-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Applicability of the Glasgow-Blatchford score in predicting low-risk patients with upper gastrointestinal bleeding – first data from the Czech Republic
Použitelnost Glasgow-Blatchford skóre při predikci nízkorizikových pacientů s krvácením do horní části gastrointestinálního traktu – první data z České republiky
Úvod: Krvácení do horního gastrointestinálního traktu (GIT) patří mezi život ohrožující stavy, které vyžadují aktivní přístup a dynamický management akutní péče. Gastroskopie představuje zásadní diagnostický a terapeutický krok. Bohužel mnoho menších nemocnic nemá možnost provést gastroskopii mimo pracovní dobu. Glasgow-Blatchford skóre (GBS) představuje nástroj umožňující odhad vývoje stavu pacienta s krvácením do horního GIT. Evropská guidelines doporučují ambulantní řešení pacientů s GBS rovno 0 nebo 1. Cílem naší studie bylo posoudit použitelnost GBS na české populaci a zhodnotit bezpečnost vyšší hranice GBS pro řešení pacientů v ambulantním režimu.
Metody: Retrospektivní sběr dat pacientů, kteří byli hospitalizováni a endoskopováni v Nemocnici Boskovice pro symptomy krvácení z horního GIT od října 2018 do prosince 2019.
Výsledky: Data hodnotící celkový průběh onemocnění naznačují, že optimální GBS pro stanovení pacienta s nízkým rizikem je ≤ 3, ale s přihlédnutím k endoskopickým nálezům lze za bezpečné pro ambulantní léčbu považovat GBS ≤ 2. GBS ≥ 10 pak předpovídá závažný celkový průběh nemoci a závažné endoskopické nálezy.
Závěr: Dle našich dat lze zvýšit hodnotu GBS na 2 pro ambulantní řešení pacientů, což by mohlo snížit délku hospitalizace a tlak na urgentní endoskopie. Tyto závěry je nutné ověřit dalším výzkumem – ideálně multicentrickou prospektivní studií s velkým množstvím analyzovaných pacientů.
Konflikt zájmů: Autoři deklarují, že text článku odpovídá etickým standardům, byla dodržena anonymita pacientů a prohlašují, že v souvislosti s předmětem článku nemají finanční, poradenské ani jiné komerční zájmy.
Publikační etika: Příspěvek nebyl dosud publikován ani není v současnosti zaslán do jiného časopisu pro posouzení. Autoři souhlasí s uveřejněním svého jména a e-mailového kontaktu v publikovaném textu.
Dedikace: Článek není podpořen grantem ani nevznikl za podpory žádné společnosti.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bio medicínských časopisů.
Klíčová slova:
gastrointestinal – bleeding – Glasgow-Blatchford score – gastroscopy – risk stratification – cost reduce – outpatient
Authors: Tesarikova P. 1; Kunovsky L. 2,3; Kovalcikova P. 4; Trnova A. 5; Stiburek O. 1; Trna J. 1,2
Authors place of work: Department of Internal Medicine, Boskovice Hospital, Czech Republic 1; Department of Gastroenterology and Internal Medicine, Faculty of Medicine, Masaryk University and University Hospital, Brno, Czech Republic 2; Department of Surgery, Faculty of Medicine, Masaryk University and University Hospital, Brno, Czech Republic 3; Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic 4; Department of Anaesthesiology, Boskovice Hospital, Czech Republic 5
Published in the journal: Gastroent Hepatol 2020; 74(4): 319-326
Category: Klinická a experimentální gastroenterologie: původní práce
doi: https://doi.org/10.14735/amgh2020319Summary
Introduction: Upper gastrointestinal (GI) bleeding is a medical emergency that requires rapid assessment and dynamic management. Gastroscopy represents a crucial part of the diagnostic and therapeutic process. Unfortunately, in many hospitals emergency endoscopy is not easily available outside working hours. The Glasgow Blatchford score (GBS) predicts the outcome of patients at present. The European guidelines recommend outpatient management for a GBS of 0 or 1. The aim of our study was to validate the applicability of GBS in a population of Czech patients and to evaluate whether extending the GBS allows for early discharge while keeping the patient safe.
Methods: Retrospectively collected data of patients who underwent gastroscopy in the endoscopy ward of Boskovice Hospital for symptoms of upper GI bleeding between October 2018 and December 2019.
Results: Data based on the overall course of the disease suggest that the optimal GBS for determining a low-risk patient is 3, but concerning the endoscopic findings, GBS ≤ 2 should be considered safe for outpatient management. A GBS ≥ 10 predicts a severe overall course of the disease and a severe endoscopic finding.
Conclusion: According to our data, the GBS could be extended to 2 for safe outpatient management, which might reduce the length of stay at the hospital and the pressure for urgent endoscopies. Further studies with more patients are necessary.
Introduction
Upper gastrointestinal (GI) bleeding is a medical emergency that requires rapid assessment and dynamic management. With an incidence of 47/100,000, it remains a significant cause of hospital admission and is associated with significant morbidity and 30-day mortality [1]. Several studies have shown that 90–95% of patients are admitted to hospitals regardless of the severity of their upper GI bleeding for observation and endoscopy. This leads to inconvenience for the patients, increased expense and a greater risk of hospital-acquired complications [2].
In patients with suspected upper GI bleeding, it is recommended to perform early gastroscopy within the first 24 hours [3]. However, emergency endoscopy is not available at all hospitals around the clock. In order to stratify the risk of complications, rebleeding, clinical intervention and death, several clinical scores have been designed and recommended by international guidelines [4,5]. The two most widely used scores are the Rockall (RS) and Glasgow Blatchford (GBS) scores. As full RS relies on endoscopic findings, its use during initial patient assessment is limited. The GBS is a formal risk assessment tool for upper GI haemorrhages and uses the patient’s blood results, blood pressure, known history and presentation findings to identify how urgently patients require endoscopic therapy [6]. GBS is summarized in Tab. 1 [7].
Tab. 1. Glasgow Blatchford score (according to Blatchford et al. [7]).
Glasgow Blatchford skóre (podle Blatchford et al. [7]).![Glasgow Blatchford score (according to Blatchford et al. [7]).<br>
Glasgow Blatchford skóre (podle Blatchford et al. [7]).](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image_pdf/7e5433a8fd73b37b5b6d44189e8b8fb8.png)
The European Society of Gastrointestinal Endoscopy (ESGE) recommends the use of the GBS for pre-endoscopy risk stratification. Outpatients determined to be at very low risk based upon a GBS score of 0–1 do not require early endoscopy or hospital admission [8]. However, the use of scoring tools for the triage and management of upper GI bleeding is uncommon in everyday clinical practice.
As the management of upper GI bleeding and understanding of its stratification risk have become a hot topic among researchers, and because no data from the Czech Republic have yet been published, we have conducted a study to validate the applicability of GBS on patients treated in our institution and to evaluate whether the threshold for safe discharge of patients can be extended.
Methods
Data were collected retrospectively on consecutive patients over the age of 18 who were hospitalised at Boskovice Hospital and underwent gastroscopy for symptoms of upper GI bleeding (haematemesis and/or melaena) between October 2018 and December 2019. The inclusion criteria were patients aged 18 years or over with no other specifics. Using the electronic patient records, the clinical history, vital signs, laboratory, endoscopic results and information on patient outcomes were recorded and the GBS was calculated. The outcomes, such as the necessity of endoscopic or other therapies (surgical or radiological), rebleeding, intensive care unit (ICU) admission, the need for transfusion and 30-day mortality were also recorded. Furthermore, the severity of the endoscopic finding and the overall course of the disease were related to the GBS.
For our purposes, we divided endoscopic findings into three groups: mild (without clear cause of bleeding, mild esophagitis, gastropathy, duodenitis, peptic ulcer disease Forrest IIc–III), moderate (esophagitis with ulcer, esophageal varices with high-risk stigmata, peptic ulcer disease Forrest IIb, bleeding polyps) and severe (peptic ulcer disease Forrest I–IIa, bleeding tumour, bleeding esophageal varices, Dieulafoy lesion) (Tab. 2).
Tab. 2. Endoscopic findings.
Endoskopické nálezy.
The overall course of the disease was defined as mild (no need for endoscopic intervention or surgery, without blood transfusions, no ICU admission), as moderate (endoscopic intervention, ICU admission or transfusion) or severe (death, surgery, rebleeding) (Tab. 3).
Tab. 3. Overall course of disease.
Celkový průběh nemoci.
The data were analysed using basic descriptive statistics (the mean, the median, the minimum, the maximum, the absolute frequency and the relative frequency), the receiver operating characteristic (ROC) analysis and the area under a curve (AUC) were calculated. The sensitivity and specificity were determined.
This is a service improvement-based project using retrospectively collected data with no impact on patient care. Ethical approval was therefore not required and there were no conflicts of interest.
Results
Ninety-four consecutive patients, who met the inclusion criteria and for whom all necessary data were available, were analysed. All patients were hospitalised at Boskovice Hospital and underwent gastroscopy here for symptoms of upper GI bleeding.
Totally, 54 (57.4%) of the included patients were men and 40 (42.6%) of them were women; the mean age was 71 years. The presenting complaints of these patients were melaena (52; 55.3%), hematemesis (16; 17%), hematemesis and melaena (7; 7.4%) and collapse (12; 12.8%).
Eleven (11.7%) patients had no clear source of bleeding found in gastroscopy, 68 (72.3%) patients had one source of bleeding, 15 patients (16%) had more possible sources of bleeding. The most common was peptic ulcer disease (34; 36.2%), esophagitis (29; 30.9%) and gastropathy/duodenitis (19; 20.2%).
Totally, 42 (44.7%) patients had mild endoscopic findings, 29 (30.9%) patients had moderate findings and 23 (24.5%) patients had a severe endoscopic finding. Most of the patients (73.4%) did not require endoscopic intervention, while 19 (20.2%) patients needed endoscopic intervention and 6.4% were not endoscopically treatable.
Overall, 15 (16%) patients experienced rebleeding, 60 (63.8%) patients needed blood transfusion (the mean was six transfusions) and six (6.4%) patients underwent surgery.
Thirty-two (34%) patients were admitted to ICU, 16 (17%) patients died.
The overall course of the disease was mild in 27 patients (28.7%), moderate in 40 (42.6%) and severe in 27 (28.7%) patients.
The descriptive statistics for the population are summarized in Tab. 4.
Tab. 4. Descriptive statistics of patients with upper gastrointestinal bleeding (N = 94).
Deskriptivní statistika pacientů s krvácením do horní části gastrointestinálního traktu (n = 94).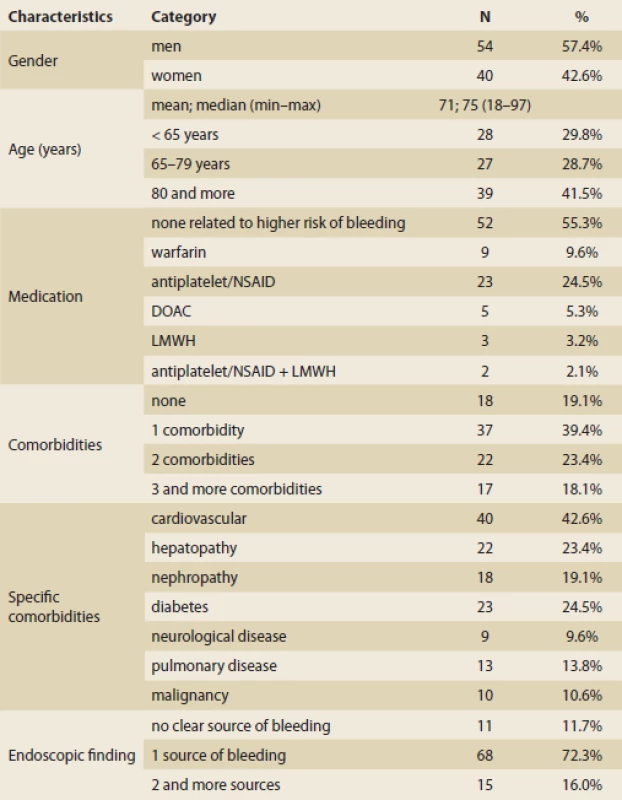
NSAID – nonsteroidal anti-infl ammatory drug, DOAC – direct oral anticoagulant, LMWH – low-molecular-weight heparin Tab. 5. – continuing. Descriptive statistics of patients with upper gastrointestinal bleeding (N = 94).
– pokračování. Deskriptivní statistika pacientů s krvácením do horní části gastrointestinálního traktu (n = 94).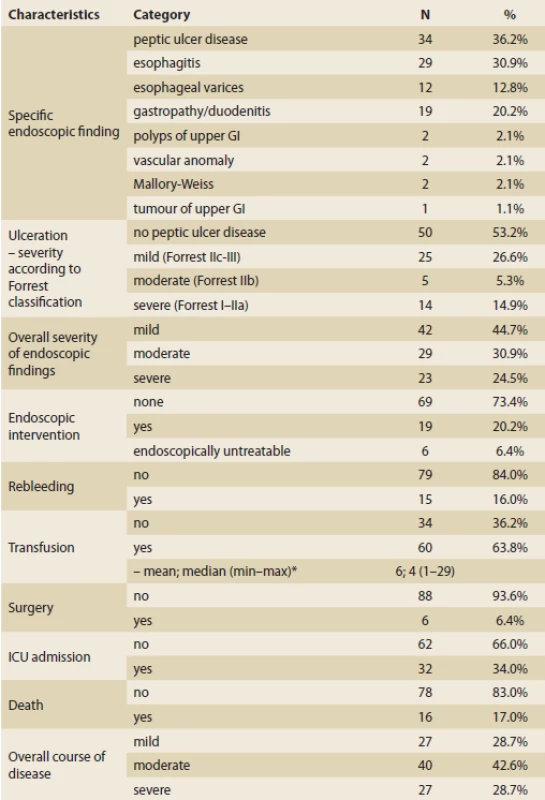
* number of transfusions is summarised in the patients who needed at least one (N = 60)
GI – gastrointestinalThe mean GBS was 9 points, the median of GBS was 12. All patients with GBS ≤ 3 had a mild course of the disease and all patients with GBS ≥ 11 had a moderate or severe course. As the GBS increased, the probability of the severity of the endoscopic finding increased (Tab. 5). The ROC curve indicated 0.973 for the prediction of a mild overall course of the disease, hence the GBS was deemed good for discrimination of low-risk patients from the clinical point of view. Using maximization of the sum of sensitivity and specificity, the optimal GBS would be 6 (sensitivity 92.6%, specificity 89.6% and overall prediction accuracy 90.4%) (Fig. 1). However, only a third of patients with a GBS of 6 had a mild course of the disease (Tab. 6). Therefore, the threshold with the highest specificity needs to be used for potential outpatient treatment, i.e. GBS = 3 (specificity 100%, sensitivity 74.1%).
Tab. 6. Overall results.
Celkové výsledky.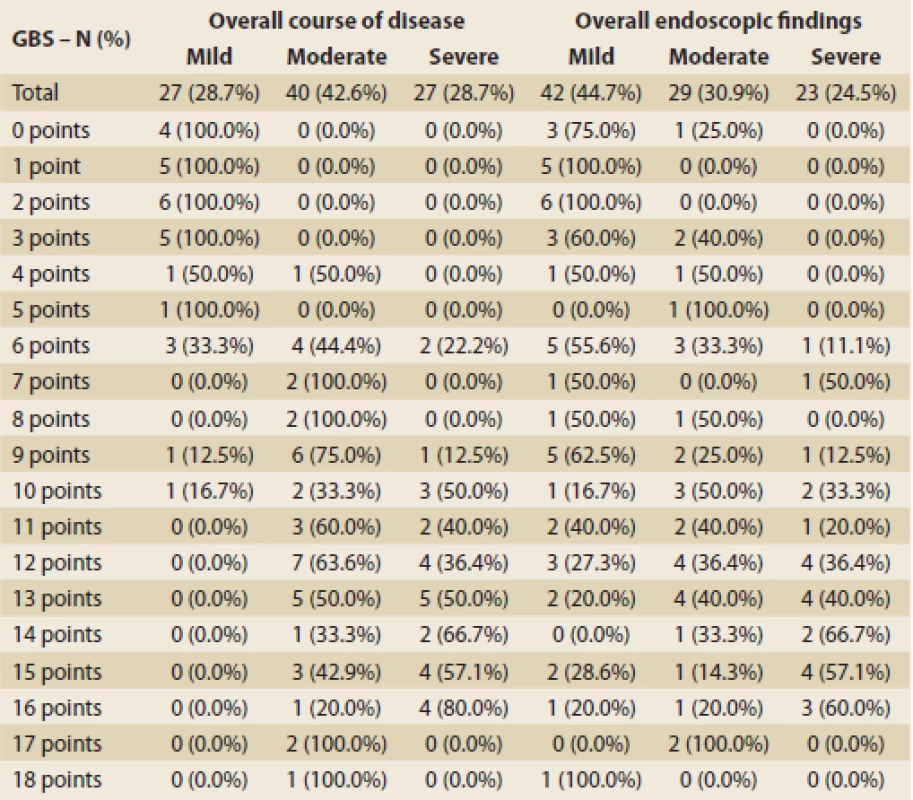
GBS – Glasgow Blatchford score Fig. 1. ROC analysis for prediction of mild course of disease – optimal threshold – maximization of sensitivity and specificity.
ROC analýza pro predikci mírného průběhu onemocnění – optimální práh – maximalizace senzitivity a specificity.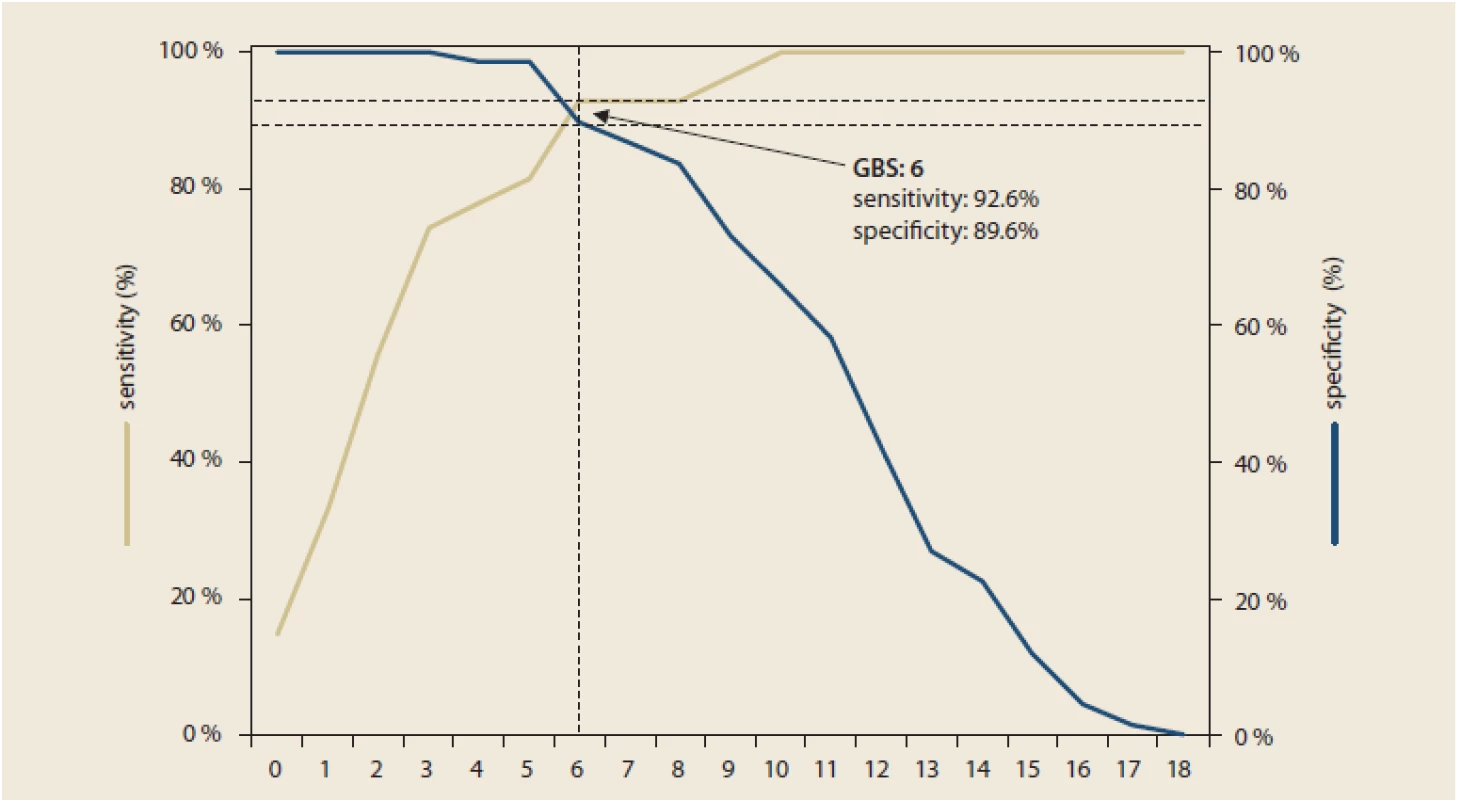
ROC – receiver operating characteristic
Glasgow-Blatchford score (GBS) – thresholdsTab. 7. Optimal GBS for prediction mild overall course – sensitivity, specificity, overall accuracy.
Optimální GBS pro predikci mírného celkového průběhu – senzitivita, specificita, celková přesnost.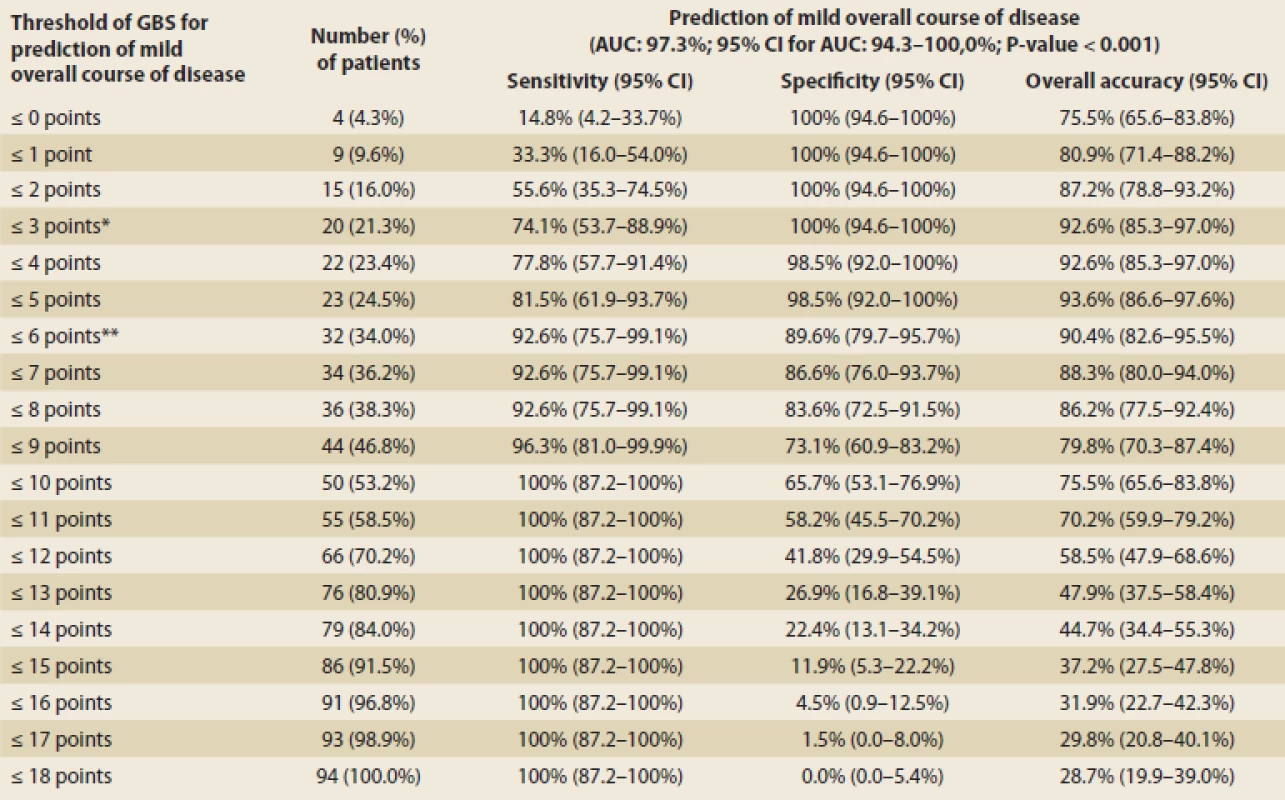
* optimal threshold with consideration of clinical benefit ** optimal threshold using maximization of the sum of sensitivity and specificity GBS – Glasgow Blatchford score, AUC – area under curve, CI – confidence interval The ROC curve indicated 0.781 for the prediction of a severe overall course, hence the GBS was deemed sufficient for the discrimination of high-risk patients from the clinical point of view. Using maximization of the sum of sensitivity and specificity, the optimal GBS would be 10 (sensitivity 88.9%, specificity 61.2% and overall prediction accuracy 69.2%). Evidently, determining the optimal GBS limit for predicting of a severe overall course of the disease is not as clear as for predicting a mild overall course of the disease.
The ROC curve indicated 0.757 for prediction of a mild endoscopic finding, hence the GBS was deemed as moderately good for the discrimination of low-risk patients from the endoscopic point of view. Using maximization of the sum of sensitivity and specificity, the optimal GBS would be 9 (sensitivity 71.4%, specificity 73.1% and overall prediction accuracy 72.3%). However, considering the clinical consequences with the imperative to accurately detect patients with mild endoscopic findings, the threshold with the highest specificity needs to be used for potential outpatient treatment, i.e. GBS = 2 (specificity 98.1%, sensitivity 33.3%).
The ROC curve indicated 0.771 for severe endoscopic finding, hence the GBS was deemed as moderately good from the endoscopic point of view. Using maximization of the sum of sensitivity and specificity, the optimal GBS would be 10 (sensitivity 87%, specificity 57.8% and overall prediction accuracy 64.9%).
In summary, based on the data from the overall course of the disease, we suggest that the optimal GBS for determining a low-risk patient is 3, but concerning the endoscopic findings, we consider GBS ≤ 2 to be safe for outpatient management. On the other end of the spectrum, GBS ≥ 10 predicts a severe overall course of the disease and severe endoscopic finding, therefore an intensive approach to the patient should be considered. The results are summarized in Tab. 5.
Discussion
Acute upper GI bleeding is a frequent cause of acute admission to the hospital. Gastroscopy represents an essential part of the diagnostic and therapeutic process, but immediate endoscopy is not always available due to the absence of endoscopists/interventionists outside working hours. The guidelines recommend the use of risk stratification tools in upper GI bleeding to facilitate accurate triage and assist in clinical decisions, such as endoscopic timing and the level of care. It is important for physicians to identify upper GI bleeding patients who are at a high risk of mortality or rebleeding [9].
Three main groups of scoring system can be established: the scores that only require endoscopic parameters (Forrest classification), those that incorporate clinical and endoscopic parameters (RS, Baylor bleeding score, Cedar-Sinai Medical Center Predictive Index (CSMCPI), Progetto Nazionale Emorragia Digestiva (PNED)) and those based only on clinical parameters (GBS, AMIS65, T score) [4,10]. The two most widely used scores are the RS and the GBS. The RS is designed to predict mortality. The components of the score are age, shock, comorbidity and the diagnosis and presence for stigmata of recent haemorrhage at endoscopy. As the full RS relies on endoscopic findings, its use during initial patient assessment is limited. A pre-endoscopy RS (pRS) can be calculated by omitting the endoscopic criteria, but this may decrease the predictive power of the score [11]. The GBS was derived with the aim of identifying patients who needed treatment [7]. The components of the score are urea, haemoglobin, systolic blood pressure, heart rate, presenting features and comorbidities. The GBS does not require endoscopy findings to be calculated, which allows it to be performed by busy clinicians before endoscopy [11]. ESGE recommends the use of the GBS for pre-endoscopy risk stratification. The outpatients determined to be at very low-risk based upon a GBS score of 0–1 do not require early endoscopy or hospital admission [8].
The median of the GBS in our patient cohort was 12 and only nine patients (21%) had a GBS of 0 and/or 1, showing that only a small number of patients could be discharged from the emergency department with early scheduled outpatient follow-up. As the population ages, elderly people have more comorbidities, for which points are added to the GBS and then it is impossible to score 0 or 1. Therefore, there are studies that recommended to increase the GBS threshold further [5,6,12–17]. Based on the data from the overall course of the disease, we suggest that the optimal GBS for determining a low-risk patient is 3, but concerning the endoscopic findings, we consider patients with a GBS of ≤ 2 to be safe for outpatient management. That is consistent with several studies which advocate that the patients presenting with a non-variceal upper GI bleed with GBS ≤ 2 form a subgroup of low-risk patients eligible for outpatient management [5,6,12–17]. A GBS of 3 was also previously mentioned as a possible safe threshold, but Chatten et al. described that two people with a GBS of 3 from their study group died within 30 days of presentation; so they recommend that a GBS of ≤ 2 should be considered as safe [6]. Masaoka et al, Srirajaskanthan et al. and Recio-Ramirez et alstudied retrospectively 93, 174 and 60 patients attending emergency departments with GI bleeding. All three studies found that patients could be deemed low-risk and suitable for outpatient management with a GBS cut-off of ≤ 2 [13,15,16].
The mean age of patients in our study was 71 years. Stephens et al. defined patients at low risk using the criteria of a GBS ≤ 2 and age less than 70 years. This classification allowed 10.5% of patients with upper GI bleeding to be safely managed in community [14].
Although endoscopic treatment was not required with a GBS of 0–5, having a low score does not exclude a pathology that necessitates follow-up (e. g. Barrett’s oesophagus). Therefore, it is essential that these patients still undergo endoscopic investigation to avoid missing findings that could jeopardise patient safety.
The GBS encompasses aspects of the history, blood results and observations that are taken as routine upon a patient’s admission to hospital. Specialist knowledge is not required to calculate or interpret the resulting score, which is simply a number ranging from 0 to 23. Therefore, it can be applied in emergency departments and acute medical units to facilitate early discharge [6].
Moreover, a high GBS could point to patients requiring an intensive approach (urgent endoscopy, transfer of the patient to a hospital with round-the-clock endoscopic services and ICU admission) [18,19]. Our data accordingly shows that a GBS ≥ 10 is associated with a severe overall course of disease and with a severe endoscopic finding. This advocates for performing endoscopy as soon as possible. Lim et al. suggest that the cut-off for urgent endoscopy is a GBS score of ≥ 12 [20]. Ko et al. indicated that the risk of significant bleeding requiring intervention increased in patients with a GBS > 9 [18]. Stanley et al presented findings that a GBS ≥ 7 had the highest combination of sensitivity and specificity for predicting endoscopic treatment or 30-day mortality, respectively, but the positive predictive value for both is low. Therefore, the clinical utility of the GBS to direct care in high-risk patients seems limited when compared to low-risk patient distinction [21].
Boskovice Hospital is a small hospital with limited possibility of emergency endoscopy. The mean time to endoscopy was 25.7 hours, 67 patients (71.3%) underwent endoscopy within 24 hours. These data are similar to those from Slovakia (the only similar study on this topic from Central and East European region), where the mean time to endoscopy was 25.01 hours and 68.8% patients underwent endoscopy within 24 hours [22]. Patients admitted to the hospital were all pretreated with proton pump inhibitors; therefore, those admitted outside working hours have more time for the effect of conservative treatment. Moreover, 80% of all upper GI bleeding stop spontaneously [23]. All these facts may explain why 73.4% patients did not require endoscopic treatment.
The primary limitations of our study are its retrospective unicentric design and a relatively small number of included patients. Larger multicentric prospective studies are needed to reach definitive conclusions.
Conclusion
Upper GI bleeding is a medical emergency. The GBS was created to stratify the risk of complications and the severity of the overall course of the disease.
Based on our retrospectively collected data, we suggest that the patients presenting with an upper GI bleeding with a GBS ≤ 2 could be considered for safe outpatient management.
As it represents an easy-to-use tool, we recommend using the GBS scale routinely to increase the number of patients with upper GI bleeding who could be discharged from the emergency department, which would in turn free up inpatient beds and save costs.
Confl ict of Interest: The authors declare that the article/ manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no
fi nancial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/ manuscript has not been published or is currently being submitted for another review. The authors agree to publish their name and e-mail in
the published article/ manuscript.
Dedication: The article/ manuscript is not supported by a grant nor has it been created with the support of any company.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for bio medical papers.Submitted/doručeno: 17. 7. 2020
Accepted/přijato: 14. 8. 2020
Assoc. Prof. Jan Trna MD, PhD
Department of Internal Medicine
Boskovice Hospital
Otakara Kubina 179
680 01 Boskovice
Czech Republic
Zdroje
1. Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2019; 42–43 : 101610. doi: 10.1016/j.bpg.2019.04.003.
2. Banister T, Spiking J, Ayaru L. Discharge of patients with an acute upper gastrointestinal bleed from the emergency department using an extended Glasgow-Blatchford Score. BMJ Open Gastroenterol 2018; 5 (1): e000225. doi: 10.1136/bmjgast-2018-000225.
3. Lau JY, Yu Y, Tang RS et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med 2020; 382 (14): 1299–1308. doi: 10.1056/NEJMoa1912484.
4. Monteiro S, Gonçalves TC, Magalhães J et al. Upper gastrointestinal bleeding risk scores: who, when and why? World J Gastrointest Pathophysiol 2016; 7 (1): 86–96. doi: 10.4291/wjgp.v7.i1.86.
5. Shafaghi A, Gharibpoor F, Mahdipour Z et al. Comparison of three risk scores to predict outcomes in upper gastrointestinal bleeding; modifying Glasgow-Blatchford with albumin. Rom J Intern Med 2019; 57 (4): 322–333. doi: 10.2478/rjim-2019-0016.
6. Chatten K, Purssell H, Banerjee AK et al. Glasgow Blatchford Score and risk stratifications in acute upper gastrointestinal bleed: can we extend this to 2 for urgent outpatient management? Clin Med (Lond) 2018; 18 (2): 118–122. doi: 10.7861/clinmedicine.18-2-118.
7. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000; 356 (9238): 1318–1321. doi: 10.1016/S0140 - 36 (00) 02816-6.
8. Gralnek IM, Dumonceau JM, Kuipers EJ et al.Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015; 47 (10): a1–a46. doi: 10.1055/s-0034-1393172.
9. Kim MS, Choi J, Shin WC. AIMS65 scoring system is comparable to Glasgow-Blatchford score or Rockall score for prediction of clinical outcomes for non-variceal upper gastrointestinal bleeding. BMC Gastroenterol 2019; 19 (1): 136. doi: 10.1186/s12876-019-1051-8.
10. Bakhtavar HE, Morteza Bagi HR, Rahmani F et al. Clinical scoring systems in predicting the outcome of acute upper gastrointestinal bleeding; a narrative review. Emerg (Tehran) 2017; 5 (1): e36.
11. Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2019; 42–43 : 101610. doi: 10.1016/j.bpg.2019.04.003.
12. Aquarius M, Smeets FG, Konijn HW et al. Prospective multicenter validation of the Glasgow Blatchford bleeding score in the management of patients with upper gastrointestinal hemorrhage presenting at an emergency department. Eur J Gastroenterol Hepatol 2015; 27 (9): 1011–1016. doi: 10.1097/MEG.0000000000000402.
13. Recio-Ramírez JM, Sánchez-Sánchez MP, Peña-Ojeda JA et al. The predictive capacity of the Glasgow-Blatchford score for the risk stratification of upper gastrointestinal bleeding in an emergency department. Rev Esp Enferm Dig 2015; 107 (5): 262–267.
14. Stephens JR, Hare NC, Warshow U et al. Management of minor upper gastrointestinal haemorrhage in the community using the Glasgow Blatchford Score. Eur J Gastroenterol Hepatol 2009; 21 (12): 1340–1346. doi: 10.1097/MEG.0b013e3 2831bc3ec.
15. Srirajaskanthan R, Conn R, Bulwer C et al. The Glasgow Blatchford scoring system enables accurate risk stratification of patients with upper gastrointestinal haemorrhage. Int J Clin Pract 2010; 64 (7): 868–874. doi: 10.1111/j.1742-1241.2009. 02267.x.
16. Masaoka T, Suzuki H, Hori S et al. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol 2007; 22 (9): 1404–1408. doi: 10.1111/j.1440-1746.2006.04762.x.
17. Schiefer M, Aquarius M, Leffers P et al. Predictive validity of the Glasgow Blatchford Bleeding Score in an unselected emergency department population in continental Europe. Eur J Gastroenterol Hepatol 2012; 24 (4): 382–387. doi: 10.1097/MEG.0b013e3283505965.
18. Ko IG, Kim SE, Chang BS et al. Evaluation of scoring systems without endoscopic findings for predicting outcomes in patients with upper gastrointestinal bleeding. BMC Gastroen-terol 2017; 17 (1): 159. doi: 10.1186/s12876-017-0716-4.
19. Lu M, Sun G, Huang H et al. Comparison of the Glasgow-Blatchford and Rockall Scores for prediction of nonvariceal upper gastrointestinal bleeding outcomes in Chinese patients. Medicine (Baltimore) 2019; 98 (21): e15716. doi: 10.1097/MD.0000000000015716.
20. Lim LG, Ho KY, Chan YH et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy 2011; 43 (4): 300–306. doi: 10.1055/s-0030-1256110.
21. Stanley AJ, Laine L, Dalton HR et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study BMJ 2017; 356: i6432. doi: 10.1136/bmj.i6432.
22. Koller T, Sekáč J, Huorka M et al. Prognostické faktory a načasovanie endoskopie u akútneho nevarikózneho krvácania z horného gastrointestinálneho traktu. Gastroent Hepatol 2011; 65 (5): 286–293.
23. Dítě P, Novotný I, Kroupa R et al. Akutní nevarikózní krvácení do horní čísti trávicího traktu. Čes a Slov Gastroent a Hepatol 2007; 61 (1): 6–10.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2020 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
-
Všetky články tohto čísla
- Klinická a experimentální gastroenterologie
- Electrogastrography in systemic sclerosis – a pilot study
- Portopulmonální hypertenze u pacientů indikovaných k transplantaci jater – zkušenosti transplantačního centra IKEM
- EUS navigované gastrointestinální anastomózy – nové možnosti terapeutické endoskopie
- Akutní pankreatitida u pacientů po transplantaci jater
- Applicability of the Glasgow-Blatchford score in predicting low-risk patients with upper gastrointestinal bleeding – first data from the Czech Republic
- Autonómna dysregulácia pri syndróme dráždivého čreva, funkčnej dyspepsii a globus pharyngeus – prehľad literatúry a pilotné výsledky
- Whippleova choroba – dvě kazuistiky
- Akutní poškození ledvin v gastroenterologii
- Výběr z mezinárodních časopisů
- Kreditovaný autodidaktický test: klinická a experimentální gastroenterologie
- Jorveza® – očekávaný preparát k léčbě eozinofilní ezofagitidy
- Hemové železo v substituci sideropenie a sideropenní anémie u pacientů s IBD
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- EUS navigované gastrointestinální anastomózy – nové možnosti terapeutické endoskopie
- Jorveza® – očekávaný preparát k léčbě eozinofilní ezofagitidy
- Whippleova choroba – dvě kazuistiky
- Applicability of the Glasgow-Blatchford score in predicting low-risk patients with upper gastrointestinal bleeding – first data from the Czech Republic
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



