-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The influence of microscopic inflammation at resection margins on early postoperative endoscopic recurrence after ileocaecal re-section for Crohn’s disease
Authors: Poredská K. 1; Kunovsky L. 1,2; Marek F. 2; Kala Z. 2; Prochazka V. 2; Dolina J. 1; Zboril V. 1; Kovalcikova P. 3; Pavlik T. 3; Ja-Bandziev P. 4; Pavlovsky Z. 5; Vlazny J. 5; Mitas L. 2
Authors place of work: Department of Gastroenterology and Internal Medicine, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Department of Surgery, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Pediatrics, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 4; Department of Pathology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 5
Published in the journal: Gastroent Hepatol 2021; 75(1): 29-37
Category: IBD: Originální článek
Summary
Background and Aims: The pathogenesis and risk factors for early postoperative endoscopic recurrence of Crohn’s disease (CD) remain unclear. Thus, this study aimed to identify whether histological inflammation at the resection margins after an ileocaecal resection influences endoscopic recurrence.
Methods: We have prospectively followed up patients with CD who underwent ileocaecal resection at our hospital between January 2012 and January 2018. The specimens were histologically analysed for inflammation at both of the resection margins (ileal and colonic). We evaluated whether histological results of the resection margins are correlated with endoscopic recurrence of CD based on colonoscopy 6 months after ileocaecal resection. Second, we assessed the influence of known risk factors and preoperative therapy on endoscopic recurrence of CD.
Results: A total of 107 patients were included in our study. Six months after ileocaecal resection, 23 patients (21.5%) had an endoscopic recurrence of CD. The histological signs of CD at the resection margins were associated with a higher endoscopic recurrence (56.5% versus 4.8%, p < 0.001). Disease duration from diagnosis to surgery (p = 0.006) and the length of the resected bowel (p = 0.019) were significantly longer in patients with endoscopic recurrence. Smoking was also proved to be a risk factor for endoscopic recurrence (p = 0.028).
Conclusions: Histological inflammation at the resection margins was significantly associated with a higher risk of early postoperative endoscopic recurrence after an ileocaecal resection for CD.
Keywords:
Crohn’s disease – ileocaecal resection – early postoperative endoscopic recurrence
Introduction
Despite the era of biologics, surgical treatment of Crohn’s disease (CD) still plays an important role. Up to 80% of patients need surgery during their lifetime [1,2]. Moreover, early postoperative endoscopic recurrence (EPER) of the disease occurs in approximately 70% of patients within the first year after the surgery [2,3]. As EPER precedes clinical symptoms, its detection is essential in the further management of postoperative CD [2,4]. The exact pathogenesis of EPER is unknown. However, according to the literature, the risk factors include smoking, previous intestinal surgery, absence of prophylactic treatment, penetrating disease at index surgery, perianal location, granulomas in the resection specimen, and myenteric plexitis [5]. Although some recent data have suggested that histopathology results of the resection margins could predict EPER [1,6,7], such relationship remains to be further established. Recurrence prevention concerning patient selection as well as postoperative treatment and its timing remains a clinical challenge, as no standard recommended algorithm exists [1,8–10]. Hence, this study aimed to identify whether histological inflammation at the resection margins influences EPER in patients with CD after an ileocaecal resection (ICR), and to assess the influence of the aforementioned risk factors and preoperative therapy on EPER.
Materials and methods
Patient selection
In this prospective study, we have included patients with CD who underwent ICR between January 2012 and January 2018 at the University Hospital in Brno. ICR was performed by either the open or the laparoscopic approach. The resection extended to macroscopically uninvolved tissue, and a primary anastomosis was constructed. All the surgeries were performed by three surgeons who are experienced in colorectal and IBD surgery. Ileocolonoscopy was performed 6 months after ICR. Patients who underwent other resection procedures, such as a hemicolectomy, multiple ileal resections, and stricturoplasties, or had either a permanent or a diverting loop ileostomy, were excluded. Moreover, patients with any postoperative prophylactic treatment before the postoperative ileocolonoscopy check-up and those with missing data were also excluded.
All patients provided written informed consent and procedures were performed in accordance with the ethical standards according to the Declaration of Helsinki.
Variables analysed
We analysed the general characteristics of the patients, including age, gender, the Montreal classification of CD (including perianal disease), and the disease duration from diagnosis to ICR. In regard to the surgery, we evaluated the type of approach (open or laparoscopic), length of the resected bowel, type of anastomosis (end-toend or side-to-side), and postoperative complications (according to the Clavien-Dindo classification) [11].
Histopathological analysis of the resected tissue was performed by an experienced IBD pathologist. As there is no histopathology score validated for CD [12], the pathologist marked the resection margin as positive if the following signs of CD were noted: architectural distortion, inflammation activity (mild: cryptitis in <25% crypts; moderate: cryptitis in >25% of crypts; severe: with ulcerations), granulomas, ulcerations, erosions, fibrosis, neuronal hyperplasia, Paneth cells metaplasia, or signs of chronic inflammation (transmural inflammation with lymphoid aggregates and basal plasmacytosis). When assessing the resection margins, the pathologist always evaluated both the ileal and the colonic margins and assessed for the aforementioned signs of CD. Specimens with inflammation in the ileal, colonic, or both resection margins were counted as positive.
Regarding EPER, ileocolonoscopy 6 months after the ICR was performed and endoscopic recurrence was evaluated using Rutgeert’s score (positive EPER was defined as Ri ≥2). The use of preoperative medication (≤12 weeks before ICR) as well as smoking and any family history of CD as possible risk factors were also analysed.
Statistical analysis
EPER was the primary endpoint of this study. The difference in the primary endpoint between the two study groups according to the histological inflammation at the resection margins was evaluated using a univariate logistic regression model.
No sample size calculations were performed before the study started. However, the number of patients in both the study arms (90 patients with negative and 17 patients with positive resection margins) as well as the observed difference in the primary endpoint ensured a statistical power of the univariate logistic regression model of >99%.
Standard frequency tables and summary statistics, i.e. means, standard deviation, median, minimum, and maximum, were used to describe the baseline demographic and clinical characteristics. Statistical significance of the differences for categorical variables was assessed using Fisher’s exact test. Comparisons of continuous variables were performed using the Mann–Whitney U test. The secondary outcomes were evaluated using univariate and multivariate logistic regression models. A p <0.05 was considered statistically significant for all analyses. All statistical analyses were performed with SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 107 patients, who provided written informed consent, were included in this study. Thirty-six patients were excluded because of a different intestinal resection or prophylactic treatment administered before postoperative ileocolonoscopy. The baseline characteristics of the study group are listed in Tab. 1.
Tab. 1. Baseline characteristics of the patients, subdivided in the two groups according to the histological signs of inflammation at the resection margins, expressed as median (minimum–maximum) or frequency (percent). 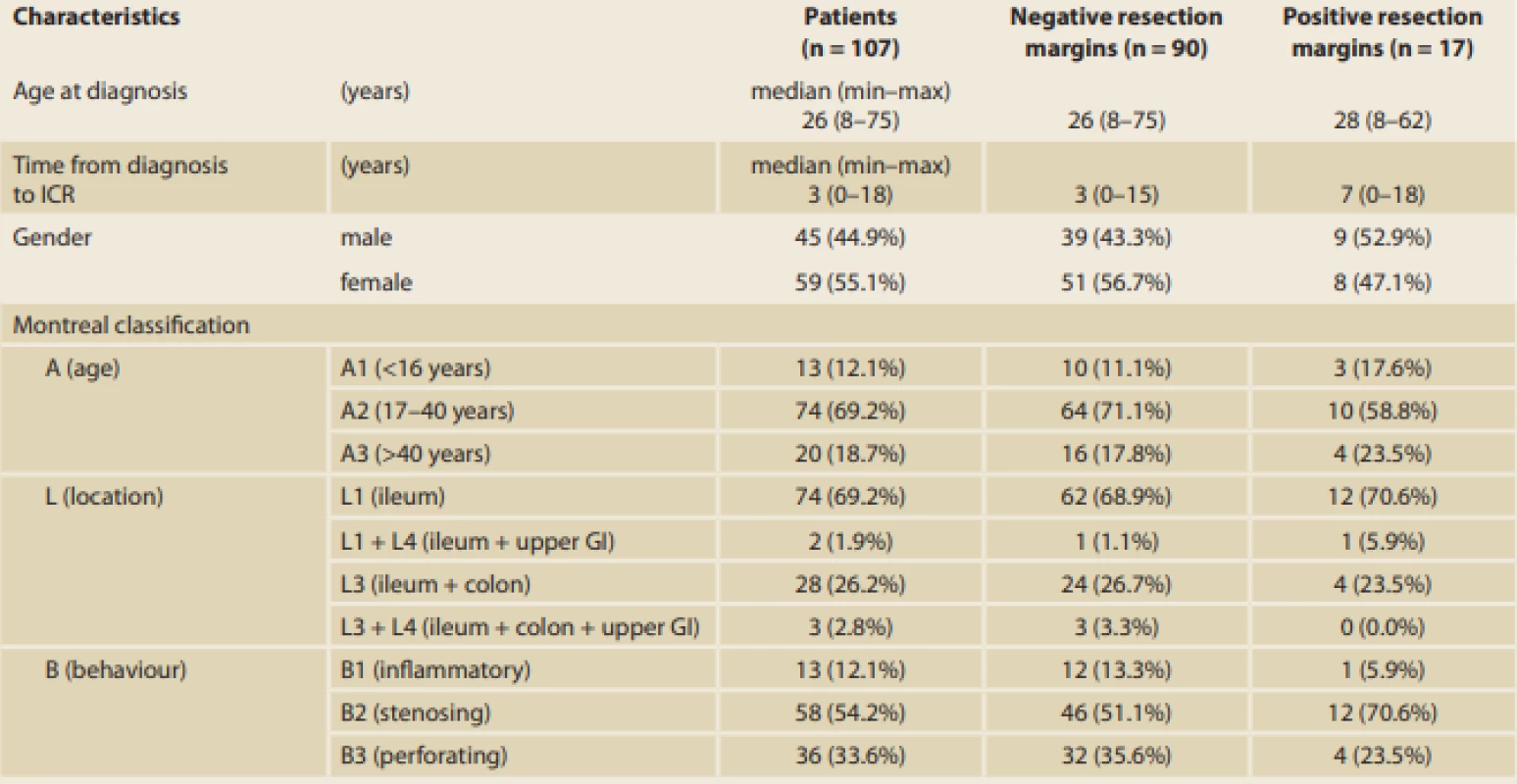
ICR – ileocaecal resecion; GI – gastrointestinal tract; 5-ASA – 5-aminosalicylates; GSC – glucocorticoids; AZA – azathioprine; MTX – methotrexate; IBD – inflamma Tab. 1– continuing. Baseline characteristics of the patients, subdivided in the two groups according to the histological signs of inflammation at the resection margins, expressed as median (minimum–maximum) or frequency (percent). 
ICR – ileocaecal resecion; GI – gastrointestinal tract; 5-ASA – 5-aminosalicylates; GSC – glucocorticoids; AZA – azathioprine; MTX – methotrexate; IBD – inflammatory bowel disease. For EPER, ileocolonoscopy was performed 6 months after ICR, and the endoscopic results were evaluated using Rutgeert’s score as shown in Tab. 2. With reference to the primary outcome of the study, histological results showed that inflammation at the resection margins has a significant statistical influence on EPER (56.5% versus 4.8%, p < 0.001; Fig. 1). The histological signs of inflammation in the resection margins were as follows: ileal (I)-positive and colonic (C)-negative in 21.7%; I-negative and C-positive in 13.0%; and I-positive and C-positive in 21.7%. In particular, the risk of EPER was 26-fold higher in patients with positive than in those with negative histology (odds ratio [OR] 26.00, 95% confidence interval [CI] 7.09–95.33) (Tab. 3, 4).
Fig. 1. Influence of histologically inflamed resection margins on endoscopic recurrence. 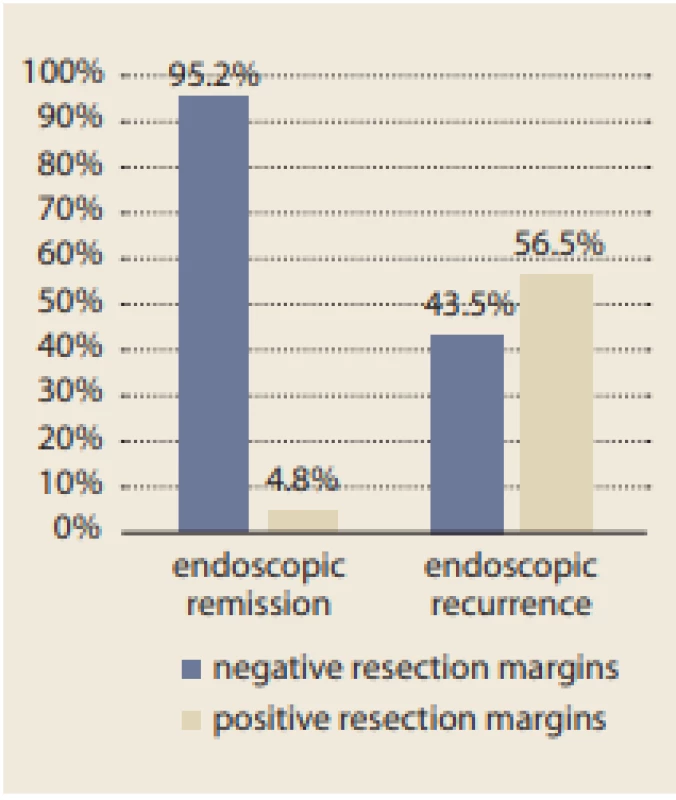
Tab. 2. Assessment of EPER using Rutgeerts score (Ri) 6 months after ICR. 
EPER – early postoperative endoscopic recurrence; ICR – ileocaecal resection. Tab. 3. Influence of the variables on EPER. Results expressed as median (minimum–maximum) or frequency (percent). 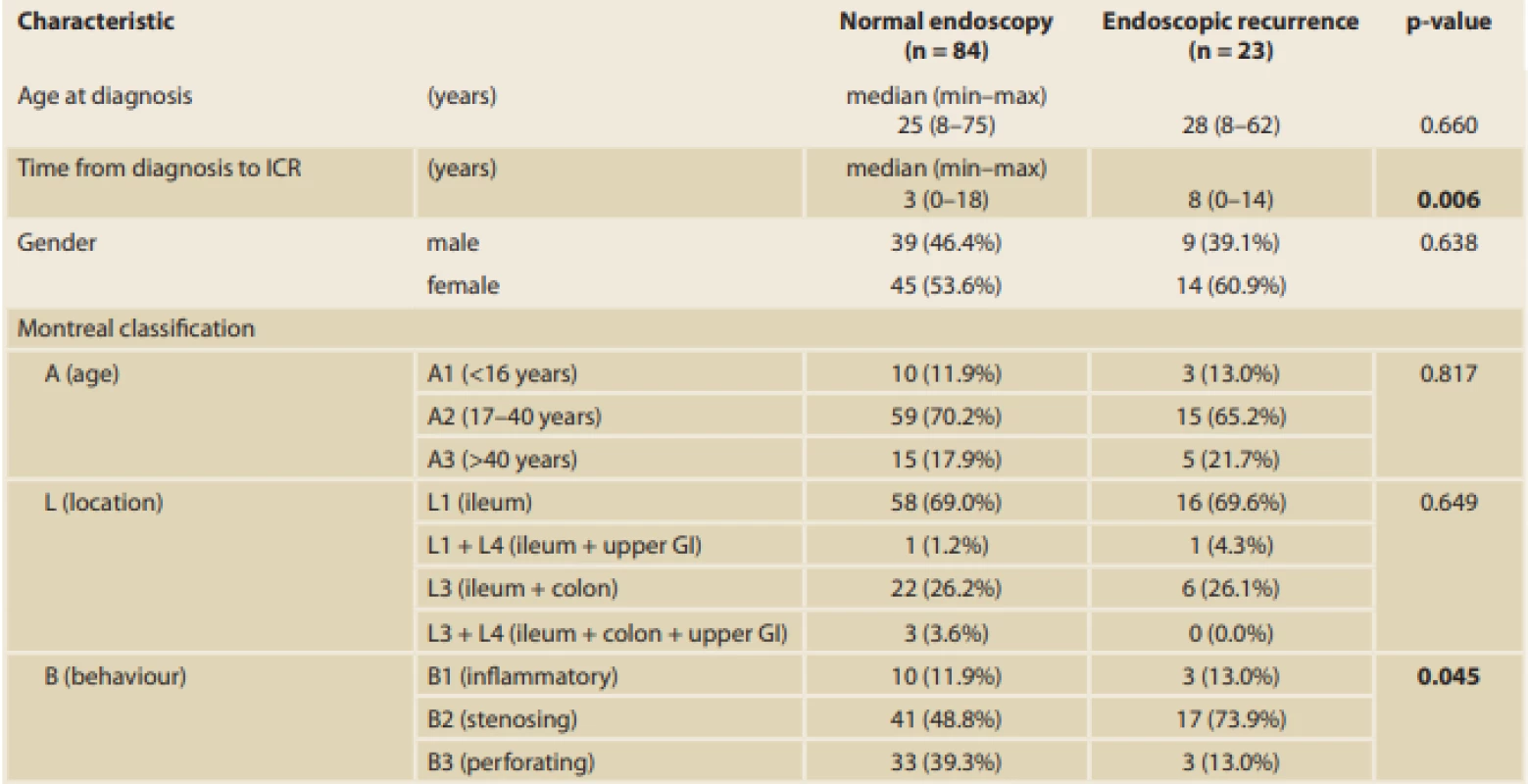
EPER – early postoperative endoscopic recurrence; ICR – ileocaecal resection; GI – gastrointestinal tract; 5-ASA – 5-aminosalicylates; GCS – glucocorticoids; AZA – azathioprine; MTX – methotrexate; IBD – infl ammatory bowel disease Tab. 3 – continuing. Influence of the variables on EPER. Results expressed as median (minimum–maximum) or frequency (percent). 
EPER – early postoperative endoscopic recurrence; ICR – ileocaecal resection; GI – gastrointestinal tract; 5-ASA – 5-aminosalicylates; GCS – glucocorticoids; AZA – azathioprine; MTX – methotrexate; IBD – infl ammatory bowel disease Tab. 4. Associations between endoscopic recurrence of CD and categorical variables (standard logistic regression models, n = 107). 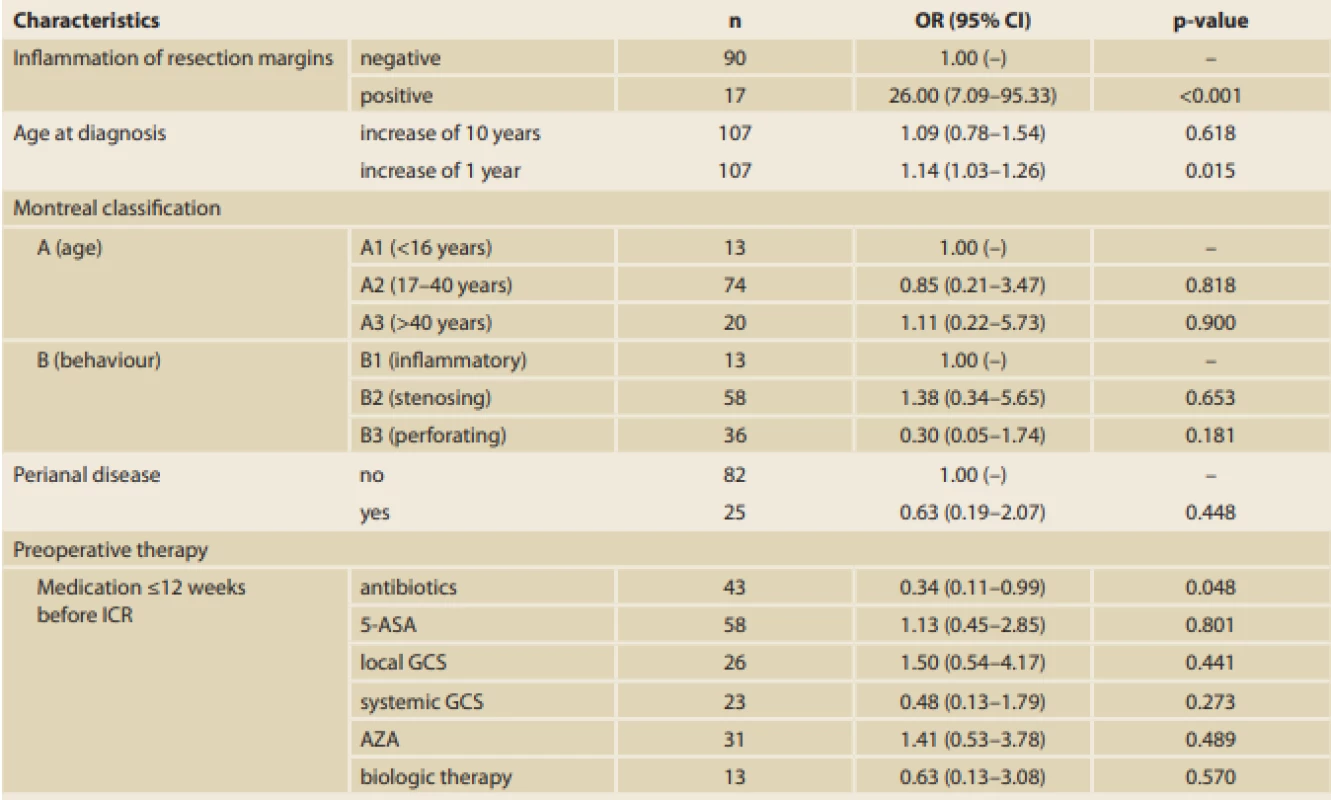
CD – Crohn’s disease; ICR – ileocaecal resection; 5-ASA – 5-aminosalicylates; GCS – glucocorticoids; AZA – azathioprine; MTX – methotrexate; OR – odds ratio; CI – confi dence interval A significant difference in the time from diagnosis of the disease to the ICR between patients with endoscopic recurrence (median 8 years [0–14 years]) and those with endoscopic remission (median 3 years [0–18 years]) (p = 0.006) was found. Thus, the longer the disease duration, the higher the risk of EPER (every year, the risk increases by 14%; OR 1.14, 95% CI 1.03–1.26; p = 0.015). Moreover, a significant difference in terms of smoking, which was identified as a risk factor for endoscopic recurrence, between patients with recurrence and those with remission was found (p = 0.028) (Tab. 3, 4).
With respect to disease phenotype, the number of patients who developed EPER was significantly higher in those with stenosing behaviour (73.9%) than in those with perforating behaviour (13%) (p = 0.045). Perianal involvement was present only in four patients (17.4%) with EPER; however, the results were not significant (p = 0.582) (Tab. 3, 4).
Another significant difference was found in terms of the length of the resected bowel; the longer the resection needed, the more likely EPER should occurr (median 30 cm [5–80 cm] in patients with endoscopic recurrence versus 25 cm [6–70 cm] in patients with endoscopic remission; p = 0.019). On average, patients with EPER required a 7 cm longer resection. With reference to the surgical approach, 66 of 107 (61.7%) procedures were open surgeries and 41 (38.3%) were performed laparoscopically. Of 43 surgeries that were initially started laparoscopically, two had to be converted to open approach (the conversion rate was 4.6%). Postoperative complications showed no statistical influence on EPER (p = 0.333) (Tab. 3).
Furthermore, out of 107 specimens, 36 specimens were positive for granulomas (33.6%). In the group with positive resection margins (n = 17), 10 specimens (58.8%) had granulomas. Moreover, a significant correlation between the presence of granulomas and EPER was found (60.9% versus 26.2%; p = 0.003) (Tab. 1, 3). Regarding preoperative therapy, the use of antibiotics at <12 weeks before the surgery decreased EPER by up to 66% (OR 0.34, 95% CI 0.11–0.99; p = 0.048) (Tab. 3, 4).
According to the multivariate analysis results, positive resection margins have the greatest influence on EPER (OR 26.46, 95% CI 6.42–109.06; p < 0.001). A statistically significant effect of disease duration (OR 1.20, 95% CI 1.02–1.41; p = 0.031) and stenosing disease behaviour (OR 0.08, 95% CI 0.01–0.87; p = 0.038) was also found (Tab. 5). The groups were comparable in the other analysed variables (demographic characteristics, other surgical characteristics, and preoperative therapy, with the exception of use of antibiotics and family history of IBD).
Tab. 5. Multidimensional logistic regression model (n = 107) of the relationship between selected variables and recurrence of Crohn’s disease. 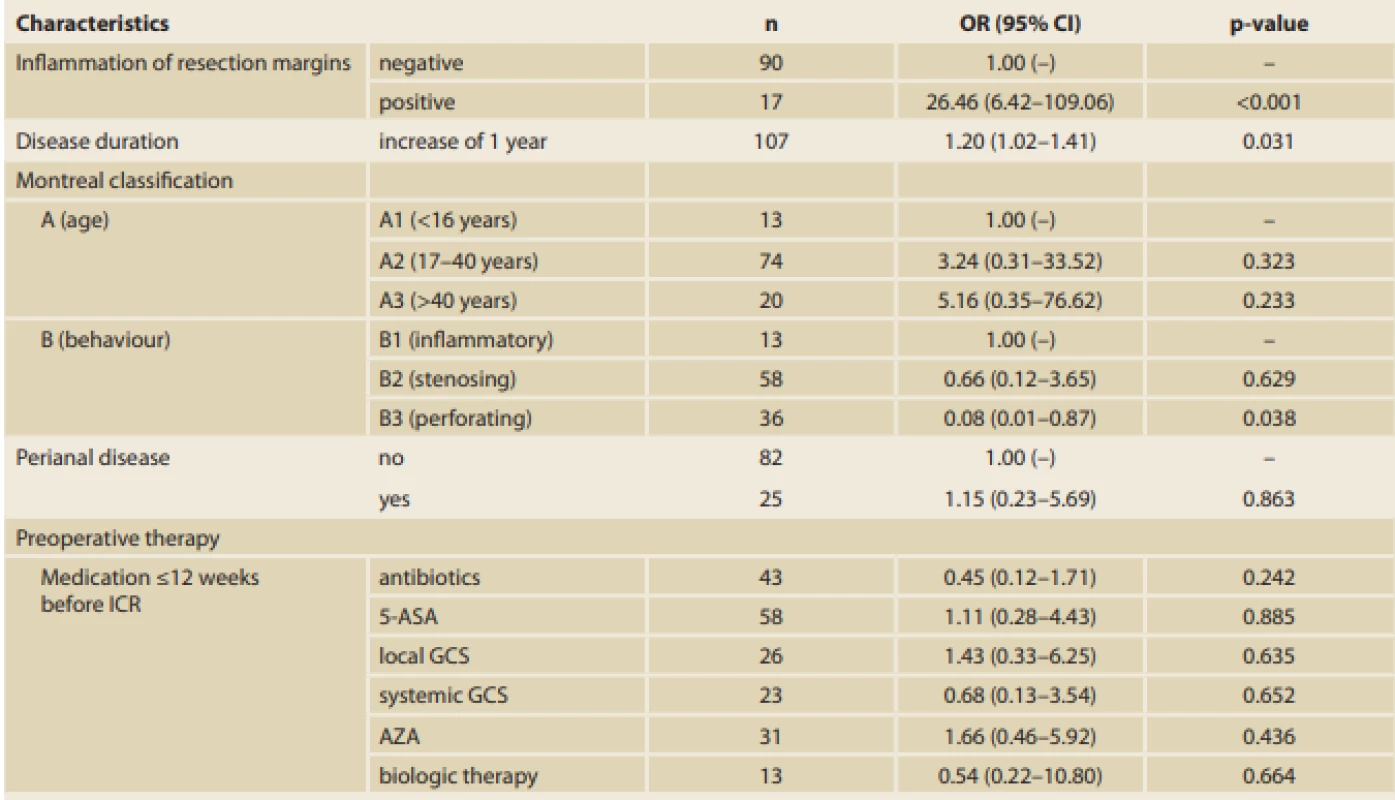
CD – Crohn’s disease; ICR – ileocaecal resection; 5-ASA – 5-aminosalicylates; GCS – glucocorticoids; AZA – azathioprine; MTX – methotrexate; OR – odds ratio; CI – confi dence interval Discussion
Disease recurrence after surgical resection in CD has been studied since the end of the 20th century [13]. The incidence of postoperative recurrence differs considerably in the literature and ranges from 10% to 90%, depending on the used definition of recurrence (i.e. clinical, endoscopic, radiological, or surgical recurrence), study designs, and postoperative therapy [2,3,14,15]. In our cohort, we have investigated EPER in patients without any postoperative therapy. EPER occurred in 21.5% of patients in our study.
It has been proven that endoscopic recurrence both precedes and predicts clinical relapse [3,4]. Currently, postoperative ileocolonoscopy within the first year following surgery is recommended [4,5]. We performed ileocolonoscopies 6 months after ICR, and the findings were assessed according to Rutgeert’s score.
A diagnosis of EPER seems to be crucial for further postoperative management to prevent any flare-up of the disease [16]. According to the current guidelines, the risk factors used as predictors of postoperative recurrence include smoking, previous intestinal surgery, absence of prophylactic treatment, penetrating disease at index surgery, perianal location, granulomas in the resection specimen, and myenteric plexitis.4 Smoking appears to be the only consistently reported risk factor that increases the risk of postoperative recurrence by 2.5-fold [4,16,17]. In our study, we have also confirmed that smoking is a risk factor (among non-smoking patients, 52.4% had endoscopic remission and 21.7% had endoscopic recurrence; p = 0.028). Nevertheless, a clear identification of patients at a higher risk of EPER, who would benefit from more aggressive and financially demanding therapy, remains a clinical challenge, as no standard for postoperative treatment exists [1,8–10].
The primary aim of our study was to identify whether thistological inflammation at the resection margins after ICR could be considered a significant risk factor for EPER. In the literature, data concerning this assumption are inconsistent [4]. The relevant studies, mainly published in the late 1990s, reported no relationship between the microscopic involvement of the resection margins and postoperative recurrence [18–21]. However, the frequently cited study of Fazio et al,19 who conducted a randomised controlled trial of 152 patients, showed that microscopic CD at the resection margins is correlated with surgical recurrence in the long-term follow-up (up to 96 months). The types of operation in their study population included not only primary ileocolic resection (49%) but also secondary resection of ileocolic anastomosis (46%), as well as other types. The patients did not receive any postoperative immunoprophylaxis [19]. In our cohort, we have specifically assessed endoscopic recurrence after 6 months only in patients who had primary ICR. Consistent with the study of Fazio et al, our patients did not receive any postoperative therapy before ileocolonoscopy. Furthermore, recent studies have confirmed that histological inflammation at the resection margin is associated with a higher risk of postoperative recurrence [1,6,7]. The retrospective study of Bobanga et al, including 142 patients, investigated the different aspects of postoperative recurrence after ileocaecal resection between adolescents and adults; multivariate analysis showed that positive resection margins are predictive of endoscopic recurrence in the adult group (p = 0.007) [1]. Concerning the histological assessment of the resection margins, we have always evaluated both the ileal and the colonic margins. On the other hand, previous studies have either assessed only the proximal margin or the margins have not been further specified [1,6,19]. Disease duration as a risk factor is not uniformly accepted [5,8,9]. However, our results imply that the longer the disease duration, the higher the risk of EPER. The longer duration could be explained by the more conservative therapeutic approach at our hospital, which would in turn lead to more complicated cases with a higher EPER incidence.
In relation to the length of the resected bowel, current data are also equivocal. Our results showed that the patients with EPER needed, on average, a 7 cm longer resection, which could be attributed to disease severity. Nonetheless, several studies reported that the length of the resected bowel does not significantly affect EPER [1,9,22]. Moreover, other variables associated with surgery (surgical approach, type of anastomosis, postoperative complications) did not show any significant effect on EPER. The guidelines state that perforating disease is an independent risk factor (level 2 evidence) [5]. However, conflicting data with regard to the early recurrence of perforating disease exist based on the meta-analysis by Similils et al [23]. Similarly, our data showed that the number of patients who developed EPER was fewer among those with a perforating behaviour of the disease than in those with a stenosing disease (p = 0.045).
Perianal disease is also a risk factor according to the guidelines [5]; nevertheless, the cited studies in the guidelines included patients who had different types of surgeries (not only ICR), presumably because of a more extensive type of the disease. Moreover, patient follow-up after surgery for perianal disease is much longer (up to years), which could be attributed to a cumulative frequency of perianal disease [4]. This could explain why our data did not confirm perianal disease as a risk factor for EPER (p = 0.582) as our cohort included only EPER assessed 6 months after the ICR.
The current guidelines also reported on histological features, such as presence of granulomas and myenteric plexitis, as risk factors for EPER [5]. In our study, we have confirmed that granulomas in the specimens are significantly correlated with EPER (p = 0.003). Myenteric plexitis has been reported as a risk factor only recently in the guidelines (level 3 evidence) [5,24]. During the initiation of our study in 2012, myenteric plexitis was not widely accepted as a risk factor; thus, this histological sign was not evaluated by our pathologist. We have included this as a limitation of our study.
As previously mentioned, preoperative therapy with antibiotics (e. g. metronidazole) administered <12 weeks before the surgery decreases the risk of EPER. However, in a multidimensional analysis, the results were not statistically significant and have to be further verified. In the literature, only the possible protective effect of the postoperative antibiotic therapy was discussed [25,26].
According to the multivariate analysis, positive resection margins have the greatest influence on EPER. A statistically significant effect of disease duration and stenosing disease behaviour (as discussed previously) was also found. However, with regard to the small number of patients and presentation of characteristics in our cohort, such a multivariate analysis is quite unstable, which could be seen, for example, in the 95% CI for OR of the resection margins.
Our study has some limitations. The group of patients in our cohort was specifically chosen (patients on prophylactic therapy or who had different types of surgery were excluded), which could in turn decrease the generalisability of the study. Moreover, the patients were followed up 6 months after ICR only, and we have not included myenteric plexitis as a risk factor in this study.
In conclusion, in our prospective study of 107 patients, we have shown that microscopic inflammation at the resection margins after ICR is associated with EPER. Although validation of this finding by larger studies is needed, our results may contribute to the identification of patients with a high risk of EPER, who could benefit from a more aggressive and targeted postoperative therapy. Consensus on a clear and definite recommendation remains to be reached, to identify the best practice for these patients.
Funding
This work was supported by the Ministry of Health, Czech Republic – conceptual development of research organisation (FNBr 65269705 – Sup 16/ 19).
Conflict of Interest
All authors declare that they have no conflicts of interest.
Author Contributions
KP: study concept and design, analysis and interpretation of data, writing, literature review; LK: concept and design of the study, review of data and the manuscript, consultant; FM: consultant, concept and design of the study, literature review; ZK: consultant, review of the manuscript; VP: consultant, review of data, concept and design of the study, literature review; JD: consultant, review of the manuscript; VZ: consultant, review of the data and the manuscript; PK: statistical analysis; TP: statistical analysis; PJ: consultant, review of the manuscript; ZP: histological analysis; JV: histological analysis; LM: consultant, literature review. All authors have made scientific contribution to the study design and discussion and have read and approved the final version of the manuscript. Moreover, all authors had full access to all the data in this study and had final responsibility for the decision to submit for publication.
Lumir Kunovsky, MD, PhD
Department of Surgery
University Hospital Brno
Faculty of Medicine, Masaryk University
Jihlavská 20
625 00 Brno
Czech Republic
Acknowledgement
Our thanks go to the Journal of Crohn’s and Colitis for their kind consent to publish this article.
Original source: Journal of Crohn‘s and Colitis 2020; 14(3): 361–368. doi: 10.1093/ ecco-jcc/ jjz153
Zdroje
1. Bobanga ID, Bai S, Swanson MA et al. Factors influencing disease recurrence after ileocolic resection in adult and pediatric onset Crohn’s disease. Am J Surg 2014; 208(4): 591–596. doi: 10.1016/ j.amjsurg.2014.06.008.
2. De Cruz P, Kamm MA, Prideaux L et al. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis 2012; 18(4): 758–777. doi: 10.1002/ ibd.21825.
3. Rutgeerts P, Geboes K, Vantrappen G et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99(4): 956–963. doi: 10.1016/ 0016-5085(90)90613-6.
4. Gionchetti P, Dignass A, Danese S et al. ECCO. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 2: surgical management and special situations. J Crohns Colitis 2017; 11(2): 135–149. doi: 10.1093/ ecco-jcc/ jjw169.
5. Bemelman WA, Warusavitarne J, Sampietro GM et al. ECCO-ESCP consensus on surgery for Crohn’s disease. J Crohns Colitis 2018; 12(1): 1–16. doi: 10.1093/ ecco-jcc/ jjx061.
6. Hay I, Lynch L, Saffouri E et al. AODTU-010 ileal inflammation at the resection margin is associated with an increased risk of recurrence of post-operative ileal Crohn’s disease over a 10-year follow up. Gut 2017; 66: A50. doi: 10.1136/ gutjnl-2017-314472.95.
7. Amesfoort J, Koens L, Bemelman W et al. The prognostic impact of radical resection margins on the recurrence of Crohn’s disease. J Crohns Colitis 2017; 11(Suppl 1): S412–S413. doi: 10.1093/ ecco-jcc/ jjx002.774.
8. Yamamoto T, Watanabe T. Strategies for the prevention of postoperative recurrence of Crohn’s disease. Colorectal Dis 2013; 15(12): 1471–1480. doi: 10.1111/ codi.12326.
9. Fornaro R, Caratto E, Caratto M et al. Post-operative recurrence in Crohn’s disease. Critical analysis of potential risk factors. An update. Surgeon 2015; 13(6): 330–347. doi: 10.1016/ j.surge.2015.04.002.
10. Bordeianou L, Stein SL, Ho VP et al. Immediate versus tailored prophylaxis to prevent symptomatic recurrences after surgery for ileocecal Crohn’s disease? Surgery 2011; 149(1): 72–78. doi: 10.1016/ j.surg.2010.03.009.
11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240(2): 205–213. doi: 10.1097/ 01.sla.0000133083.54934.ae.
12. Sturm A, Maaser C, Calabrese E et al. European Crohn’s and Colitis Organisation (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR). ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 2019; 13(3): 273–284. doi: 10.1093/ ecco-jcc/ jjy114.
13. Rutgeerts P, Geboes K, Vantrappen G et al. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut 1984; 25(6): 665–672. doi: 10.1136/ gut. 25.6.665.
14. De Cruz P, Kamm MA, Hamilton AL et al. Efficacy of thiopurines and adalimumab in preventing Crohn’s disease recurrence in high-risk patients – a POCER study analysis. Aliment Pharmacol Ther 2015; 42(7): 867–879. doi: 10.1111/ apt.13353.
15. Singh S, Garg SK, Pardi DS et al. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology 2015; 148(1): 64–76.e2. doi: 10.1053/ j.gastro.2014.09.031.
16. De Cruz P, Kamm MA, Hamilton AL et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet 2015; 385(9976): 1406–1417. doi: 10.1016/ S0140-6736(14)61908-5.
17. Yamamoto T, Keighley MR. The association of cigarette smoking with a high risk of recurrence after ileocolonic resection for ileocecal Crohn’s disease. Surg Today 1999; 29(6): 579–580. doi: 10.1007/ BF02482360.
18. Botti F, Carrara A, Antonelli B et al. The minimal bowel resection in Crohn’s disease: analysis of prognostic factors on the surgical recurrence. Ann Ital Chir 2003; 74(6): 627–633.
19. Fazio VW, Marchetti F, Church M et al. Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. A randomized controlled trial. Ann Surg 1996; 224(4): 563–571: discussion 571–573. doi: 10.1097/ 000 00658-199610000-00014.
20. Fazio VW, Marchetti F. Recurrent Crohn’s disease and resection margins: bigger is not better. Adv Surg 1999; 32 : 135–168.
21. Kotanagi H, Kramer K, Fazio VW et al. Do microscopic abnormalities at resection margins correlate with increased anastomotic recurrence in Crohn’s disease? Retrospective analysis of 100 cases. Dis Colon Rectum 1991; 34(10): 909–916. doi: 10.1007/ BF02049707.
22. Cunningham MF, Docherty NG, Coffey JC et al. Postsurgical recurrence of ileal Crohn’s disease: an update on risk factors and intervention points to a central role for impaired host-microflora homeostasis. World J Surg 2010; 34(7): 1615–1626. doi: 10.1007/ s00268-010-0504-6.
23. Simillis C, Yamamoto T, Reese GE et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol 2008; 103(1): 196–205. doi: 10.1111/ j.1572-0241.2007.01548.x.
24. Lemmens B, de Buck van Overstraeten A, Arijs I et al. Submucosal plexitis as a predictive factor for postoperative endoscopic recurrence in patients with Crohn’s disease undergoing a resection with ileocolonic anastomosis: results from a prospective single-centre study. J Crohns Colitis 2017; 11(2): 212–220. doi: 10.1093/ ecco-jcc/ jjw135.
25. Rutgeerts P, Van Assche G, Vermeire S et al. Ornidazole for prophylaxis of postoperative Crohn’s disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2005; 128(4): 856–861. doi: 10.1053/ j.gastro.2005.01.010.
26. Doherty G, Bennett G, Patil S et al. Interventions for prevention of post-operative recurrence of Crohn’s disease. Cochrane Database Syst Rev 2009; 4: CD006873. doi: 10.1002/ 1465 1858.CD006873.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecná
Článek Kvíz z klinické praxeČlánek Editorial
Článok vyšiel v časopiseGastroenterologie a hepatologie
Najčítanejšie tento týždeň
2021 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
-
Všetky články tohto čísla
- Dát či nedat vakcínu proti covid-19 pacientům s IBD?
- Kvíz z klinické praxe
- Idiopatické střevní záněty mají negativní dopad na reprodukční chování pacientů: první multicentrický průzkum v České republice
- Editorial
- Obezita a idiopatické střevní záněty
- The influence of microscopic inflammation at resection margins on early postoperative endoscopic recurrence after ileocaecal re-section for Crohn’s disease
- Komentář ke článku „The influence of microscopic in-flammation at resection margins on early postoperative endoscopic recurrence after ileocaecal resection for Crohn’s disease“ (Journal Crohn’s Colitis 2020; 14(3): 361–368
- Psychologické aspekty a možnosti psychologických intervencií u pacientov s nešpecifickými zápalovými ochoreniami čreva
- Posterior reversible encephalopathy syndrom u dítěte s ulcerózní kolitidou
- Rifaximin v terapii idiopatických střevních zánětů
- Vzácná příčina mnohočetných vředů tenkého střeva
- (Ne)tradičně komplikovaná pankreatitida
- Neuroendokrinní tumor periapendikulární oblasti dna céka – kazuistika
- Inhibitory protonové pumpy – známe je dobře? Jsou skutečně tak bezpečné? – část 3
- Výběr z mezinárodních časopisů
- Správná odpověď na kvíz Diferenciální diagnóza terminální ileitidy: Meckelův divertikl v atypické lokalizaci
- Gastroenterologie a hepatologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Vzácná příčina mnohočetných vředů tenkého střeva
- Psychologické aspekty a možnosti psychologických intervencií u pacientov s nešpecifickými zápalovými ochoreniami čreva
- Inhibitory protonové pumpy – známe je dobře? Jsou skutečně tak bezpečné? – část 3
- Neuroendokrinní tumor periapendikulární oblasti dna céka – kazuistika
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



