-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
PRESERVATION OF VENOUS OUTFLOW IMPROVES TRANSVERSE RECTUS ABDOMINIS MUSCULOCUTANEOUS FLAP SURVIVAL FOLLOWING VASCULAR DELAY
Autoři: D. Tsoutsos 1; A. Gravvanis 1; D. Kakagia 1,2; S. Ghali 3; A. Papalois 1
Působiště autorů: Department of Plastic Reconstructive Surgery, Athens General Hospital “G. Gennimatas”, Athens 1; Department of Plastic Surgery, Democritus University in Thrace, Alex/polis, Greece, and 2; Department of Plastic Surgery, The Royal Free Hospital, London, UK 3
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 51, 1, 2009, pp. 11-14
INTRODUCTION
The benefits of the application of vascular delay in restorative surgery have already been recognized and acknowledged, though its pathophysiology is not yet fully understood (7). Vascular delay has been clinically applied to the superior pedicled transverse rectus abdominis musculocutaneous flap for breast reconstruction following mastectomy for patients at high risk of complications (2, 20). Surgical methods used to induce vascular delay vary according to individual empirical strategies and include the selective ligation of one or both stems of the deep inferior epigastric vessels with or without occlusion of the corresponding superficial epigastric vessels (10, 16, 17). However, regardless of which vessels undergo ligation, it has been customary to ligate both the artery and the corresponding vein together (18).
The inferiorly based transverse rectus abdominis musculocutaneous flap has been widely investigated as an appropriate experimental model for the study of vascular delay (3, 4, 8), despite the fact that it is not directly analogous to the human equivalent (9).
Previous attempts at selective vascular delay involving preservation of ipsilateral venous outflow have shown no improvement over combined arterial and venous interruption (18). In the same study, selective venous division did not result in a significant increase in flap survival, indicating the importance of adequate venous outflow.
We therefore hypothesized that TRAM flap survival could be improved with selective ligation of arterial inflow with preservation of venous outflow not only in the superior ipsilateral pedicle but also in both superior and inferior contralateral pedicles.
METHODS
Animals
Thirty-six Wistar rats, 16–20 weeks old, weighing 280–320 grams were used. The animals were housed in an approved animal care center with 12 hour light cycles and provided with standard rodent chow and water ad libitum. All animal studies were approved by the Greek and European Community guidelines regulating animal research (Reg No: 4221/ 31-12-2002).
Anaesthesia
Animal anaesthesia was induced with intramuscular administration of a mixture consisting of 13cc ketamine (Ketalar 50mg/ml), 2.5cc Midazolam (Dormicum 5mg/ml) and 1cc atropine (Atropine Sulphate 1mg/ml). The adequacy of the anaesthesia was monitored prior to commencing surgery and at regular intervals throughout the operative procedure by response to forepaw stimulation. If minimal or no response was observed following firm pinching of the forepaw, then an appropriate level of anaesthesia was judged to have been achieved. Other parameters monitored throughout the anaesthetic and post-operatively until recovery included respiration and skin colour / turgor. Prior to coming round from the anaesthesia the rats received an intra-peritoneal injection of buprenorphine 0.05 mg/kg.
Experimental Protocol
Rats were randomly assigned to one of three groups consisting of 12 animals each, differentiated according to the type of vascular ligation selected for the first experimental stage in order to achieve the delay phenomenon.
Group A: was used as a control group, and no vessels were ligated.
Group B: The right inferior epigastric vessels were preserved, while 3 arteries and 3 veins were ligated. The right deep superior and left inferior and left superior deep vessels were ligated.
Group C: The right inferior epigastric vessels were preserved, while 3 arteries and 1 vein were ligated. The right superior vessels (artery and vein) as well as the left superior and inferior epigastric arteries (veins preserved) were ligated.
During the first stage of this procedure, small 1 cm horizontal skin incisions were made to the left and right of the xiphoid process using the operating microscope for ligation of the left and right deep superior epigastric vessels respectively (Fig. 1). A similar incision was made in the anterior rectus sheath and the muscle fibres spread in order to expose the superior epigastric vessels. For ligation of the left deep inferior epigastric vessels a small 1 cm horizontal skin incision was made in the left iliac fossa. Identification of the iliac vessels and preparation of the inferior epigastric vessels at their point of origin was carried out, again using the operating microscope. This was followed by ligation of the vascular pedicle (Group B) or selective ligation of the artery (Group C) with nylon 8-0 stitches.
Fig. 1. First stage of the delay procedure: horizontal skin incisions 1 cm long are used to expose deep superior epigastric vessels (bilaterally) and left deep inferior epigastric vessels 
Following a delay period of 1 week (15), the second stage of the procedure was carried out. This involved the preparation and elevation of the transverse rectus abdominis musculocutaneous (TRAM) flap based on the right inferior epigastric vessels. The skin paddle was of similar dimensions in all the experimental animals (Fig. 2A). The skin paddle was elevated with careful preservation of all musculocutaneous perforators from the right rectus abdominis muscle. The right rectus abdominis muscle was dissected from its origin superiorly at the right rib arc and detached from the ipsilateral abdominal wall and from the linea alba. The preparation of the right TRAM flap continued inferiorly to the right deep inferior epigastric vessels which were preserved (Fig. 2B). Finally, the flap was photographed (Sony Cybershot DSCF717 5.0 megapixel digital camera) and inset in its original position with 4-0 silk.
Fig. 2. Second stage: In all animal groups the skin paddle is elevated with preservation of all musculocutaneous perforators from the right rectus abdominis muscle only. A. The skin paddle extends from a line connecting both rib arcs to the xiphoid process to a line connecting the two anterior-superior iliac spines. B. The rectus abdominis muscle is dissected, preserving the blood supply from the right deep inferior epigastric vessels 
In control group A the musculocutaneous flap based on the right inferior epigastric vessels did not undergo an initial surgical delay procedure. In addition, venous patency in all group C vascular pedicles was confirmed with an Acland’s test prior to proceeding with the second stage.
Ninety-six hours later the animals were re-anaesthetised to evaluate skin island viability. The skin islands were harvested and mounted onto a wooden frame. They were then photographed (Sony Cybershot DSCF717 5.0 megapixel digital camera) and the images analyzed using SigmaScan (SPSS, Inc., Chicago, IL) to quantify and calculate flap survival. The area of flap survival divided by the total flap area multiplied by 100 was used to calculate the percentage of flap survival. Results are expressed as a mean ± standard deviation.
Statistical analysis
Statistical analysis was performed using the SigmaStat statistical program version 3.1 (SPSS Science, Chicago, IL). An analysis of variance (ANOVA) was used to evaluate differences between the experimental and control groups. A value of p<0.05 was considered significant. An additional post hoc test (Tukey’s Test) was carried out to verify any pairwise differences between groups.
RESULTS
All animals tolerated the surgical procedure and recovered smoothly from the anaesthesia. Table 1 illustrates the survival percentage of the flap with regard to the type of selective delay.
The percentage of survival in control group A was 50±6%; group B showed a percentage survival of 60±4% and group C 85±4% (see Table 1).
Tab. 1. Flap survival rates depending on the type of vascular delay in the three groups of animals 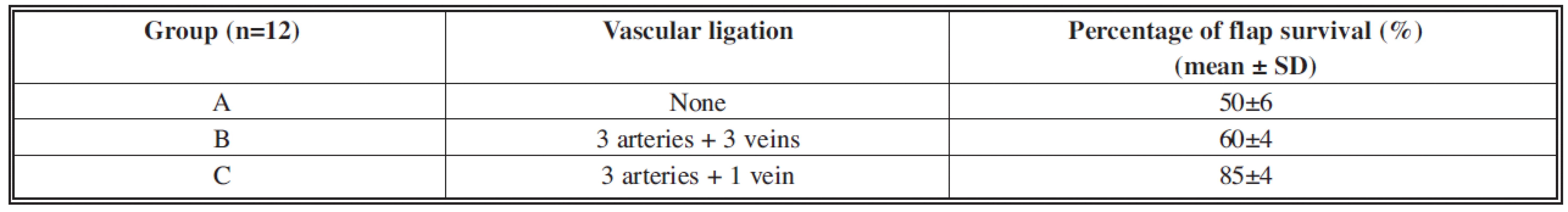
Variance analysis indicated a statistically significant difference in the survival percentage between groups C and A, and between groups C and B (p<0.05, ANOVA).
The occlusion of the three vascular pairs in group B improved the survival percentage in comparison to the control group A, but not to a statistically significant level (p>0.05, ANOVA). The selective occlusion of both the arteries on the left (opposite the flap blood supply side) with simultaneous preservation of the corresponding veins in group C improved the flap survival percentage to a statistically significant degree compared to groups A and B.
Necrosis was observed in the control group A in zones II, III and IV (Fig. 3A). Flap necrosis was mainly observed in group B in zone IV (left outer part of the flap, opposite the right rectus abdominis muscle) which should theoretically have the poorest blood supply (Fig. 3B), and it is worth noting that none of the animals in group C exhibited necrosis in this zone (Fig. 3C).
Fig. 3. Representative samples of the flaps from all three animal groups (A: control group, B: group B, C: group C) immediately after insetting (1) and 48 hours later (2). 
DISCUSSION
The transverse rectus abdominis musculocutaneous flap is one of the most commonly used flaps in breast reconstruction following mastectomy (5). Various techniques have been used to improve the blood supply to the flap in order to safely transfer large skin islands. Techniques include the use of a “double pedicle” (11), based on the superior epigastric vessels with or without simultaneous microvascular anastomoses of the inferior epigastric vessels (1), and the use of a free musculocutaneous flap based on the inferior epigastric vessels (19) or inferior epigastric vessel perforators (12). In spite of advances made in this field and increased experience in free tissue transfer, the superior epigastric vessel pedicled island flap is often applied to patients at high risk of complications (2). In this context, there have been a number of both experimental (3, 6, 8, 9, 15, 18) and clinical (2, 10, 16, 17, 20) attempts at applying the delay phenomenon to the rectus abdominis flap.
The rat transverse rectus abdominis musculocutaneous flap was the first model used to study the delay phenomenon in 1978 (6) and is analogous in many ways to the human TRAM flap with respect to myocutaneous perforators and dominant vs. non-dominant pedicles. It has since proven to be a reliable and consistently reproducible model for the study of the delay phenomenon.
The choice of a 1-week-time interval between the delay and flap elevation in the present study was not arbitrary but based on experimental work by Ozgentas et al. (15) as well as the clinical work of Restifo et al. (16, 17), who provided evidence that one week is sufficient an interval for the benefits of a delay procedure to occur.
A number of theories have been suggested as to the cause of the delay phenomenon (7). McFarlane et al. (13) suggested that the delay phenomenon is based on gradual reduction of blood supply, resulting in ischaemia, which in turn stimulates the development of collateral blood flow. On the other hand Myers et al. (14) put forward the assumption that the sudden increase in tissue pCO2 causes vascular dilation and an increase in the flap blood supply. More recent interpretation of the delay phenomenon comes from Taylor et al. (20), who suggest that after division of the dominant vascular stem of an angiosome (e.g. the deep superior to the rectus abdominis) the choke vessels connecting it with its neighbouring angiosome dilate, and as a result the blood supply to this neighbouring angiosome increases.
In addition Taylor et al. (20) have suggested that insufficient venous outflow causes the division of the venous part of the vascular pair resulting in blood reflux into the secondary vascular pair. On these grounds we decided to obstruct the arterial inflow to the contralateral left rectus abdominis muscle with division of the left superior and inferior epigastric arteries while maintaining venous outflow by preserving the left superior and inferior veins. In other words, we achieved arterial flow into one angiosome (right rectus) with the preservation of the inferior epigastric artery, and we ceased the flow of the superior and inferior epigastric arteries into the neighbouring angiosome (left rectus) while maintaining venous outflow (superior and inferior epigastric veins).
Accordingly we demonstrated that zone IV, with the poorest blood supply, exhibited no necrosis in any group C animals. This indicates that preservation of the venous outflow of zone IV with simultaneous reduction of its blood supply results in increased blood supply. This conclusion is further supported by direct comparison of group C to group B, where there was no venous outflow in zone IV.
Sano et al. (18) demonstrated in the rat rectus abdominis muscle model that selective ligation of the ipsilateral superior epigastric artery bears the same results as ligation of the superior epigastric vascular pair (artery and vein). They also demonstrated that selective ligation of the superior epigastric vein had a detrimental effect on flap viability, since the survival percentage in this case was no different to that of a control group where delay was not performed. The authors did not apply any kind of delay to the angiosomes on the contralateral side (left rectus abdominis muscle) and therefore did not observe any remarkable improvement to zone IV. Limiting contralateral arterial inflow while preserving contralateral venous outflow therefore appears to preserve the ischaemia required to stimulate vascular delay while simultaneously preventing venous congestion and therefore flap necrosis. Further experiments are planned to address the relative contribution of each of the contralateral venous outflow channels to flap survival.
There is no doubt that clinical interest in the delay phenomenon applied to the rectus abdominis muscle, mainly regarding breast reconstruction, is focused on increasing blood supply to zone IV. Surgical delay techniques applied herein have the disadvantage of excess surgical manipulations, but this is balanced by increased viability of the musculocutaneous flap and especially by the outstanding increased survival rate of zone IV. We conclude that selective preservation of venous outflow improves TRAM Flap survival following vascular delay and should be considered in high risk patients undergoing autologous breast reconstruction with a pedicled musculocutaneous flap.
Address for correspondence:
D. Kakagia, M.D, PhD., FEBOPRAS
Democritus University In Thrace
7, P. Kirillou Str,
68100 Alex/polis
Greece
E-mail: despoinakakagia @yahoo.com
Zdroje
1. Civelek B., Kargi E., Akoz T., Sensoz O. Turbocharge or supercharge? Plast. Reconstr. Surg., 102, 1998, p. 1303.
2. Codner MA., Bostwick J., 3rd, Nahai F., Bried JT., Eaves FF. TRAM flap vascular delay for high-risk breast reconstruction. Plast. Reconstr. Surg., 96, 1995, p. 1615-1622.
3. Dunn RM., Mancoll J. Flap models in the rat: a review and reappraisal. Plast. Reconstr. Surg., 90, 1992, p. 319-328.
4. Dunn RM., Huff W., Mancoll J. The rat rectus abdominis myocutaneous flap: a true myocutaneous flap model. Ann. Plast. Surg., 31, 1993, p. 352-357.
5. Elliott LF., Hartrampf CR., Jr. Tailoring of the new breast using the transverse abdominal island flap. Plast. Reconstr. Surg., 72, 1983, p. 887-893.
6. Finseth F., Cutting C. An experimental neurovascular island skin flap for the study of the delay phenomenon. Plast. Reconstr. Surg., 61, 1978, p. 412-420.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článek ČESKÉ SOUHRNY
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2009 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Možnosti využití metamizolu v léčbě akutních primárních bolestí hlavy
-
Všetky články tohto čísla
- PRESERVATION OF VENOUS OUTFLOW IMPROVES TRANSVERSE RECTUS ABDOMINIS MUSCULOCUTANEOUS FLAP SURVIVAL FOLLOWING VASCULAR DELAY
- THE EFFICACY OF MAGNESIUM SULFATE ON RESOLVING SURGICALLY PROVOKED VASOSPASM OF THE FLAP PEDICLE IN AN EXPERIMENT
- AWARD OF THE G. WHITAKER INTERNATIONAL BURNS PRIZE FOR 2009 PALERMO, ITALY
- THE EFFECT OF BLOOD AROUND A FLAP PEDICLE ON FLAP PERFUSION IN AN EXPERIMENTAL RODENT MODEL
- USE OF PIEZOELECTRIC BONE SCALPEL IN HAND AND RECONSTRUCTIVE MICROSURGERY
- MANAGEMENT OF INFECTED TIBIAL FRACTURES AND CHRONIC TIBIAL OSTEOMYELITIS BY MUSCLE FLAP TRANSFER: A COMPARISON OF TWO SERIES OF PATIENTS
- ČESKÉ SOUHRNY
- G. WHITAKER INTERNATIONAL BURNS PRIZE – PALERMO (Italy)
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- USE OF PIEZOELECTRIC BONE SCALPEL IN HAND AND RECONSTRUCTIVE MICROSURGERY
- MANAGEMENT OF INFECTED TIBIAL FRACTURES AND CHRONIC TIBIAL OSTEOMYELITIS BY MUSCLE FLAP TRANSFER: A COMPARISON OF TWO SERIES OF PATIENTS
- THE EFFICACY OF MAGNESIUM SULFATE ON RESOLVING SURGICALLY PROVOKED VASOSPASM OF THE FLAP PEDICLE IN AN EXPERIMENT
- ČESKÉ SOUHRNY
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



