-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
ENZYMATIC NECROLYSIS OF ACUTE DEEP BURNS – REPORT OF PRELIMINARY RESULTS WITH 22 PATIENTS
Autoři: J. Koller; P. Bukovčan; M. Orság; R. Kvalténi; I. Gräffinger
Působiště autorů: Slovak Republic ; Department of Burns and Reconstructive Surgery, University Hospital Bratislava, Ružinov Hospital, Bratislava
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 50, 4, 2008, pp. 109-114
INTRODUCTION
Early excision has become the method of choice for treatment of acute deep burns over the last few decades (1, 2). One of the major drawbacks of the surgical removal of necrotic tissues is that much healthy, viable tissue is removed together with the necrotic areas. Therefore a more selective method for necrotic tissue removal, which would preserve and not harm the viable tissues, has been sought. One of the most promising options seems to be enzymatic debridement. In 1943 Cooper (3) published an article on enzymatic debridement of burns by a mixture of papain, cystein hydrochloride and sodium salicylate. Various proteolytic enzymes of bacterial origin, such as clostridial collagenase, streptokinase and streptodornase, were used for enzymatic debridement in the 1950s (4, 5, 6) with controversial efficacy. Proteases derived from B. subtilis were used in the 1970s and were marketed as Travase®, manufactured by Flint Laboratories (7, 8). The results of all the above methods have been highly variable. The enzymes were effective only in a moist environment, and the debridement took quite a considerable time – which varied from days to weeks. Hence, under these conditions, the spread of infection was facilitated, and infection was found to be the most frequent complication of such debridements. Enzymatic debridement with these agents was slow, not effective enough, could not be used for large surfaces, and was accompanied by high frequency of adverse events and complications (9).
First reports on the use of a pineapple stem-derived proteolytic enzyme mixture (bromelain) for burn debridement were published by Klein (10, 11). He used commercially available lyophilized enzyme with good but inconsistent results. With the development of more effective extraction methods (12), the pineapple stem enzyme mixture known as Debridase was used by Rosenberg (13) for burn debridement in a group of 130 patients during the years from 1984 to 1999.
MATERIAL AND METHODS
The Bratislava Burn Department participated in two multicenter prospective randomized clinical studies with a new enzymatic debriding agent under a name of Debrase Gel DressingTM (DGD) assigned for early debridement of acute deep burns. The clinical trials were approved by the Ethical Committee of the Ružinov Hospital. The first study, which was performed during the years 2002 and 2003 and which was focused on the efficacy and safety of the drug, included 15 patients. The second study, which started in 2006 and which is still ongoing, has so far included 7 patients. Altogether 22 patients have been included in the two studies.
Inclusion criteria for both the studies included acute deep partial thickness and full thickness burns from 5% to 30% of the total body surface area, which were indicated for treatment by early excision. The age of eligible patients in the first study was between 18 and 60 years; in the second study it was between 4 and 55 years. Patients with burns older than 48 hours, with serious concomitant diseases, and those with severe allergies were excluded, as were alcoholics and drug users. Patients with severe inhalation injuries and women in fertile age with positive pregnancy tests were also excluded. In the first study only one target wound (TW) could be treated, and burns of the face and hands could not be included among target wounds.
DebraseTM (MediWound Ltd) is a purified substance derived from specially-made bromelain raw material. The enzyme is supplied as a sterile purified freeze-dried substance. DGD contains very active proteolytic enzymes isolated and purified from pineapple stems and fruits. The enzymes are capable of dissolving and removing necrotic tissue within a couple of hours and at the same time they do not damage the potentially viable tissue. DGD consists of Debrase powder, which is mixed and rehydrated just before application by a hydrating agueous gel. Two or five grams of Debrase powder are mixed with 20 or 50 grams of hydrating gel directly before application and used to treat 100 cm2 or 250 cm2 of burn eschar respectively.
Patient randomization
Informed written consent was obtained from each eligible patient before randomization. Online electronic management of patient case record files (eCRFs) was used. This also applies to randomization of the patients for the study, which was provided online centrally. In the first study there were three arms – DGD application, standard of care (SOC), and gel only application. In the second study there were two arms only – DGD application and SOC groups. Baseline vital functions and blood samples for biochemistry and hematology investigations were obtained before treatment in either of the groups.
Wound care before application
In SOC groups standard wound care of the department was employed, followed by either surgical interventions (tangential excision followed by autograft wound closure), or use of biological skin substitutes for coverage and/or promotion of autolytic debridement of deep partial thickness burns. All the full thickness areas were treated by early excision and autograft closure. Late autografting was done in areas which did not heal within 3 weeks.
In the DGD and/or gel only group (first study only) the wounds assigned for treatment were cleansed several times by mild antiseptic solution (Betadine soap) which was followed by complete removal of all the remnants of devitalized epidermis. Thereafter wet sterile saline or antibacterial solution soaks were applied to the wound for 2 hours (soaking period).
Application method
DGD was applied at the bedside, or in a special room for dressing changes for burn patients. Following removal of the dressing used for initial soaking, the wound edges were covered by a protective layer of sterile vaseline and the wound surface covered by a layer of DGD 1.5 to 3 mm thick, spread evenly with a sterile wooden spatula. The treated area was then covered by a sterile occlusive film spread over the wound and fixed with a loose sterile fluffy gauze outer dressing, held in place by elastic bandages. This dressing was left in place for 4 hours. The treated area needed to be kept warm (35–37°C).
Removal of DGD from the wound
The dressing was aseptically removed followed by removal of the protective vaseline barrier with the use of a sterile spatula. In thicker eschar another spatula was used to remove the dissolved parts of the eschar from the wound by scraping. The wound was rinsed several times with sterile saline followed by scraping of the wound with sterile gauze. The efficacy of debridement was evaluated and new soaking dressing with antibacterial solution was applied for 2 hours.
Removal of post-debridement soaking dressing and assessment of debridement efficacy
Following removal of the post-treatment soaking dressing, the efficacy of debridement was estimated as a percentage of the necrosis removed. Debridement efficacy more than 90% was judged as excellent, efficacy between 65% and 90% as good, and less than 65% as poor. If debridement was less than 65% the debridement could be repeated once more with a new application of DGD followed by another 2 hours of soaking.
Post-debridement wound management
Wound management post debridement offered quite a wide range of options, depending on the decision of the surgeon. The options included topical antibacterial treatment by silversulfadiazine cream (SSD), wound coverage by allografts, xenografts, cultured allogenic keratinocytes. Selective partial autografting of the very deep dermal and full thickness areas was possible as well, leaving the more superficial areas to heal spontaneously. We used all the above methods except SSD. Continuous wound monitoring with adequate therapeutic response to changes in the healing process was provided.
Evaluation
Enzymatic debridement was compared with the standard of care methods used at the facilities participating at the multicenter clinical trial. Primary endpoint of the study included the percentage of the original wound which required surgical excision following enzymatic debridement. Secondary endpoint was the time to complete wound closure and the extent of the original wound which required autologous skin grafting. Other investigations included measurement of basic vital signs, pain assessment by a 10-grade visual analogue scale, measurements of blood loss (number of blood transfusions required), basic hematological and biochemical parameters, occurrence of local and systemic adverse reactions and take of eventually applied skin autografts.
RESULTS
Altogether 22 patients were selected for the study. One patient was eliminated from the study following selection because he fell out of the time frame, and one patient was excluded during the study due to development of delirium tremens of alcoholic origin.
The age range was between 19 and 69 years (with exceptional approval for the oldest patient). The DGD group included 10 patients, the SOC group 7 patients, and the gel only group 3 patients (Table1).
Tab. 1. Patients included in the study 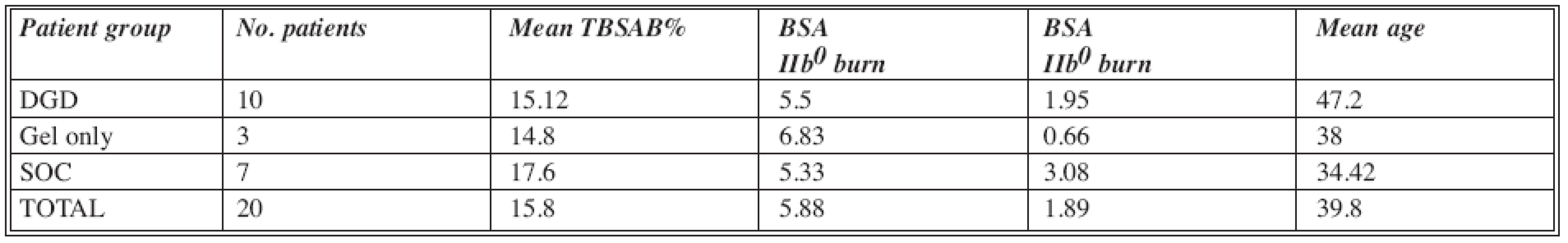
Abbreviations: DGD= Debrase Gel Dressing; SOC= Standard of Care; TBSAB= Total Body Surface Area Burn; BSA= Body Surface Are Gel only group of patients
In all the three patients who received just gel instead of DGD no debridement was achieved. All these patients were, according to the study protocol, treated exactly the same way as the SOC patients.
DGD group of patients
In the DGD group of patients (Table 2) the debridement efficacy varied between 50% and 100%. In 8 out of 10 patients the efficacy was more than 90% (excellent). In one patient (No. 1) it was 80%, and in this case the only repeated debridement was provided, with an efficacy of 30%. In this patient an additional surgical debridement of 15% of the target wound was required as well, and almost the entire target wound (TW) needed closure by autograft. The lowest efficacy – 50% (TW1) and 70% (TW2) was observed in one patient (No. 10) where it was assumed that the burns were more superficial than they appeared clinically. This fact was also confirmed by the observation that the wounds healed within 19 (TW1) and 32 (TW2) days respectively, without the need for surgical intervention.
Tab. 2. DGD group of patients 
Abbreviations: TW= Target Wound; XG1= non-meshed xenografts; XG2= meshed xenografts; ALL= meshed allografts; AG= split thickness meshed autografts; KCA= cultured allogenic keratinocytes Scar score: 0= none; 1= mild; 2= moderate; 3= severe BSA= Body Surface Area In most of the patients the pain score during debridement was mild (range 1 to 3); in 3 patients it was moderate (range 4 to 5) and could easily be handled by the administration of analgetic medications. The scar score was assessed after complete healing at monthly follow-up visits. The scores in the table were estimated at the 3rd monthly visit. In most of the cases scarring was mild, while in only 2 cases it was moderate. The most common adverse reaction (AR) was temperature elevation – in almost all the patients. The second most frequent AR was deepening of parts of the treated wound from deep dermal to full thickness injuries, which required finally moderate amounts of skin grafting. No severe infectious or other complications were observed. The progress of wound healing was comparable to the standard of care treatment patients.
SOC group of patients
The standard of care patients were treated according to the protocols used in our burn department (Table 3). Initial wound care consisted of surgical wound cleansing and debridement of loose devitalized remnants of epidermis. Wound areas with dermal burns which were not excessively deep were temporarily covered by non-meshed fresh frozen xenografts, which were kept in place until most of the wounds were healed. In areas where deepening of the wounds was observed and necrotic skin was present, the patients were subjected to surgical, mostly tangential excisions and wound closure by autologous skin grafts. All the subdermal (third degree) burns were excised surgically and grafted by skin grafts. The wound care and follow up was identical to the DGD group.
Tab. 3. SOC group of patients 
Abbreviations: XG1= non-meshed xenografts; ALL= meshed allografts; AG= split thickness meshed autografts; KCA= cultured allogenic keratinocytes Scar score: 0= none; 1= mild; 2= moderate; 3= severe BSA= Body Surface Area DISCUSSION
Various enzymes have been used in order to remove necrotic tissues from the wounds of different origin. The enzymes were derived from plants such as papain and bromelain; from bacteria-like sutilains, streptokinase-streptodornase and various other bacterial collagenases. However, this type of debridement had many limitations. The efficacy of all the above-mentioned enzymes was usually not sufficient for early removal of all the necrotic tissues, which resulted in necessity of prolonged exposures and repeated applications. The wound surface assigned for debridement was also limited due to the possible toxicity of the products. Moreover, the use of enzymes was not recommended in the early phases of wound treatment. Prolonged and repeated exposures led to increased risk of severe wound infections, as has been observed during the treatment. All these factors led to considerable prolongation of healing of the wounds and more scarring (9, 14, 15, 16, 17).
Surgical treatment of deep burns by early excision became more popular in the last few decades and was used preferentially in most of the burn centers. The advantages included considerable shortening of morbidity, reduction of mortality and reduction of infection and hypermetabolic response. The overall healing time was reduced significantly (1, 2). The drawbacks of surgical excision are that it is an invasive procedure accompanied by significant perioperative blood loss and postoperative pain. Selectivity of the procedure is low, since surgical excision entails the removal of a considerable amount of healthy viable tissue along with the necrotic tissues. Compared to surgical excision, enzymatic debridement was more selective but much more time consuming in the past.
According to Rosenberg (13), the ideal debridement agent or method should have the following attributes:
- Safety; i.e. without any systemic adverse effects and minimal, if any, bleeding.
- Selectivity; resulting in removal of the necrotic eschar without affecting the surrounding viable tissue, thus permitting accurate diagnosis of the extent of the original damage.
- Effective; removing the entire eschar, preferably in a single application.
- Rapid; resulting in rapid reduction of the infection risk and permitting sequential debridement of large areas over a short time span.
- Simple to use and cost-effective.
Rosenberg’s study (13) of 130 burn patients with 332 deep dermal and full thickness burns proved good safety, selectivity, efficacy and rapidity of the debridement. In our study on a more limited number of patients the similar favorable effects of DGD debridement have been observed. In most of the DGD group patients the entire burn eschar was digested within 4 hours following application. Selectivity of the debridement was proved, particularly in deep partial thickness burns, where enzymatic debridement could be used also as a diagnostic procedure to differentiate between partial and full thickness burns (Fig. 1–6). This would prevent unnecessary surgical procedures, which could also remove much of the potentially viable tissues and create full thickness injuries instead of partial thickness wounds with a good potential for spontaneous healing enhanced by biological dressings. In our patients all the three types of post-debridement wound coverage (i.e. meshed skin allografts, xenografts, or cultured allogeneic keratinocytes) together with meticulous wound care provided good healing for the selectively debrided partial thickness wounds. In full thickness injuries the graft take at the debrided areas was comparable with the take of grafts following excision procedures. The number of patients treated at our department by DGD was not yet sufficient for statistical evaluation of the results.
Fig. 1. Deep dermal to full thickness burns on the trunk at admission 
Fig. 2. The same burns following debridement by DGD. Narrow red margin at the lower wound edges are superficial burns which were not harmed by the enzyme. Left two thirds of the wound were selectively debrided deep dermal burns, the distal third of the wound were mostly full thickness areas with few dermal remnants 
Fig. 3. Details of the same burn. Debrided deep dermal burn shows very nice viable white dermis with red patches representing capillary loops on top of viable dermal papillae 
Fig. 4. Deep dermal burns of the left hand and lower arm at admission 
Fig. 5. According to the first study protocol, the lower arm only was debrided by DGD. Note the selectivity of the debridement which saved all the potentially viable healthy tissues 
Fig. 6. Detail of the same wound showing the differences between nondebrided (left margin) and debrided areas of the wound 
The study was sponsored by MediWound Ltd.
Conflict of interest declaration
None of the authors had any financial interests in the company.
Address for correspondence:
Assoc. Prof. Ján Koller, M.D., Ph. D.
Teaching Department for Burns and Reconstructive Surgery
University Hospital Bratislava, Ružinov Hospital
Ružinovská 6
826 06 Bratislava, Slovak Republic
E-mail: koller@ruzinov.fnspba.sk
Zdroje
1. Janzekovic Z. A new concept of early excision and immediate grafting of burns. J. Trauma, 10, 1970, p. 1103–1108.
2. Herndon DN., Barrow RE., Rutan RL., Rutan TC. A comparison of conservative versus early excision therapies in severely burned patients. Ann. Surg., 209, 1989, 547–553.
3. Cooper GR., Hodge GB., Beard JW. Enzymatic debridement in the local treatment of burns. Am. J. Dis. Child, 65, 1943, p. 909–911.
4. Connell JF., Rousselot LM. The use of proteolytic enzymes in the debridement of the burn eschar. Surg. Forum, 4, 1953, p. 422–427.
5. Teitelmann SL., Movitz D., Zimmerman LM. Enzymatic debridement of necrotic surfaces. Ann. Surg., 136, 1952, p. 261–271.
6. Moserová J., Koníčková Z., Holuša R. The effect of collagenase on thermally damaged skin. In: Matter P., Barclay TL., Koníčková Z. (Eds.) Research in Burns. Transactions of the Third International Congress on Research in Burns, held in Prague, September 20–25, 1970. Bern: Hans Huber Publishers, 1971, p.174–178.
7. Garrett TA.: Bacillus subtilis protease: a new topical agent for debridement. Clin. Med., 76, 1969, p. 11–15.
8. Pennisi VR., Capozzi A. Travase: observations and controlled study of the effectiveness in burn debridement. Burns, 1, 1974, p. 274–278.
9. Klasen HJ. The non-operative removal of necrotic tissues from burn wounds. In: Klasen HJ. History of Burns, Rotterdam: Erasmus Publishing, 2004, p. 453–480.
10. Klein GKV. Enzymatic debridement of third degree burns in animals with bromelains – a preliminary report. J. Maine Med. Assoc., 55, 1964, p. 169–171.
11. Klein GKV. Historical development of Bromelain in the treatment of burn wounds. In: May SR., Dogo G. (eds.) Care of the Burn Wound. Basel: Karger, 1985, p. 90–96.
12. Houck JC., Chang CM., Klein G. Isolation of an effective debriding agent from the stems of pineapples plants. Int. J. Tissue React., 5, 1983, p. 125–134.
13. Rosenberg L., Lapid O., Bogdanov-Berezovski A., Glesinger R., Krieger Y, Silberstein E., Sagi A., Judkins K., Singer A.J. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns, 30, 2004, 843–850.
14. Holubec K., Karfík V. Chirurgické léčení popálenin. Praha: Vojenskozdravotnická knihovna (svazek 29), 1956, p. 92.
15. Zimmermann WE. Topical Treatment of Burn with Collagenase. Physio-Pathology and Treatment of Burns. Bruxelles: Presses Académique Européenne, 1964.
16. Köpp FH. Kritische Bemerkungen zum Problem der enzymatischen Wundbehandlung unter besonderer Berücksichtigung von Verbrennungsverletzungen. Med. Welt, 2, 1965, p. 1990–1995.
17. Šimko Š, Koller J. Popáleniny. Martin: Osveta, 1992, p. 191.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2008 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Možnosti využití metamizolu v léčbě akutních primárních bolestí hlavy
-
Všetky články tohto čísla
- CONGRESS HISTORY OF THE INTERNATIONAL SOCIETY OF BURN INJURIES
- LATE COMPLICATION OF RESPIRATORY THERMAL INJURIES
- ENZYMATIC NECROLYSIS OF ACUTE DEEP BURNS – REPORT OF PRELIMINARY RESULTS WITH 22 PATIENTS
- HEMOCOAGULATION DISORDERS IN EXTENSIVELY BURNED PATIENTS: PILOT STUDY FOR SCORING OF THE DIC
- ČESKÉ SOUHRNY
- Contens
- INDEX
- ETHICAL PROBLEMS IN BURN MEDICINE
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- ENZYMATIC NECROLYSIS OF ACUTE DEEP BURNS – REPORT OF PRELIMINARY RESULTS WITH 22 PATIENTS
- CONGRESS HISTORY OF THE INTERNATIONAL SOCIETY OF BURN INJURIES
- HEMOCOAGULATION DISORDERS IN EXTENSIVELY BURNED PATIENTS: PILOT STUDY FOR SCORING OF THE DIC
- ETHICAL PROBLEMS IN BURN MEDICINE
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



