-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Breast reconstruction with autologous abdomen-based free flap with prior abdominal liposuction – a case-based review
Authors: Hagiga A. 1; Rajiah E. 1; Ali M. 2; Shalabi M. 1; Mellington A. 1; Jones M. 1
Authors place of work: Queen Victoria Hospital NHS Foundation Trust, East Grinstead, United Kingdom 1; Barts Health NHS Trust, London, United Kingdom 2
Published in the journal: ACTA CHIRURGIAE PLASTICAE, 64, 1, 2022, pp. 31-38
doi: https://doi.org/10.48095/ccachp202231Introduction
Over recent years, liposuction has become the most commonly used surgical procedure to refine male and female body contours. In the last four decades, the incidence of breast cancer has increased [1]. Of these patients, there are more women requiring breast reconstruction who have undergone liposuction in the past.
In current practice, autologous abdomen-based free flap breast reconstruction is considered the gold standard as it provides a more natural result and improves the abdominal contour. Deep inferior epigastric perforator (DIEP) reconstruction allows the transfer of subcutaneous tissue to the breast without sacrificing the rectus muscle or fascia [2]. When planning an abdominal-based free flap breast reconstruction, previous abdominal scars as a result of laparotomy, caesarean section or liposuction are an important risk factor in determining flap survival [3].
If there has been previous liposuction to the donor site selected for a perforator based flap, there is a concern that a number of perforators may be fibrosed or damaged [4]. However, there is little in the literature regarding perforator flaps from previously liposuctioned donor sites.
The authors report on their experience and review the current literature on the autologous abdominal-based free flap for breast reconstruction after previous liposuction of the abdominal wall.
Methods
Five different databases Medline (PubMed), Scopus, Web of Science, Cochrane Library and Embase, were interrogated from inception to November 2021 by two independent investigators. The following keywords were used: (liposuction) OR (lipoaspirate) OR (liposculpting) OR (lipectomy)) AND ((abdominal-based autologous) OR (DIEP) OR (SIEP) OR (TRAM) OR (muscle-sparing transverse rectus abdominis myocutaneous) OR (sparing transverse rectus abdominis myocutaneous) OR (deep inferior epigastric perforator) OR (superficial inferior epigastric perforator flap)). Additionally, forward snowballing of retrieved studies was performed to identify further eligible studies for inclusion. Inclusion criteria were studies using abdominal autologous free flap for breast reconstruction. Exclusion criteria included non-abdominal based free flaps of breast reconstruction or surgical technique and non-English articles were also excluded. No studies were excluded based on study design or quality.
Case Presentations
Case 1
A 49-year-old lady diagnosed with right-sided breast cancer underwent mastectomy followed by chemotherapy and radiotherapy in 2013. The following year, a delayed latissmus dorsi (LD) free flap and expander insertion was performed. Subsequently the right breast expander was exchanged for an implant and a left mastectomy and implant insertion was also performed in the same year. She had liposuction in 2015 to the thigh and lower abdomen for lipomodelling of her right breast after LD reconstruction. In 2017, the patient presented with a concern regarding a discrepancy between the right and left breast of one and a half cup sizes. This had implications in finding appropriately sized bras which were often uncomfortable. Relevant past medical history includes hypertension and she was a smoker and had a body mass index (BMI) of 31. On examination, the left breast was well contoured but with grade 1 capsular contracture of the implant. The right breast had a larger LD skin paddle and underlying implant with grade 2 capsular contracture. On examination the abdomen showed suitability for consideration of deep inferior epigastric perforator (DIEP) flap with sufficient soft tissue, minimal rectus divarication and no surgical scars. CT angiogram showed a suitable vessel for reconstruction despite the previous liposuction (Fig. 1). In January 2019, the patient underwent removal of the right LD flap and implant which was replaced with a DIEP free flap. The left breast implant was also removed. There were no immediate postoperative complications, and she was discharged 2 days postoperative. The patient is still under ongoing follow-up for lipomodelling of the right flap and at a later stage is planned for nipple tattooing. Overall, the patient was satisfied with the postoperative outcome.
Fig. 1. Computed tomography angiography (0.63 mm) showing perforators (white arrows) for deep inferior epigastric perforators reconstruction. 
Case 2
A 59-year-old lady diagnosed with grade 2 lobular cancer ER8, HER2 negative and negative sentinel lymph node and completed chemotherapy and radiotherapy treatment in 2017. She was a non-smoker with a BMI of 20.8 and had undergone previous abdominal liposuction for aesthetic reasons. On assessment, there was sufficient soft tissue on her abdomen for breast reconstruction. CT angiogram was done and showed good periumbilical perforators. In 2019, the patient had left delayed bi-pedicled DIEP. She had an uneventful postoperative recovery. She complained of scar tightening; however intraoperatively, only fibrosis to the abdominal wall was noticed. The patient is pleased with the aesthetic results.
Case 3
A 62-year-old lady was diagnosed with bilateral grade III breast cancer, triple positive (HER2, ER, and PR), BRCA negative in 2010. She underwent bilateral mastectomies with immediate reconstruction using expanders and post-operatively had chemotherapy, radiotherapy and Herceptin. Unfortunately, she developed an infection of the right expander and had an LD flap and implant reconstruction. She had liposuction to the abdomen as a donor site for breast symmetrisation. In 2019, MRI showed evidence of bilateral intracapsular rupture and presented to our outpatient department for consideration of subsequent reconstruction of her breasts. On assessment, she was deemed suitable for DIEP reconstruction. Computed tomography angiography (CTA) was performed preoperatively for assessment of perforators. In 2020, she had removal of the right LD flap and bilateral implants with bilateral DIEP flap reconstruction. Intraoperatively she was found to have extensive fibrosis with difficult dissection around the perforator. She is happy with the results and listed for lipofilling, nipple reconstruction and tattooing at a later stage (Fig. 2, 3).
Fig. 2. Preoperative deep inferior epigastric perforators reconstruction photographs. 
Fig. 3. Deep inferior epigastric perforators reconstruction – 6 weeks postoperative. 
Results
Initial database searches identified 802 articles. After duplicates were removed, this resulted in 381 articles (Scheme 1). These articles were screened by title and abstract, which resulted in 10 studies eligible based on the aforementioned inclusion criteria. One study was excluded due to insufficient information regarding patient factors and measured outcomes. Therefore, nine studies were included in this review (Tab. 1).
Tab. 1. Included studies demographic and outcomes. 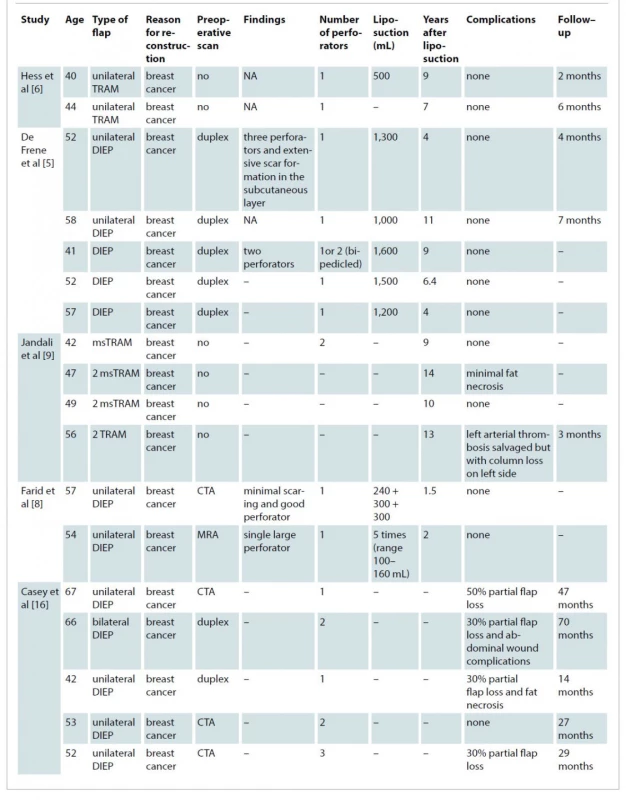

CTA – computed tomography angiography, DIEP – deep inferior epigastric perforator, MRA– magnetic resonance angiogram, msTRAM – muscle–sparing transverse rectus abdominis myocutaneous, NA – not available The nine studies reviewed 37 patients in total undergoing abdominal based free flap with a prior history of abdominal liposuction. Thirty patients (81.1%) underwent DIEP reconstruction, six patients had transverse rectus abdominis myocutaneous (TRAM) flap and 1 patient had a unilateral superficial interior epigastric artery (SIEA) flap. The age ranged from 32 to 73 years (mean of 51.7 years). The mean length of reconstruction after liposuction was 8.9 years, ranging from 1.5 to 23 years reported in 23 patients. The reconstructed breast volume was reported in nine patients in four studies with a mean volume of 1,026.6 mL (range 500–1,600 mL) [5–8].
Twelve patients (32.43%) had complications. Seven patients (18.9%) had partial loss of the flap. Studies that reported complications post free flap reconstruction did not report liposuction volume and the time between liposuction and autologous abdominal flap reconstruction. Twenty patients (54.05%) had CTA pre-operatively for the assessment of the perforators. Four patients did not undergo preoperative imaging for assessment of perforators. Of these, one patient had an arterial thrombus, which was salvaged with re-anastomosis [9]. Of the 20 patients imaged with CTA pre-
-operatively, the median number of perforators suitable for the reconstruction was 2, ranging from 1 to 5 perforators. This included patients who had conventional liposuction.
Discussion
The literature draws upon two main groups of patients undergoing autologous abdominal breast reconstruction with a background of previous liposuction. These are patients undergoing cosmetic liposuction and patients who have liposuction in conjunction with implant-based reconstruction. With an increasing number of abdominal-based autologous free flap reconstructions and liposuction procedures, it is imperative to identify the risks of liposuction and how it may adversely affect free flap reconstruction.
A major concern regarding abdominal liposuction is the risk of damage to perforators. This is illustrated in the literature through studies that have shown the effect of liposuction on the perforators. Salgarello et al [10] studied six patients who had superficial subdermal liposuction perioperatively, showing no changes in the number of perforators preoperatively. However, 6 months postoperatively, these perforators were found to have decreased in diameter. With the evolution of liposuction techniques, Blondeel et al conducted a comparative study between ultrasound-assisted liposuction and conventional liposuction on cadavers and concluded there was no evidence of superiority of one technique over the other with regard to damage to perforators [11]. Another study showed loss of perforators after conventional liposuction due to the vacuum effect of liposuction [12]. Furthermore, fibrosis secondary to liposuction may result in difficult perforator dissection, which increases the risk of damage intraoperatively when raising the flap. Complications related to breast reconstruction flap failure vary from patient-related such as smoking, overweight, radiation, to surgical-related reasons such as arterial or venous thrombosis [13].
Most patients included in this review had CTA as a main preoperative assessment tool. Although no patients were excluded based on their preoperative scan, included studies reported good perforator suitable for anastomosis. In our case series, perforators were screened by CTA prior to reconstruction. One patient had fibrosis which made intra-operative dissection difficult. However, there were no resulting complications from this.
It is imperative to have a detailed history from the patient and a preoperative scan through a suitable modality such as CTA or magnetic resonance angiography (MRA) to identify perforators and measure their diameter. Currently, CTA is the gold standard preoperative scan and is superior to MRI, particularly in detecting perforators. However, MRA eliminates the risk of radiation exposure. Moreover, the accuracy of the intramuscular course of a perforator is better assessed within MRA. Also, it may be considered more suitable for patients with liposuction as fibrosis is a factor which may affect the choice of perforator and is better appreciated on MRA. However, CTA has better spatial resolution in with the ability to visualise perforators as small as 0.6 mm compared to MRA which can detect 2 mm perforators. We changed our protocol in our unit to MRA for surgical planning
Another method of screening is fluorescence which is more relevant in intraoperative assessment of flap perfusion [14,15]. Casey et al performed a study using indocyanine green laser angiography to assess the perfusion of the flap after liposuction intraoperatively. This aided intraoperative examination of the flap perfusion and to dissect hypoperfused and nonviable tissue and demonstrated improved outcomes in patients who had liposuction prior to DIEP reconstruction [16].
The literature shows controversies on the injury to the perforators after liposuction. Some studies found no damage to the perforators [17]. Other studies showing decrease in the number of perforators [18]. İnceoğlu et al found that 57.8% of abdominal perforators were not detected using coloured doppler at 2 weeks and 3 months postoperative in patients who had conventional liposuction [19]. There was no difference in vascular damage between conventional liposuction and ultrasound assisted liposuction [11].
Upon reviewing our included cases, there were limitations; we could not identify the volume of liposuction that patients had previously. Regarding the studies included in our literature search, these mainly comprised case reports and case series, which are liable to selection bias. Additionally, the majority of studies did not provide information about liposuction volume.
Overall, thorough clinical assessment and pre-operative imaging remain the mainstay for identifying suitability for a patient for autologous abdominal-based breast reconstruction following previous abdominal liposuction. Therefore, following the identification of this group of patients, clinicians ought to conduct a detailed consultation explaining the risks and preoperative examination of the donor site. Surgeons should highlight the postoperative risks of scarring and dimpling, which might worsen after reconstruction, which may, in turn, have adverse effects on the cosmetic outcome due to fat necrosis. A systematic review showed the rate of fat necrosis in patients who had free flap reconstruction is 11.3% with higher necrosis in patients who underwent DIEP reconstruction [20]. However, there are no studies that report long-term fat necrosis on patients who had liposuction prior to breast reconstruction, Moreover, confounding factors such as surgeons’ experience, patient BMI, and other abdominal operations should be considered.
A systematic review on autologous breast reconstruction showed a pooled partial necrosis rate of 6% [21] whereas the partial necrosis rate in our review was 19.9%.
A direct comparison however cannot be drawn here owing to a smaller number of patients in the present review and selection bias as the patients had all undergone liposuction and had a proportionately higher rate of partial flap necrosis compared to the review by Kelley et al [21].
Moreover, more in vivo studies are recommended to assess the optimum time of abdominal-based breast reconstruction after liposuction. Also, prospective, multi-centre studies are recommended to assess the long-term volume of the flap, the rate of fat necrosis and whether it is higher compared to patients who have not undergone liposuction.
Conclusion
Our case series and review of the literature show successful cases with abdominal-based breast reconstruction. However, it is imperative to perform a detailed assessment of patients pre-operatively and fully outline the risks, specifically the consequences of potential damage to abdominal perforators following liposuction. Moreover, further research in this area should be targeted towards identifying the optimal timing for autologous abdominal-based reconstruction following liposuction. A careful combination of preoperative scanning, hand held Doppler and clinical examination reduce the chance of an unsuccessful outcome.
Declaration of conflicting interests: Ahmed Hagiga, Elizabeth Rajiah, Mohamed Aly, Mohamed Shalabi, Andrew Mellington, Martin Jones declare that they have no conflict of interest. The authors declare that this study has received no financial support. All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: A written informed consent was obtained from all patients in the study.
Role of authors: All authors have been actively involved in the planning, preparation, analysis and interpretation of the findings and processing of the article with the same contribution.
Ahmed Hagiga
Queen Victoria Hospital NHS
Foundation Trust
East Grinstead
United Kingdom, RH19 3DZ
e-mail: ahmed.tarekhagiga@nhs.net
Submitted: 19. 12. 2021
Accepted: 8. 2. 2022
Zdroje
1. Schneider AP., Zainer CM., Kubat CK., et al. The breast cancer epidemic: 10 facts. Linacre Q. 2014, 81(3): 244–277.
2. Granzow JW., Levine JL., Chiu ES., et al. Breast reconstruction with the deep inferior epigastric perforator flap: history and an update on current technique. J Plast Reconstr Aesthet Surg. 2006, 59(6): 571–579.
3. Roostaeian J., Yoon AP., Sanchez IS., et al. The effect of prior abdominal surgery on abdominally based free flaps in breast reconstruction. Plast Reconstr Surg. 2014, 133(3): 247e–255e.
4. Mankowski PJ., Kanevsky J., Lessard AS., et al. Free deep inferior epigastric perforator flap after abdominal liposuction: reconsidering a contraindication. Plast Aesthet Res. 2015, 2 : 311–314.
5. De Frene B., Landuyt KV., Hamdi M., et al. Free DIEAP and SGAP flap breast reconstruction after abdominal/gluteal liposuction. J Plast Reconstr Aesthet Surg. 2006, 59(10): 1031–1036.
6. Hess CL., Gartside RL., Ganz JC. TRAM flap breast reconstruction after abdominal liposuction. Ann Plast Surg. 2004, 53(2): 166–169.
7. Dammacco MAD., Deneuve S., Mouttet D., et al. Successful breast reconstruction with deep inferior epigastric perforator flap in high risk breast cancer patient after abdominal liposuction: technique and case report. Eur J Surg Oncol. 2019, 45(2): e104–e105.
8. Farid M., Nicholson S., Kotwal A., et al. DIEP breast reconstruction following multiple abdominal liposuction procedures. Eplasty. 2014, 14: e47.
9. Jandali S., Nelson JA., Wu LC., et al. Free transverse rectus abdominis myocutaneous flap for breast reconstruction in patients with prior abdominal contouring procedures. J Reconstr Microsurg. 2010, 26(9): 607–614.
10. Salgarello M., Barone-Adesi L., Cina A., et al. The effect of liposuction on inferior epigastric perforator vessels: a prospective study with color Doppler sonography. Ann Plast Surg. 2005, 55(4): 346–351.
11. Blondeel PN., Derks D., Van Landuyt KH., et al. The effect of ultrasound-assisted liposuction and conventional liposuction on the perforator vessels in the lower abdominal wall. Br J Plast Surg. 2003, 56(3): 266–271.
12. Young W., Cohen AR., Ransohoff J. Acute physiological effects of ultrasonic vibrations on nervous tissue. Neurosurgery. 1981, 8(6): 689–694.
13. Thorarinsson A., Fröjd V., Kölby L., et al. Patient determinants as independent risk factors for postoperative complications of breast reconstruction. Gland Surg. 2017, 6(4): 355–367.
14. Chae MP., Hunter-Smith DJ., Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg. 2015, 4(2): 164–178.
15. Wade RG., Watford J., Wormald JCR., et al. Perforator mapping reduces the operative time of DIEP flap breast reconstruction: a systematic review and meta-analysis of preoperative ultrasound, computed tomography and magnetic resonance angiography. J Plast Reconstr Aesthet Surg. 2018, 71(4): 468–477.
16. Casey WJ., Connolly KA., Nanda A., et al. Indocyanine green laser angiography improves deep inferior epigastric perforator flap outcomes following abdominal suction lipectomy. Plast Reconstr Surg. 2015, 135(3): 491e–497e.
17. Teimourian B., Kroll SS. Subcutaneous endoscopy in suction lipectomy. Plast Reconstr Surg. 1984, 74(5): 708–711.
18. Ozcan G., Shenaq S., Baldwin B., et al. The trauma of suction-assisted lipectomy cannula on flap circulation in rats. Plast Reconstr Surg. 1991, 88(2): 250–258.
19. İnceoğlu S., Özdemir H., İnceoğlu F., et al. Investigation of the effect of liposuction on the perforator vessels using color Doppler ultrasonography. Eur J Plast Surg. 2005, 21(1): 38–42.
20. Khansa I., Momoh AO., Patel PP., et al. Fat necrosis in autologous abdomen-based breast reconstruction: a systematic review. Plast Reconstr Surg. 2013, 131(3): 443–452.
21. Kelley BP., Ahmed R., Kidwell KM., et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol. 2014, 21(5): 1732–1738.
22. Zavlin D., Jubbal KT., Elsworth 4th WA., et al. Breast reconstruction with DIEP and SIEA flaps in patients with prior abdominal liposuction. Microsurgery. 2018, 38(4): 413–418.
23. Papas Y., Bou-Merhi J., Odobescu A., et al. Partial DIEP flap loss in a patient with history of abdominal liposuction. Ann Chir Plast Esthet. 2021, 66(3): 257–260.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článek Editorial
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2022 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Editorial
- Efficacy of pedicled anterolateral thigh flap for reconstruction of regional defects – a record analysis
- Depression and anxiety disorders in patients with carpal tunnel syndrome after surgery – a case control study
- Recurrence of breast ptosis after mastopexy – a prospective pilot study
- Development of a questionnaire for a patient-reported outcome after nasal reconstruction
- Breast reconstruction with autologous abdomen-based free flap with prior abdominal liposuction – a case-based review
- Superficial circumflex iliac artery perforator flap on extremity defects – case series
- Emergency evacuation low-pressure suction for the management of extravasation injuries – a case report
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Superficial circumflex iliac artery perforator flap on extremity defects – case series
- Efficacy of pedicled anterolateral thigh flap for reconstruction of regional defects – a record analysis
- Recurrence of breast ptosis after mastopexy – a prospective pilot study
- Breast reconstruction with autologous abdomen-based free flap with prior abdominal liposuction – a case-based review
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




