-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
preLekára.sk / Odborné časopisy / Česká a slovenská neurologie a neurochirurgie / 2017 - Online onlyCase of Adult Escherichia Coli Meningitis
Kazuistika meningitidy dospělých způsobená bakterií Escherichia coli
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: Ch. Chang-Hua 1; Y. Hua-Cheng 2; L. Li-Jhen 3
Authors place of work: Division of Infectious Disease, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan 1; Department of Neurosurgery, Changhua Christian Hospital, Changhua, Taiwan 2; Infection Control Committee, Changhua Christian Hospital, Changhua, Taiwan 3
Published in the journal: Cesk Slov Neurol N 2017; 80/113(Online only): 0
Category: č. 5
Summary
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Dear editors,
bacterial meningitis causes potentially life-threatening neurological complications [1]. In contrast to Streptococcus pneumoniae and Neisseria meningitis [2], Escherichia coli (E. coli) rarely causes adult meningitis, accounting for approximately 1% of meningitis cases [2,3], although this disease is a healthcare-associated infection and occurs especially aftertraumatic craniocerebral injury or as a neurosurgical procedure complication [4]. Furthermore, E. coli meningitis (EcM) patients have higher mortality rate than those with other bacterial meningitis [3,5]. However, patients admitted to long-term care facilities (LTFCs) almost always have preexisting risk factors to EcM (e.g. advanced age, cancer or diabetes mellitus history). Furthermore, their disease typically occurs secondary to a distant or contiguous infection focus (e.g. urinary tract or gastrointestinal infection or otitis media) [3,5]. However, reported cases of adult EcM in LTFCs are limited.
We describe a case of a 49-year-old man admitted at a LTCF who previously suffered from cerebral vascular accident with multiple infarction haemorrhages over the right cerebellum and respiratory failure. He had hydrocephalus and neurogenic bladder and underwent ventriculoperitoneal (VP) shunting and cystostomy, respectively, as treatments 6 years ago. He was admitted to our institute because of changes in consciousness and low blood pressure. Initially, he had a body temperature of 38.6 °C, pulse rate of 132/min and blood pressure of 80/ 50 mm Hg; his respiratory rate was equivalent to the ventilator rate. Neurological examination was difficult to perform but no significant abnormalities (e.g., meningeal signs) or new neurological deficits were observed. Physical examination of the respiratory system revealed coarse breathing sounds. Laboratory results on the 1st admission day showed markedly increased white blood cell (WBC) count (12,200/ μL), C-reactive protein (7.65 mg/ dL), and serum glucose levels (160 mg/ dL). No definite new and active lung lesion was observed on the initial chest imaging. However, brain computed tomography (CT) displayed one left prefrontal ovoid lesion (size 31 mm). Hence, an immediate lumbar puncture was conducted. Cerebral spinal fluid examination revealed WBC, protein, and glucose levels of 1,120/ cm3, 192 mg/dL (normal range, 15–45 mg/dL), and 2 mg/dL (normal range, 40–70 mg/dL), respectively. A central nervous system infection was suspected; antimicrobial treatment with ceftazidime (2 g intravenous (IV) every 8 hours) and vancomycin (500 mg IV every 6 hours) was started. On the 2nd admission day, E. coli was identified from blood and CSF specimens using matrix-assisted laser desorption/ ionization - time-of-flight mass spectrometry (bioMérieux, Hazelwood, MO). An antimicrobial drug susceptibility test [6] was conducted through the bioMérieux VITEK 2 system (bioMérieux). The minimal inhibition concentration levels were listed at Tab. 1 (Supplementary Tab. 1 online). After pathogen identification, we performed additional diagnostics to identify the primary infection focus. Although the patient was vegetarian, his past medical history showed absence of significant predisposing factors (e.g., previous EcM), except for craniotomy and VP shunting. Furthermore, chest X-ray revealed old lesions, and abdominal lesions were detected during the brain CT conducted at admission. However, these results did not reveal the primary focus. Thus, gallium scan was initially performed, followed by pelvis CT. The CT findings indicated abscess formation in the left psoas muscle and pericecal region that was complicated with fluid collection around the left abdominal wall and the VP shunt catheter tip. Thus, emergent operation was performed to aspirate the psoas muscle abscess under sonographic guidance. Furthermore, abdominal wall abscess debridement and VP shunt removal were conducted. The abdominal abscess culture showed polymicrobes (e.g., E. coli, Klebsiella pneumoniae, and Candida albicans). The antimicrobial regimen was changed from ceftazidime and vancomycin to imipenem - cilastatin (250 mg IV every 8 hours) and fluconazole (200 mg IV daily). However, imipenem - cilastatin was replaced with meropenem (2 m IV every 8 hours) because of a tonic-clonic seizure attack after 5 days of medication administration. Despite the treatment, the patient’s condition deteriorated. Specifically, he required mechanical ventilation that resulted in ventilator-associated pneum-onia. His general condition continued to worsen and this had prompted his family to request discharge due to medical futility.
Tab. 1. Evidence-based literature review of risk factors and management of adult E. coli meningitis. 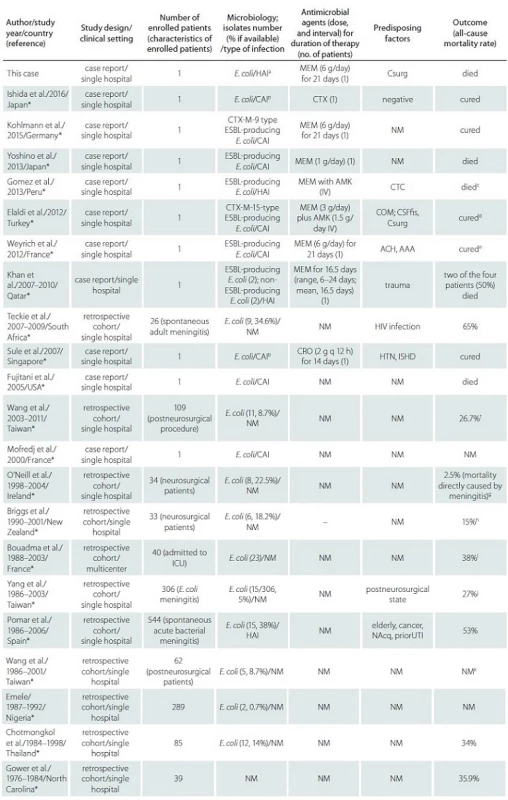
AAA – aortic mycotic aneurysms; Abx – antibiotics; ACH – alcoholism; AMK – amikacin; CAI – community-associated infection; CIP – cipro-floxacin; COM – chronic otitis media; CRO – ceftriaxone; CSFfi s – cerebrospinal fluid fistula; Csurg – cranial surgery; CTC – corticosteroids; CTX – cefotaxime; ESBL – extended-spectrum β-lactamase; HAI – healthcare-associated infection; HTN – hypertension; ICU – intensive care unit; ISHD – ischemic heart disease; MEM – meropenem; NAcq – nosocomial acquisition; NM – not mentioned; priorUTI – urinary tract infection as distant focus of infection; trauma, accidental and neurosurgical trauma of the head and spine. a Although the CSF culture was negative in our patient, E. coli was detected in his blood and urine cultures. b Although CSF culture was negative, we diagnosed and treated the condition as E. coli meningitis based on the results of the CSF analysis and positive blood culture. c Principal conclusion from this reference: Strongyloides stercoralis infection should be excluded in adults with spontaneous E. coli meningitis, especially if the patient experienced gastrointestinal symptoms and had a history of traveling to an endemic area. d Principal conclusion from this reference: Empiric antibiotic therapy with carbapenem can be started before the culture results are obtained,primarily in areas where ESBL epidemiology is well known. e Meningitis was cured, but the patient died during an operation. f Principal conclusion from this reference: IV antibiotic therapy is a useful treatment for postneurosurgical Gram-negative bacillary meningitis or ventriculitis. g Principal conclusion from this reference: The median duration of treatment was 19.2 days, and 20% of cases were caused by organism resistant to the third-generation cephalosporins. h Principal conclusion from this reference: Recommended initial treatment was IV ceftriaxone and amikacin. Treatment for at least 14 days afterthe last positive CSF culture guaranteed cure. i Principal conclusion from this reference: Five patients had strongyloidiasis, and 40% of E. coli were resistant to aminopenicillins. j Principal conclusion from this reference: Diabetes mellitus and post-neurosurgical status were the common predisposing factors. E. coli meningitis that are not susceptible to third-generation cephalosporin have emerged since 2001.Four patients who were not administered with appropriate antibiotic treatment died, and the other 11 patients were given appropriate antibiotic treatment. k Principal conclusion from this reference: Nine out of 62 organisms were resistant to third-generation cephalosporins. Increase incidence of oxacillin-resistant Staphylococcus infection was observed in patients with postneurosurgical nosocomial meningitis. * Complete list of references online only. We report the first case of an adult EcM from a LTCF in Changhua County. Some studies on healthcare-associated meningitis reported that patients are particularly prone to this disease because of subsequent microorganism penetration owing to traumatic craniocerebral injury or neurosurgical procedures [2,4,5]. In our patient, there was an evidence of possible healthcare-associated meningitis, in which E. coli originated from the psoas muscle abscesses and was transmitted via the VP shunt. Considering that adult EcM is rarely suggested as a cause of a central nervous system infection, we conducted an evidence-based literature review. Worldwide, 112 adult patients with EcM were reported (Supplementary Tab. 2 online). The crude mortality rate ranged from 65% [7] to 2.5% [8]. The majority of EcM cases was observed in patients with predisposing risk factors, such as advanced age [5], cancer history [5], nosocomial acquisition [5], cerebrospinal fluid fistula [9], cranial surgery [9], chronic otitis media [9], and trauma [10]. However, our patient did not display any of these risk factors except from craniectomy and VP shunt insertion 7 years ago. Moreover, approximately 75% of the cases [3,4] occurred secondary to a distant or contiguous infection (e.g. urinary tract infection). Thus, the diagnostics in this case were extended after pathogen identification to identify the primary infection focus, resulting in the diagnosis of previously unrecognized psoas muscles abscesses. Consequently, our report emphasizes the importance of searching for the source of adult EcM, especially if no typical risk factors are identified.
EcM in adult patients is a rare and fatal disease that usually has risk factors. Performing an extended diagnostic examination to exclude underlying diseases and possible precedent infections is advised if no typical risk factors or the original focus are identified.
Supplementary Tab. 1. Susceptibilities of this isolate to the antimicrobial agents. 
Notes: * The Clinical and Laboratory Standards Institute minimum inhibitory concentration (MIC) breakpoints for Enterobacteriaceae were applied for all antimicrobial agents except for flomoxef and tigecycline Reference: Clinical and Laboratory Standards Institute. 2016. M100-S26: performance standards for antimicrobial susceptibility testing, 26th informational supplement. CLSI, Wayne, PA. ** Interpretation of tigecycline MIC results was determined according to the recommendations of the US Food and Drug Administration given in the package insert for treating Enterobacteriaceae (susceptible ≤ 2 μg/mL; resistant, ≥ 8 g/ml) I – intermediate; MIC – minimal inhibitory concentration; S – susceptible; R – resistant. Supplementary Tab. 2. Evidence-based literature review of risk factors and management of adult E. coli meningitis. We conducted an evidence-based literature review using the keywords “Escherichia coli,” “meningitis,” and “central nervous system infection.” Only adults with E. coli meningitis were enrolled in this Table. 
Abbreviations: AAA – aortic mycotic aneurysms; Abx – antibiotics; ACH – alcoholism; AMK – amikacin; CAI – community-associated infection; CIP – ciprofloxacin; COM – chronic otitis media; CRO – ceftriaxone; CSFfis – cerebrospinal fluid fistula; Csurg – cranial surgery; CTC – corticosteroids; CTX – cefotaxime; ESBL – extended-spectrum β-lactamase; HAI – healthcare-associated infection; HTN – hypertension; ICU – intensive care unit; ISHD – ischemic heart disease; IV – intravenous; MEM – meropenem; NAcq – nosocomial acquisition; NM – not mentioned; priorUTI – urinary tract infection as distant focus of infection; trauma, accidental and neurosurgical trauma of the head and spine. a Although the CSF culture was negative in our patient, E. coli was detected in his blood and urine cultures. b Although CSF culture was negative, we diagnosed and treated the condition as E. coli meningitis based on the results of the CSF analysis and positive blood culture. c principal conclusion from this reference: Strongyloides stercoralis infection should be excluded in adults with spontaneous E. coli meningitis, especially if the patient experienced gastrointestinal symptoms and had a history of traveling to an endemic area. d principal conclusion from this reference: Empiric antibiotic therapy with carbapenem can be started before the culture results are obtained, primarily in areas where ESBL epidemiology is well known. e Meningitis was cured, but the patient died during an operation. f principal conclusion from this reference: IV antibiotic therapy is a useful treatment for postneurosurgical Gram-negative bacillary meningitis or ventriculitis. principal conclusion from this reference: The median duration of treatment was 19.2 days, and 20% of cases were caused by organism resistant to the third-generation cephalosporins. h principal conclusion from this reference: Recommended initial treatment was IV ceftriaxone and amikacin. Treatment for at least 14 days after the last positive CSF culture guaranteed cure. i principal conclusion from this reference: Five patients had strongyloidiasis, and 40% of E. coli were resistant to aminopenicillins. j principal conclusion from this reference: Diabetes mellitus and post-neurosurgical status were the common predisposing factors. E. coli meningitis that are not susceptible to third-generation cephalosporin have emerged since 2001. Four patients who were not administered with appropriate antibiotic treatment died, and the other 11 patients were given appropriate antibiotic treatment. k principal conclusion from this reference: Nine out of 62 organisms were resistant to third-generation cephalosporins. Increase incidence of oxacillin-resistant Staphylococcus infection was observed in patients with postneurosurgical nosocomial meningitis Acknowledgements
The authors thank the Changhua Christian Hospital for their kind gift of clinical Escherichia coli.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Chen Chang-Hua, MD
Division of Infectious Disease
Department of Internal Medicine
Changhua Christian Hospital
Nanhsiao Street
Changhua 500
Taiwan
e-mail: 76590@cch.org.tw
Accepted for review: 21. 2. 2017
Accepted for print: 11. 5. 2017
Zdroje
1. Barshak MB, Kasper DL. Intraabdominal Infections and Abscesses, In: Kasper DL, et al. Harrison's Principles of Internal Medicine. New York: McGraw-Hill Education 2015.
2. van de Beek D, de Gans J, Spanjaard L et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351(18):1849–59.
3. Bouadma L, Schortgen F, Thomas R et al. Adults with spontaneous aerobic Gram-negative bacillary meningitis admitted to the intensive care unit. Clin Microbiol Infect 2006;12(3):287–90.
4. van de Beek D, Brouwer MC, Thwaites GE et al. Advances in treatment of bacterial meningitis. Lancet 2012;380(9854):1693–1702. doi: 10.1016/S0140-6736(12)61186–6.
5. Pomar V, Benito N, López-Contreras J et al. Spontaneous gram-negative bacillary meningitis in adult patients: characteristics and outcome. BMC Infect Dis 2013;13 : 451. doi: 10.1186/1471-2334-13-451.
6. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-sixth Informational Supplement M100-S26. CLSI, Wayne: Clinical and Laboratory Standards Institute 2016.
7. Teckie G, Karstaedt A. Spontaneous adult Gram-negative bacillary meningitis in Soweto, South Africa. Int J Infect Dis 2015;30 : 38–40. doi: 10.1016/j.ijid.2014.10.006.
8. O'Neill E, Humphreys H, Phillips J et al. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother 2006;57(2):356–9.
9. Elaldi N, Gozel MG, Kolayli F et al. Community-acquired CTX-M-15-type ESBL-producing Escherichia coli meningitis: a case report and literature review. J Infect Dev Ctries 2013;7(5):424–31. doi: 10.3855/jidc.2820.
10. Khan FY, Abukhattab M, Anand D. Nosocomial Escherichia coli meningitis in adults: Report of four cases and literature review. J Neurosci Rural Pract 2013;4(3):349–51. doi: 10.4103/0976-3147.118800.
11. Ishida K, Noborio M, Nakamura M, et al. Spontaneous Escherichia coli bacterial meningitis mimicking heatstroke in an adult. Clinical Case Reports 2016 ;15;4(4):323-6
12. Kohlmann R, Nefedev A, Kaase M, et al. Community-acquired adult Escherichia coli meningitis leading to diagnosis of unrecognized retropharyngeal abscess and cervical spondylodiscitis: a case report. BMC infectious diseases 2015 12;15 : 567.
13. Yoshino Y, Seo K, Koga I, et al. A case of community-onset bacterial meningitis due to extended-spectrum beta-lactamase producing escherichia coli. Journal of Medical Cases 2013;4(7):511–4.
14. Gomez JB, Maque Y, Moquillaza MA, et al. E. coli Meningitis Presenting in a Patient with Disseminated Strongyloides stercoralis. Case Reports in Infectious Diseases 2013;2013 : 424362.
15. Elaldi N1 Gozel MG, Kolayli F, et al. Community-acquired CTX-M-15-type ESBL-producing Escherichia coli meningitis: a case report and literature review. J Infect Dev Ctries 2013;7(5):424–31.
16. Weyrich P, Ettahar N, Legout L, et al. First initial community-acquired meningitis due to extended-spectrum beta-lactamase producing Escherichia coli complicated with multiple aortic mycotic aneurysms. Ann Clin Microbiol Antimicrob 2012;11 : 4.
17. Khan FY, Abukhattab M, Anand D. Nosocomial Escherichia coli meningitis in adults: Report of four cases and literature review. J Neurosci Rural Pract 2013;4(3):349–51.
18. Teckie G, Karstaedt A. Spontaneous adult Gram-negative bacillary meningitis in Soweto, South Africa. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases 2015;30 : 38–40.
19. Sule AA, Tai DY. Spontaneous Escherichia Coli meningitis in an adult. Crit Care & Shock 2007;10 : 148–50.
20. Fujitani S, Kamiya T, Kaufman L. Community-acquired Escherichia coli meningitis in adult. Hawaii Med J 2005;64(5):118–20.
21. Wang JH, Lin PC, Chou CH, et al. Intraventricular antimicrobial therapy in postneurosurgical Gram-negative bacillary meningitis or ventriculitis: a hospital-based retrospective study. Journal of microbiology, immunology, and infection 2014;47(3):204–10.
22. Mofredj A, Guerin JM, Leibinger F, et al. Spontaneous Escherichia coli meningitis in an adult. Scandinavian journal of infectious diseases 2000;32(6):699–700.
23. O'Neill E, Humphreys H, Phillips J, et al. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. The Journal of antimicrobial chemotherapy 2006;57(2):356–9.
24. Briggs S, Ellis-Pegler R, Raymond N, et al. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scandinavian journal of infectious diseases 2004;36(3):165–73.
25. Bouadma L, Schortgen F, Thomas R, et al. Adults with spontaneous aerobic Gram-negative bacillary meningitis admitted to the intensive care unit. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2006;12(3):287–90.
26. Yang TM, Lu CH, Huang CR, et al. Clinical characteristics of adult Escherichia coli meningitis. Japanese journal of infectious diseases 2005;58(3):168–70.
27. Pomar V, Benito N, Lopez-Contreras J, et al. Spontaneous gram-negative bacillary meningitis in adult patients: characteristics and outcome. BMC infectious diseases 2013;13 : 451.
28. Wang KW, Chang WN, Huang CR, et al. Post-neurosurgical nosocomial bacterial meningitis in adults: microbiology, clinical features, and outcomes. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia 2005;12(6):647–50.
29. Emele FE. Etiologic spectrum and pattern of antimicrobial drug susceptibility in bacterial meningitis in Sokoto, Nigeria. Acta paediatrica (Oslo, Norway: 1992) 2000;89(8):942–6.
30. Chotmongkol V, Techoruangwiwat C. Community acquired-bacterial meningitis in adults. The Southeast Asian journal of tropical medicine and public health 2000;31(3):506–8.
31. Gower DJ, Barrows AA, Kelly DL Jr., et al. Gram-negative bacillary meningitis in the adult: review of 39 cases. Southern medical journal 1986;79(12):1499–502.
Štítky
Detská neurológia Neurochirurgia Neurológia
Článok vyšiel v časopiseČeská a slovenská neurologie a neurochirurgie
Najčítanejšie tento týždeň
2017 Číslo Online only- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Neuromultivit v terapii neuropatií, neuritid a neuralgií u dospělých pacientů
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
Najčítanejšie v tomto čísle- Dva případy atypického teratoidního rhabdoidního tumoru CNS a přehled literatury
- Intravenózní trombolýza po zrušení účinku dabigatranu specifickým antidotem Idarucizumabem
- Kazuistika meningitidy dospělých způsobená bakterií Escherichia coli
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



