative and quantitative features. An important qualitative characteristic of antibodies is affinity/avidity, which changes in the process of its maturation during the immune response.
This study is aimed to summarize the knowledge about avidity of selected autoantibodies in certain autoimmune diseases. The avidity of various autoantibodies differs under distinct clinical situations. High-, moderate or low-avidity may be found in biological fluids in patients with autoimmune diseases.
The avidity maturation associated with progression from low to high values typical for antibodies against exogenous antigens is not always uniform in autoimmune diseases; therefore, the avidity of each autoantibody should be judged individually. Some studies promise the possible benefit of avidity examination for the refinement of diagnosis and prediction of selected autoimmune diseases.
Key words:
affinity – anti-citrullinated protein antibodies – anti-glomerular basement membrane antibodies – anti-glutamate decarboxylase antibodies – anti-insulin antibodies – autoantibodies – avidity – onconeuronal antibodies
INTRODUCTION
Autoantibodies directed against various self-antigens are found in serum and also in some other body fluids. The determination of their presence or levels (titres) in biological fluids, especially in serum, has great clinical relevance [1]. Many of them can be used as an important biomarker for diagnosis, prediction and prognosis for various autoimmune-mediated diseases.
Autoantibodies against certain autoantigens comprise a heterogeneous group of immunoglobulins, which differ in their qualitative features such as specificity or affinity and avidity.
Affinity is defined as the strength of various non-covalent interactions between a single epitope of an antigen and a single antibody paratope [2, 3]. Because the immunoglobulin molecules are multivalent and antigens usually carry more epitopes, the term avidity or functional affinity is used for total binding strength of interactions between a multivalent antibody and an antigen. Terminology for avidity and affinity is not always adhered uniformly. The term avidity used to be incorrectly replaced by the term affinity in some studies.
The affinity/avidity of antibodies is changed during the immune response in the process of affinity/ avidity maturation. Much information has been obtained for avidity maturation of antibodies against exogenous antigens derived from pathogens. Initially, low avidity of antibodies increases after primary infection upon secondary antigen exposure [2]. The consequence of avidity maturation against antigens derived from pathogens is an increased efficiency of humoral immunity. The avidity of antibodies in some infectious diseases (for example rubella, toxoplasmosis or cytomegalovirus virus and human immunodeficiency virus infections) is often assayed as a part of laboratory examination with the aim to extend the information about the immunological status of the patient [4–9]. While the avidity maturation of anti-infectious antibodies raises the protection against the pathogens, the increase of avidity of autoantibodies may have both protective effects and injurious potential [2, 10, 11].
Several methods are used for the determination of antibody affinity and avidity. While some techniques are not suitable for routine testing, others may be utilized in routine practice. The methods for affinity and avidity determination are discussed at large in the study of Božič et al. [12] and Woznicová [7].
Although current knowledge about the avidity of autoantibodies is not so extended as that for antibodies against foreign antigens, the information has gradually grown of recent years. More detailed information about the avidity of antiphospholipid antibodies and autoantibodies against nervous tissue antigens has been already published [13–15]. Therefore this review will be focused on the selection of other autoantibodies especially those whose avidity had ever been studied in the context of their clinical relevance to the autoimmune-mediated diseases.
AVIDITY OF SELECTED AUTOANTIBODIES
AVIDITY OF AUTOANTIBODIES USED IN RHEUMATOLOGY
A wide spectrum of autoantibodies is a beneficial tool in diagnosis, monitoring and prognosis of various rheumatologic diseases. The current knowledge about avidity of anti-citrullinated protein antibodies (ACPA) and antibodies against type II collagen (CII) antibodies will be mentioned.
ANTI-CITRULLINATED PROTEIN ANTIBODIES
The common serological disease-specific biomarkers used in patients with rheumatoid arthritis (RA) are anti-citrullinated protein antibodies, which were included in the classification criteria for RA [16]. They can be found even years before the onset of clinical manifestation. The autoantigens for ACPA are proteins containing the epitopes with amino acid citrulline formed in the process of post-translation modification of arginines by enzyme peptidylarginine deiminase [17].
ACPA response in ACPA-positive healthy asymptomatic individuals displays a lower avidity while the ACPA avidity in ACPA-positive patients with joint symptoms and RA patients was low to moderately high [18]. The increase of ACPA avidity was observed in some patients during the asymptomatic phase before arthritis became apparent and no additional avidity maturation occurred after the onset of arthritis. No change in ACPA IgG avidity over time during a 5-year follow-up after disease manifestation was seen [19]. Unfortunately, ACPA avidity measurement in the pre-disease stage most likely cannot be useful for the identification of patients who will develop RA [18].
The avidity of ACPA in serum of ACPA-positive patients with early arthritis was low in comparison with the avidity of antibodies against recall antigens (tetanus toxoid, diphtheria toxoid) in the same patients [19]. Even patients with high titre of ACPA display only relatively low-avidity antibodies. The analysis of ACPA avidity in synovial fluid, which was similar to the avidity in serum, excludes the possibility that high-avidity antibodies would be retained in the inflamed joints due to the presence of antigens with citrullinated epitopes. However, the eventuality that high avidity antibodies are bound in the form of immune complexes not detectable by standard ELISA methods might also be considered.
Nevertheless, antibody avidity varied considerably among individual subjects and ACPA avidity in RA patients was related to disease characteristic activity. Surprisingly, low-avidity ACPA were associated with more severe joint destruction probably due their ability to be more potent in activating the complement system demonstrated by in vitro experiments [20]. To explain this observation by another way, authors hypothesized that ACPA may bind to one citrullinated antigen with low avidity and, because of cross reactivity, also to other so far unknown citrullinated antigens with high avidity. The antibodies initially directed against the exogenous antigen may be later expanded to autoantigens. A more recent study [21] in which the avidity measurement was performed by surface plasmon resonance showed that the avidity of ACPA to citrullinated histone peptide autoantigens was higher than avidity to exogenous citrullinated peptides derived from exogenous antigens. Moreover, the specific changes in Fab glycosylation in ACPA IgG molecules prior to the onset of arthritis were found to modulate binding avidity of ACPA to citrullinated antigens [22].
Taken together, the ACPA avidity in RA patients is characterized by a higher value without signs of future avidity maturation during disease. In spite of higher avidity, its value does not reach the value typical for anti-infectious antibodies. It is possible that ACPA avidity determination might have a significant for the assessment of RA severity considering low-avidity ACPA are associated with a higher rate of radiographic joint destruction [20].
ANTI-TYPE II COLLAGEN ANTIBODIES
The type II collagen, a major collagen type in joint cartilage, is another target for the autoimmune reaction. Anti-CII antibodies are present in serum and synovial fluid of patients with rheumatoid arthritis and juvenile idiopathic arthritis (JIA) [23, 24]. It is assumed that anti-CII antibodies play a role in the pathogenesis of JIA. Araujo et al. [25] observed that the avidity of antibodies against CII was significantly higher in JIA than in patients with ankylosing spondylitis or healthy controls. Additionally, the patients with active disease at the time of sample collection of antibodies and the polyarticular patients had anti-CII antibodies with high avidity more frequently. Insufficient blockage of immune complexes formation between anti-CII antibodies and collagen type II in joint cartilages and subsequent induction of complement activation with inflammation process may explain the association between high-avidity anti-CII antibodies and active disease [25].
AVIDITY OF AUTOANTIBODIES USED IN GASTROENTEROLOGY
Coeliac disease (CD) is systemic autoimmune disease induced by wheat gliadin (and similar prolamin proteins from related cereals) in genetically predisposed persons. Clinical manifestation of CD, maily in adults, can be different, not only gastrointestinal, typical for children. In addition to antibodies against alloantigens coeliac disease patients produce autoantibodies against transglutaminase-2 (TG2) [26, 27]. Serum TG2 antibodies are detectable in higher frequency in coeliac disease and they are used as its diagnostic marker. The tissue enzyme TG2 located in the gut wall enhances the immunostimulatory effect of gluten performing cross-linking of gliadin, and acts as a target autoantigen [27]. No evidence for avidity maturation of IgA antibodies against tissue TG2 during the development of coeliac disease was observed [28]. Clear differences in the IgA-TG2 titres between the group of children with progression to coeliac disease and the children presenting only with transient or fluctuating autoantibodies were found while no significant difference in the initial peak avidity indices between these two groups of children was observed. The avidity indices showed a significantly increasing tendency during the follow-up period without differences between both groups. High-avidity antibodies may be bound to TG2 in the gut whereas low-avidity antibodies may be released in the serum. The avidity of antibodies against transglutaminase 2 appears not to be related to antibody levels, disease stage, age or duration of exposure to gluten [29]. Similarly as ACPA, serum autoantibodies IgG and IgA directed against TG2 in CD patients also have a lower avidity in comparison with antibodies against alloantigens, in this case gliadin and E. coli [29].
Antibodies to two isoforms of carbonic anhydrase (CA) I, II – anti-CA I and anti-CA II have been considered to be involved in the pathogenesis of autoimmune chronic pancreatitis [30]. The anti-CA I and II antibodies were of low avidity but the avidity of anti-CA II was significantly higher in patients with chronic pancreatitis in comparison with other patient groups (systemic lupus erythematosus, RA, Sjögren syndrome or viral hepatitis).
AVIDITY OF AUTOANTIBODIES USED IN DIABETOLOGY
Type 1 diabetes mellitus (T1DM) is caused by a loss of the pancreatic β-cells producing insulin. The occurrence of autoantibodies against one or several pancreatic islet cell autoantigens is the characteristic feature of T1DM which corroborates the autoimmune basis of β-cell destruction [31, 32]. The usual target antigens of autoantibodies in diabetes are insulin, 65 kDa isoform of glutamic acid decarboxylase (GAD), insulinoma-associated protein 2 and zinc transporter-8 (ZnT8). The determination of islet cell autoantibodies is important for the diagnosis and prediction of diabetes. In addition to levels of these autoantibodies, their avidity have been tested with the aim to evaluate their clinical relevance especially for the assessment of the T1DM risk.
ANTI-INSULIN ANTIBODIES
Anti-insulin antibodies (IAA) used to be the first islet autoantibodies found in high titre in children who were at risk of DM development. The study of IAA characteristics showed their wide range of avidity associated with substantial differences in the interaction between IAA and insulin [33]. Bindings of high-avidity IAA required conservation of amino acid residues 8–13 of the human insulin A chain. These antibodies also reacted with proinsulin. In contrast to high-avidity IAA, low-avidity IAA bound to C-terminal residue of insulin B chain and did not interact with proinsulin.
IAA observed in T1DM patients are characterized by high avidity, but the presence of low-avidity IAA cannot be excluded [33]. On the other hand, naturally occurring polyreactive IAA in healthy subjects displayed a low to moderate avidity [34].
The measurement of IAA avidity can be helpful for the prediction of T1DM development in the preclinical phase [33]. Achenbach et al. [33] reported that IAA avidity in the first IAA-positive samples varied considerably among children of parents with T1DM, but their avidity did not significantly change during follow-up. High-avidity IAA were detected in children who synthesized IAA at a very young age and were associated with HLA DRB1*04 allele but not with IAA titre. In addition, high-avidity IAA were present in the children who subsequently produce multiple islet autoantibodies. A risk of progress into diabetes in children with high-avidity IAA was 50% within 6 years of follow-up, while no children synthesizing low-avidity IAA converted into diabetes. These observations implied that the measurement of IAA avidity in children who are only IAA-positive could be valuable in distinguishing who is more likely to develop multiple islet antibodies and to progress to T1DM [33]. The detection of high-avidity IAA might identify the increased risk of T1DM not only in first-degree relatives of patients with T1DM but also in IAA-positive school-children from the general population aged 6–17 years [35]. High-avidity IAAs correctly identified children who developed autoantibody positivity against multiple islet cell antigens or diabetes. A more recent study of Curnock et al. [36] who used a modified method for IAA avidity measurement supported previous observations. Unfortunately, IAA avidity differentiated poorly between rapid progressors to type 1 diabetes mellitus and subjects remaining unaffected or progressing slowly to clinical disease among young children (median of age = 1.5 years) from the general population carrying HLA-conferred disease susceptibility [37].
The workshop within the scope of the Diabetes Antibody Standardization Program demonstrated high concordance between laboratories in distinguishing high, moderate and low avidity IAA measured by competitive radiobinding assay in various own modifications [38]. Multiple islet autoantibody-positive and T1DM patient sera were identified by high-avidity IAA regardless of the laboratory where the avidity measurement was performed. The results of work suggested that combining avidity and titre determination significantly improved sensitivity, specificity and concordance of IAA measurement [38].
The avidity of IAA was not influenced by intranasal insulin administration in children. Ryhänen et. al [39] showed that the IAA avidity in children treated by nasal insulin was already relatively high at the beginning of the study. No further avidity maturation was observed during follow-up.
ANTI-GLUTAMATE DECARBOXYLASE ANTIBODIES
Most children who are IAA-positive also synthesize autoantibodies against intracellular enzyme glutamate decarboxylase, which catalyzes the synthesis of γ-aminobutyric acid (GABA) [40]. The target of autoantibodies against glutamate decarboxylase (GADA) predominantly comprises a conformational epitope of cytoplasmic 65 kDa isoform of glutamate acid decarboxylase (GAD65) which appears to maintain short-term requirements for increase GABA. Besides the 65 kDa isoform the larger 67 kDa isoform exists which is responsible for the constitutive production of GABA.
The investigation of GADA found a characteristic epitope specificity including major reactivity against the C-terminal (residues 445–585) and/or middle part (residues 235–244) of the GAD65 protein, which bound with high-avidity GADA [41].
Although the GADA is a diagnostic marker of T1DM, the levels of antibodies against GADA alone are not sufficient to predict progression to diabetes [42]. The examination of GADA avidity might extend the possibility of their clinical usefulness. The study in the group of children with a T1DM family history demonstrated that avidity of GADA varied between children and showed positive correlation with GADA titre [43]. The GADA avidity was higher in children who developed diabetes and in those with HLA DRB1*03 alleles. The higher GADA avidity was also associated with children who had multiple islet autoantibodies [43]. The follow-up study did not show frequent changes in GADA avidity.
The GADA response may be stratified according to GADA avidity, titre and epitope specificity and presence of other islet antibodies into distinct GADA profiles [43]. High--avidity GADA binding to the common middle and/or C-terminal of GAD65 epitopes were found in children positive with other islet antibodies. The children with similar profiles but negative to other islet antibodies frequently progress to synthesis multiple islet autoantibodies or diabetes. This finding suggests that determination of GADA profiles may improve risk assessment of type 1 diabetes especially in single GADA-positive children.
The benefit of GADA avidity determination is not limited to the subject with familial predisposition [41]. Similarly as in the children with a type 1 diabetes family history, the avidity of GADA among the children of a general school population fluctuated over almost five orders of magnitude and correlated with GADA levels and also remained relatively constant on follow-up. High-avidity GADA can identify those GADA positive children from the general population who are more likely to progress to type 1 diabetes. Likewise, an assessment of GADA avidity, their epitope specificity and levels together with occurrence of other islet antibodies allowed to define several distinct GADA profiles that can be used for further risk stratification of T1DM.
However not all studies are in good agreement with these promising results. Though Westerlund et al. [44] confirmed only a slight fluctuation in the avidity of GADA observed over time, the avidity index patterns of pre-diabetic children did not differ from those of controls throughout the follow-up. Similarly, GAD65--GAD65Ab interactions in predominantly adult T1DM patients did not vary from non-DM patients and were characterized by a relatively high affinity constant. No differences in GAD65Ab binding avidity at distinct stages of diabetes in the same group of T1DM patients were observed [45].
GADA avidity was also tested in patients with latent autoimmune diabetes in adults (LADA) [46]. In concordance with T1DM patients, GADA avidity also ranged widely in GADA-positive LADA patients. GADA avidity correlated with β-cell function and a subsequent need of insulin treatment in patients. It seems that high-avidity GADA might be a marker for impaired β-cell function and that the determination of GADA avidity might be valuable in the identification of single GADA-positive patients who are at the highest risk for requiring insulin therapy.
High titres of GADA are characteristic for patients with stiff person syndrome (SPS) which may be occasionally associated with T1DM [40]. It is supposed that antibodies against GADA, which recognize both conformational and linear epitopes in SPS, could inhibit an activity of glutamic acid decarboxylase and suppress GABA synthesis in brain [40, 47, 48]. The changes in synthesis and activity of GADA, may have an impact on the impaired function of CNS. Skorstad et al. [47] showed that intrathecally and systemically produced antibodies against GAD65 IgG in SPS are oligoclonal and are characterized by high avidity corresponding to that of high-avidity antibodies, which have undergone avidity maturation. The avidity was higher in CSF than serum in some patients.
ANTIBODIES AGAINST INSULINOMA-ASSOCIATED PROTEIN 2
Antibodies against insulinoma-associated protein 2 (tyrosine phosphatase-related islet antigen 2) (IA-2A), usually typically occurring with other islet antibodies, include another type of autoantibody associated with a high risk for T1DM. The anti-IA-2A response in children with a T1DM family history was characterized by high avidity at first appearance and by a strong association with diabetes development [49]. IA-2A avidity remained relatively constant during follow-up [44, 49]. According to Westerlund et al. [44] precipitation of T1DM is not preceded by avidity maturation of autoantibodies against IA-2A. In contrast to GADA and IAA, the IA-2A avidity could not stratify progression to T1DM risk in multiple autoantibody positive children [49]. In addition, IA-2A avidity was not associated with epitope specificity or HLA class II haplotype and titre [49].
In summary, the avidity determination of autoantibodies used in diabetology seems to have certain clinical potential. Despite some contradiction, higher avidities might be predictive for the development of diabetes not only among children with positive family history but perhaps among the general population. This promising observation must be confirmed by further studies.
AVIDITY OF KIDNEY AUTOANTIBODIES USED IN NEPHROLOGY
Several autoantibodies are assayed within immunological examination in patients with immune-mediated diseases of glomeruli. As the avidity of antineutrophil cytoplasmic antibodies and anti-dsDNA were mentioned in previous review [14], only anti-glomerular basement membrane (GBM) antibodies will be discussed.
ANTI-GLOMERULAR BASEMENT MEMBRANE ANTIBODIES
The anti-GBM antibodies directed mainly against the non-collagenous domain of the α3 chain of type IV collagen are present in linear deposition along GBM. Experimental studies performed by passive transfer of IgG from patients with Goodpasture syndrome to monkeys demonstrated the participation of these antibodies in the pathogenicity of anti-GBM antibody-mediated diseases [50].
Antibodies against the glomerular basement membrane exist in sera in patients with anti-GBM disease as well as in healthy persons [51]. However, the characteristics of natural anti-glomerular basement membrane antibodies described in healthy subjects differed from patients with anti-GBM diseases in titres and avidity. The low titres and low avidity of these autoantibodies were in normal human sera while the anti-GBM antibodies from patients with anti-GBM diseases and transplanted Alport syndrome were characterized by higher avidity [51-53]. The avidities of anti-GBM antibodies were comparable with those synthesizing against foreign antigens upon secondary antigen exposure [52]. Cui and Zhao [11] observed a great difference of anti-GBM antibody avidity in patients with anti-GBM antibody-mediated diseases. An association between anti-GBM antibody avidity and the percentage of crescent formation in all glomeruli was found. Avidity of anti-GBM antibodies independently predicted the percentage of glomerular crescents while no correlation was observed between avidity and age, antibody titre or concentration of serum creatinine on diagnosis. The authors supposed that different avidity of the anti-GBM antibodies participates in the distinct clinical presentation of disease. High-avidity anti-GBM antibodies may contribute to both rapid onset of disease and severe renal injury by their accumulation and induction of inflammatory process in the glomeruli.
It seems that the avidity maturation of the anti-GBM antibodies is necessary for damage of glomeruli and it is finished by the time that patients presented with anti-GBM disease manifestation. Serial determination of anti-GBM antibodies did not show any changes in their avidity in patients with anti-GBM disease [11, 54].
ONCONEURONAL ANTIBODIES
An extraordinary group of antibodies associated with nervous tissue are onconeuronal antibodies. Cancer antigens can induce immunity response elsewhere in the body including neuronal tissue [55]. This cellular and humoral immunity reaction may cause degeneration of neurons and glia cells.
Various onconeural antibodies such as anti-Hu, anti-Yo, anti-amphiphysin, anti-Ma2, anti-Ri, or anti-Tr are well-characterised [55]. The avidity of common onconeuronal antibodies anti-Hu and anti-Yo was studied in paraneoplastic neurological syndromes [56]. It varied greatly, probably because of various factors such as the immune status of the patients at the time of serum collection or the time of serum sampling in relation to cancer and the heterogeneity of the cancer. The anti-Yo antibodies in general had higher avidity than anti-Hu antibodies. The follow-up of time-dependent changes in anti-Hu and anti-Yo antibody avidity showed that they may fluctuate or remain stable during disease. More patients with anti-Hu antibodies had a time-dependent increase in avidity compared with patients with anti-Yo [56]. However, no association between the time course of the anti-Hu or anti-Yo avidity and the treatment of the patients was found. Initial study did not describe any association between antibody avidity and the underlying type of cancer [56]. The later study reported that high-avidity anti-Yo antibodies were mainly associated with ovarian cancer while high-avidity anti-Hu and anti-CRMP5 were found more frequently in patients with small-cell lung cancer [57].
AVIDITY OF AUTOANTIBODIES USED IN PSYCHIATRY
The presence of extracellular cerebral amyloid plaques formed by aggregated amyloid-β peptide (Aβ) is one of the hallmark lesions in Alzheimer disease (AD) [58]. Experiments carried on the murine model of AD demonstrated the significance of antibodies against Aβ in reducing of cerebral Aβ deposits [59]. It was reported that naturally occurring anti-Aβ antibodies may help to maintain amyloid-β peptide homeostasis by catalysis of Aβ hydrolysis [60]. Sera from healthy humans contain natural anti-Aβ antibodies of IgG or IgM isotypes [61]. High-avidity anti-Aβ antibodies represent less than 0.1 % of the total mass of single-donor plasma-derived human IgG [62]. According to Szabo [61] free high-avidity anti-Aβ antibodies in circulation are often masked by ligands including Aβ and anti-idiotypic antibodies. Therefore, the amount and avidity of anti-Aβ antibodies can increase after dissociation from their ligands [61].
Both the levels and the avidity of naturally occurring anti-Aβ antibodies were significantly lower in patients with Alzheimer disease (AD) in comparison with healthy controls without significant correlation between levels and avidity [10]. The presence of low levels and low-avidity anti-Aβ antibodies was also observed in patients with cerebral amyloid angiopathy (CAA)-related cerebral haemorrhage [63]. CAA is a disease that can occur in association with AD and certain familial syndromes or can be self-existent. It is characterized by deposits of Aβ peptide in small - to medium-sized cerebral vessels that can damage their wall and provoke intracerebral haemorrhage. The vessel accumulation of Aβ peptide may be the consequence of their insufficient clearance. The authors hypothesized that the low levels of low-avidity anti-Aβ antibodies may be a potential risk for the impaired mechanism of Aβ deposit removal by anti-Aβ antibodies.
CONCLUSION
The avidity of various autoantibodies differs under distinct clinical conditions. High-, low-avidity or moderate avidity of autoantibodies may be found in autoimmune diseases (Table 1).
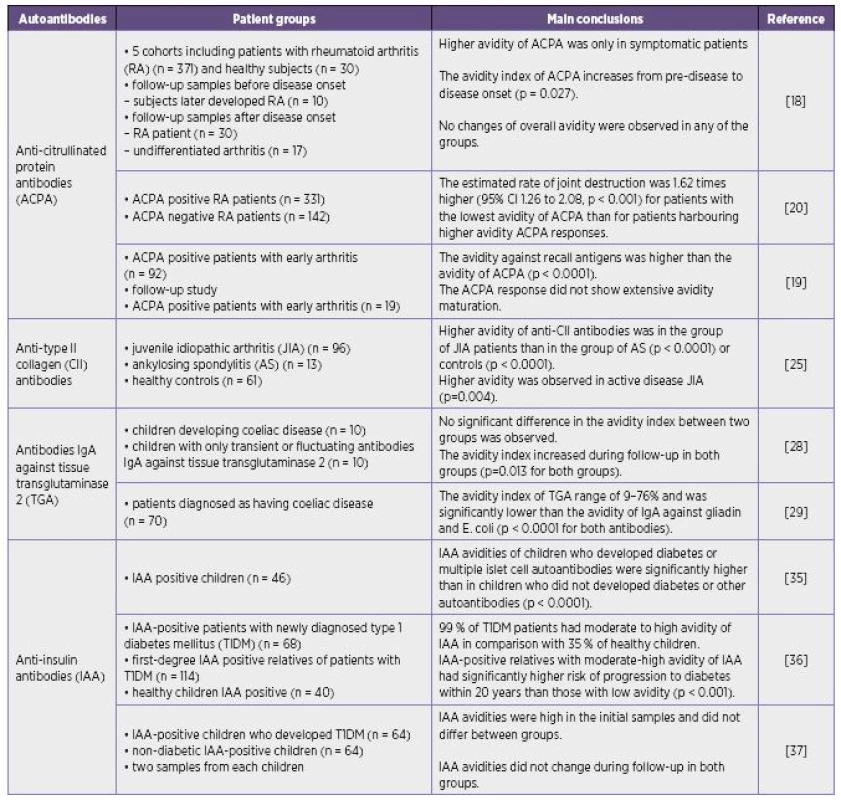
The changes in avidity of some autoantibodies in appropriate clinical conditions were similar those observed during a secondary immune response in infectious diseases although their avidity does not always reach values common in anti-infectious antibodies. High-avidity autoantibodies are supposed to have deleterious effects and may participate in the pathogenesis of certain disorders and correspond with the severity of the disease. On the other hand the findings of low-avidity autoantibodies in serum may be also associated with the more severe manifestation of disease. It is supposed that high-avidity autoantibodies may form immune complexes with their antigens in tissues so that high-avidity antibodies need not be detected in circulation and only free low-avidity antibodies are present in the serum.
Different immunological methods used for avidity determination can contribute to some discrepancies in clinical studies. So far no standard methods for the avidity determination of individual autoantibodies are at disposal.
It is obvious that interpretation of autoantibody avidity values is complicated with regards to various circumstances which influence avidity maturation, formation of immune complexes and suitable laboratory methods. The avidity of each autoantibody must be judged individually since no uniform pattern of avidity development was revealed. However, some studies promise the possible benefit of avidity examination for the refinement of diagnosis and prediction of some autoimmune diseases. A simple and reliable immunochemical assay acceptable for routine praxis will be required if the effectiveness of avidity determination will be confirmed.
Acknowledgments:
Supported by the project of Ministry of Health, Czech Republic for conceptual development of research organization RVO-VFN64165 (General University Hospital in Prague, Czech Republic) and by research project of Charles University in Prague PRVOUK P25/LF1/2.
Conflict of interest statement:
The author states that there are no conflicts of interest regarding the publication of this article.
Do redakce došlo dne 18. 1. 2016.
Adresa pro korespondenci:
MUDr. Lenka Fialová, CSc.
Ústav lékařské biochemie a laboratorní diagnostiky,
1. LF, UK a VFN
Kateřinská 32
121 08 Praha 2
e-mail: lfial@lf1.cuni.cz
Sources
1. Damoiseaux J, Andrade LE, Fritzler MJ, et al. Autoantibodies 2015: From diagnostic biomarkers toward prediction, prognosis and prevention. Autoimmun Rev, 2015;14 : 555–563.
2. Gharavi A, Reiber H. Affinity and avidity of autoantibodies. In: Peter J, Shoenfeld Y, editors. Autoantibodies. Amsterdam: Elsevier; 1996. p. 13–23.
3. Eisen HN. Determination of antibody affinity for haptens and antigens by means of fluorescence quenching. Methods Med Res, 1964;10 : 115–121.
4. Prince HE, Wilson M. Simplified assay for measuring Toxoplasma gondii immunoglobulin G avidity. Clin Diagn Lab Immunol, 2001;8 : 904–908.
5. Wilson KM, Di Camillo C, Doughty L et al. Humoral immune response to primary rubella virus infection. Clin Vaccine Immunol, 2006;13 : 380–386.
6. Štěpánová V, Plíšková L, Kubišová M, Štěpánová E, Bolehovská R, Boštíková V, Svobodová M. [The use of IgG antibody avidity assays in the diagnosis of cytomegalovirus infection]. [Article in Czech]. Epidemiol Mikrobiol Imunol, 2011;60 : 115–120.
7. Woznicová V. [Immunoglobulin G avidity in infectious diseases]. [Article in Czech]. Epidemiol Mikrobiol Imunol, 2004;53 : 4–11.
8. Shepherd SJ, McAllister G, Kean J et al. Development of an avidity assay for detection of recent HIV infections. J Virol Methods, 2015;217 : 42–49.
9. Strharsky J, Madarova L, Klement C. [Laboratory diagnosis of toxoplasmosis]. [Article in Czech]. Epidemiol Mikrobiol Imunol, 2009;58 : 51–62.
10. Jianping L, Zhibing Y, Wei Q et al. Low avidity and level of serum anti-Abeta antibodies in Alzheimer disease. Alzheimer Dis Assoc Disord, 2006;20 : 127–132.
11. Cui Z, Zhao MH. Avidity of anti-glomerular basement membrane autoantibodies was associated with disease severity. Clin Immunol, 2005;116 : 77–82.
12. Božič B, Čučnik S, Kveder T, et al. Affinity and avidity of autoantibodies. In: Shoenfeld Y, Gershwin ME, Meroni PL, editors. Autoantibodies. Amsterdam: Elsevier, 2007. p. 21–28.
13. Fialova L. Avidity of antiphospholipid antibodies – our current knowledge. Epidemiol Mikrobiol Imunol, 2014;63 : 221–225.
14. Fialova L. Avidity of autoantibodies against antigens in nervous tissue In: Costa A, Villalba E, editor. Horizons in Neuroscience Research. New York: Nova Science Publishers, 2015. p. 159–169.
15. Bozic B, Cucnik S, Kveder T, et al. Avidity of anti-beta-2-glycoprotein I antibodies. Autoimmun Rev, 2005;4 : 303–308.
16. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum, 2010;62 : 2569–2581.
17. Farid S, Azizi G, Mirshafiey A. Anti-citrullinated protein antibodies and their clinical utility in rheumatoid arthritis. Int J Rheum Dis, 2013;16 : 379–386.
18. Suwannalai P, van de Stadt LA, Radner H et al. Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum, 2012;64 : 1323–1328.
19. Suwannalai P, Scherer HU, van der Woude D, et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann Rheum Dis, 2011;70 : 373-379.
20. Suwannalai P, Britsemmer K, Knevel R, et al. Low-avidity anticitrullinated protein antibodies (ACPA) are associated with a higher rate of joint destruction in rheumatoid arthritis. Ann Rheum Dis, 2014;73 : 270–276.
21. Rossi G, Real-Fernandez F, Panza F, et al. Biosensor analysis of anti-citrullinated protein/peptide antibody affinity. Anal Biochem, 2014;465 : 96–101.
22. Rombouts Y, Willemze A, van Beers JJ, et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis, 2016; 75 : 578–585.
23. Berntson L, Nordal E, Fasth A, et al. Anti-type II collagen antibodies, anti-CCP, IgA RF and IgM RF are associated with joint damage, assessed eight years after onset of juvenile idiopathic arthritis (JIA). Pediatr Rheumatol Online J, 2014;12 : 22.
24. Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol, 2008;18 : 429–441.
25. Araujo GR, Fonseca JE, Fujimura PT, et al. Anti-type II collagen antibodies detection and avidity in patients with oligoarticular and polyarticular forms of juvenile idiopathic arthritis. Immunol Lett, 2015;165 : 20–25.
26. Makovicky PE, Makovicky PA, Jilek F. [From historical data and opinions to present challenges in the field of celiac disease]. [Article in Czech]. Epidemiol Mikrobiol Imunol, 2008;57 : 90–96.
27. Di Sabatino A, Vanoli A, Giuffrida P, et al. The function of tissue transglutaminase in celiac disease. Autoimmun Rev, 2012;11 : 746–753.
28. Westerlund A, Ankelo M, Simell S et al. Affinity maturation of immunoglobulin A anti-tissue transglutaminase autoantibodies during development of coeliac disease. Clin Exp Immunol, 2007;148 : 230–240.
29. Gelderman KA, Drop AC, Trouw LA, et al. Serum autoantibodies directed against transglutaminase-2 have a low avidity compared with alloantibodies against gliadin in coeliac disease. Clin Exp Immunol, 2014;177 : 86–93.
30. Bartolome MJ, de las Heras G, Lopez-Hoyos M. Low-avidity antibodies to carbonic anhydrase-I and -II in autoimmune chronic pancreatitis. Scientific World Journal, 2002;2 : 1560–1568.
31. Pihoker C, Gilliam LK, Hampe CS, et al. Autoantibodies in diabetes. Diabetes, 2005;54 Suppl 2:S52–61.
32. Kantarova D, Pridavkova D, Sagova I et al. [Genetic and molecular background in autoimmune diabetes mellitus]. [Article in Czech]. Epidemiol Mikrobiol Imunol, 2015;64 : 121–129.
33. Achenbach P, Koczwara K, Knopff A et al. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest, 2004;114 : 589–597.
34. Casali P, Nakamura M, Ginsberg-Fellner F, et al. Frequency of B cells committed to the production of antibodies to insulin in newly diagnosed patients with insulin-dependent diabetes mellitus and generation of high affinity human monoclonal IgG to insulin. J Immunol, 1990;144 : 3741–3747.
35. Schlosser M, Koczwara K, Kenk H et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia, 2005;48 : 1830–1832.
36. Curnock RM, Reed CR, Rokni S, et al. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol, 2012;167 : 67–72.
37. Siljander H, Harkonen T, Hermann R et al. Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes Metab Res Rev, 2009;25 : 615–622.
38. Achenbach P, Schlosser M, Williams AJ et al. Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes antibody standardization program. Clin Immunol, 2007;122 : 85–90.
39. Ryhanen SJ, Harkonen T, Siljander H et al. Impact of intranasal insulin on insulin antibody affinity and isotypes in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetes Care, 2011;34 : 1383–1388.
40. Raju R, Hampe CS Immunobiology of stiff-person syndrome. Int Rev Immunol, 2008;27 : 79–92.
41. Bender C, Schlosser M, Christen U et al. GAD autoantibody affinity in schoolchildren from the general population. Diabetologia, 2014;57 : 1911–1918.
42. Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes, 2004;53 : 384–392.
43. Mayr A, Schlosser M, Grober N, et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes, 2007;56 : 1527–1533.
44. Westerlund A, Ankelo M, Ilonen J, et al. Absence of avidity maturation of autoantibodies to the protein tyrosine phosphatase-like IA-2 molecule and glutamic acid decarboxylase (GAD65) during progression to type 1 diabetes. J Autoimmun, 2005;24 : 153–167.
45. Coco G, Chen S, Powell M, et al. Analysis of the GAD65-GAD65 autoantibody interaction. Clin Chim Acta, 2008;391 : 51–59.
46. Krause S, Landherr U, Agardh CD, et al. GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care, 2014;37 : 1675–1680.
47. Skorstad G, Hestvik AL, Torjesen P, et al. GAD65 IgG autoantibodies in stiff person syndrome: Clonality, avidity and persistence. Eur J Neurol, 2008;15 : 973–980.
48. Bjork E, Velloso LA, Kampe O, et al. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes, 1994;43 : 161–165.
49. Krause S, Chmiel R, Bonifacio E, et al. IA-2 autoantibody affinity in children at risk for type 1 diabetes. Clin Immunol, 2012;145 : 224–229.
50. Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med, 1967;126 : 989–1004.
51. Cui Z, Wang HY, Zhao MH. Natural autoantibodies against glomerular basement membrane exist in normal human sera. Kidney Int, 2006;69 : 894–899.
52. Rutgers A, Meyers KE, Canziani G, et al. High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen. Kidney Int, 2000;58 : 115–122.
53. Dougan T, Levy JB, Salama A et al. Characterization of autoantibodies from patients with Goodpasture’s disease using a resonant mirror biosensor. Clin Exp Immunol, 2002;128 : 555–561.
54. Marriott JB, Oliveira DB. Serial functional affinity of autoantibodies in anti-glomerular basement membrane disease. Clin Exp Immunol, 1994;95 : 498–501.
55. Roberts WK, Darnell RB. Neuroimmunology of the paraneoplastic neurological degenerations. Current Opinion in Immunology, 2004;16 : 616–622.
56. Totland C, Aarseth J, Vedeler C. Hu and Yo antibodies have heterogeneous avidity. J Neuroimmunol, 2007;185 : 162–167.
57. Totland C, Ying M, Haugen M, et al. Avidity of onconeural antibodies is of clinical relevance. Cancer Immunol Immunother, 2013;62 : 1393–1396.
58. Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci, 2000;924 : 17–25.
59. Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med, 2000;6 : 916–919.
60. Taguchi H, Planque S, Nishiyama Y, et al. Catalytic antibodies to amyloid beta peptide in defense against Alzheimer disease. Autoimmun Rev, 2008;7 : 391–397.
61. Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev, 2008;7 : 415–420.
62. Szabo P, Mujalli DM, Rotondi ML, et al. Measurement of anti-beta amyloid antibodies in human blood. J Neuroimmunol, 2010;227 : 167–174.
63. Cao Z, Lv J, Quan W. Low avidity and level of serum anti-Abeta antibodies in patients with cerebral amyloid angiopathy-related cerebral hemorrhage. Int J Neurosci, 2010;120 : 760–764.
Labels
Hygiene and epidemiology Medical virology Clinical microbiologyArticle was published in
Epidemiology, Microbiology, Immunology

2016 Issue 3
-
All articles in this issue
- The incidence of viral hepatitis A in the Hradec Králové Region in the Czech Republic in the last decade
- Effect of lipophosphonoxins on inhibition of bacterial colonization of bone cements
- Stenotrophomonas maltophilia as the cause of ventilator-associated pneumonia in a female patient with toxic epidermal necrolysis and Clostridium colitis: time for off-label tigecycline?
- HIV/AIDS epidemics in sub-Saharan regions in the 2010s: Regional analysis of UNAIDS data
- Avidity of selected autoantibodies – usefulness of their determination for clinical purposes
-
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex - Campylobacteriosis in the South Bohemian Region – a Recurrent Problem
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Stenotrophomonas maltophilia as the cause of ventilator-associated pneumonia in a female patient with toxic epidermal necrolysis and Clostridium colitis: time for off-label tigecycline?
- Avidity of selected autoantibodies – usefulness of their determination for clinical purposes
-
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex - HIV/AIDS epidemics in sub-Saharan regions in the 2010s: Regional analysis of UNAIDS data

