-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Characterization of swallowing disorders in myasthenia gravis through a fibre-optic endoscopic evaluation
Charakterizace poruch polykání při myasthenia gravis pomocí flexibilního endoskopického vyšetření
Cíl: Myasthenia gravis (MG) je autoimunitní onemocnění charakteristické slabostí a rychlou únavou jakýchkoli svalů pod volní kontrolou, které se při vyvinutém úsilí zhoršuje, s možným rozvojem poruch polykání. Cílem této studie bylo charakterizovat poruchy polykání u MG pomocí flexibilního endoskopického vyšetření (fiberoptic endoscopic evaluation of swallowing; FEES). Metody: Studie byla provedena hodnocením 37 pacientů s MG v období od května 2015 do listopadu 2016. Kromě analýzy klinických a demografických dat byli všichni pacienti vyšetřeni pomocí FEES. Výsledky: Vztah mezi výskytem laryngeální senzitivity a stázou ukázal, že u pacientů se sníženou senzitivitou dochází k stáze slin a kapalin a zejména ke statisticky významné stáze kašovité (p = 0,025) a pevné (p < 0,001) stravy. Závěr: U pacientů s MG se vyskytují poruchy polykání. U nemocných s generalizovaným postižením a větší progresí MG byly pozorovány změny v kontraktibilitě hltanu a snížení laryngeální senzitivity.
Klíčová slova:
myasthenia gravis – poruchy polykání – endoskopie – autoimunitní onemocnění – polykání
Authors: A. De C. A. F. De Castro; R. A. Dedivitis; D. S. Queija; L. Arakawa-Sugueno; M. A. Castro; L. L. Matos
Authors place of work: São Paulo Medical School, University, of São Paulo, São Paulo, Brazil. ; Department of Head and Neck Surgery
Published in the journal: Cesk Slov Neurol N 2021; 84/117(4): 374-380
Category: Původní práce
doi: https://doi.org/10.48095/cccsnn2021374Summary
Aim: Myasthenia gravis (MG) is an autoimmune disease characterized by weakness and rapid fatigue of any of the muscles under voluntary control, aggravated by effort, with the possibility of developing swallowing disorders. The aim of this study was to characterize swallowing disorders in MG through a fibre-optic endoscopic evaluation of swallowing (FEES). Methods: The study was carried out by evaluating 37 patients with MG in the period between May 2015 and November 2016. In addition to clinical and demographic data, all patients were examined using the FEES. Results: The association between the presence of laryngeal sensitivity and stasis showed that patients with sensitivity reduction had stasis for saliva, liquids and especially for purées (P = 0.025) and solids (P < 0.001) with statistical significance. Conclusion: MG patients present with swallowing disorders. Patients classified with generalized involvement and greater progression of MG presented with changes in pharynx contraction and reduction of laryngeal sensitivity.
Keywords:
myasthenia gravis – deglutition disorders – endoscopy – autoimmune disease – Swallowing
Introduction
Myasthenia gravis (MG) is a chronic autoimmune neuromuscular disorder characterized by weakness of the skeletal muscles [1]. In approximately 85–90% of patients with MG, antibodies against nicotinic acetylcholine receptors (AChR) are identified in the neuromuscular junctions, with a smaller group of patients having autoantibodies against muscle-specific kinase (MuSK) [2], low-density lipoprotein-related protein and even against agrin [3–5].
The most important features are weakness and fatigability of the skeletal muscles, usually occurring in a characteristic distribution. When the facial and bulbar muscles are affected, there may be a characteristic flattened smile, nasal speech and difficulty in chewing and swallowing [6].
Dysphagia is a common symptom in MG, occurring in 15–40% of patients with the generalized form of the disease and may manifest in coughing and choking during and after meals, sometimes with nasal regurgitation [6,7]. By contrast, it is very unusual for dysphagia to be the main manifestation of the disease [8–10]. Clinical presentation with changes in swallowing, speech and voice together can lead to a mistaken diagnosis of primary bulbar involvement caused by other diseases. Diagnosis in these patients can be difficult, especially if the antibodies against the acetylcholine receptors are not present or there is no clinical response to anticholinesterase drug administration [11].
When studying dysphagia, the diagnostic tools should also be able to assess the various oro-pharyngo movements that take place during swallowing in relationship to the type of bolus administered, as well as to evaluate the validity and efficacy of the relative compensation postures and manoeuvres [12].
Videofluoroscopy is currently considered the “gold standard” method for studying swallowing, which permits the investigation of all stages during swallowing [13,14].
Fibre-optic endoscopic evaluation of swallowing (FEES) has been proposed in recent years as a useful tool for studying swallowing [15,16]. In fact, FEES is now considered the first choice method of investigation due to the important advantages it offers: easy to use, well tolerated, radiation-free, possibility of bedside examination and less costly, in Europe [17]. In addition, FEES can be used for sensory testing [18].
There is a lack of studies in the literature characterizing the changes in swallowing which can be found in patients suffering from MG. Sometimes, these changes are given less attention by authors due to the clinical impact of other manifestations of the disease. However, taking into consideration the possibility of MG as a differential diagnosis, for patients with dysphagia symptoms it is crucial to prevent silent aspiration and the long-term sequelae of pulmonary infections, dehydration and malnutrition. This can be achieved through diet modification, changes in posture, swallowing exercises or if necessary, through the placement of a parenteral feeding. Morbidity and mortality may be reduced with early identification and management of neurogenic dysphagia [19].
The present study aimed to characterize the swallowing disorders in MG using the FEES.
Methods
This transversal observational study included 37 patients diagnosed with MG selected at the Neurology Outpatient Centre at the Department of Head and Neck Surgery, São Paulo Medical School. Patients were selected from May to November 2015 and subsequently called for studies from April to September 2016. Of the 55 patients selected, 4 patients died in 2015, 1 patient had no clinical conditions to perform the tests and 13 patients did not return for other reasons.
Patients with other neuromuscular, cardiovascular and respiratory diseases, episodes of clinical decompensation of their disease with in the two months prior to the study, with a prior stroke, history of mental disease or abuse of drugs and/or alcohol were not eligible for this study.
Demographic data were obtained through anamnesis and the patients’ medical records.
There were 8 men and 29 women, whose ages varied from 16 to 75. The duration of clinical complaints varied from 6 to 492 months. All patients had typical fatigable muscle weakness. Autoantibodies to muscle nicotinic AChR were present in 27 patients, whereas the others were seronegative patients with MG and two of these had serum IgG antibodies against MuSK.
In most patients, four treatment methods were used: increased neuromuscular transmission with anticholinesterase agents, surgical thymectomy, immunossuppression and short-term immunotherapies, including plasma exchange and intravenous immunoglobulin if the patient was in crisis.
Patients were evaluated and grouped according to the clinical classification proposed by Osserman et al in 1971 to define the severity of myasthenia [20]. The scale classifies adult patients as follows: group I – patients with fatigue and weakness in the external ocular muscles; group IIA – MG in a generalized but mild form, with compromised cranial muscles, lower limbs and trunk, excluding the respiratory muscles; group IIB – MG in a generalized way and moderately intense, with the presence of diplopia, palpebral ptosis, dysarthria, dysphagia, difficulty blowing, muscular weakness in the extremities and intolerance to exercise; group III – acute fulminating MG, with severe bulbar affection and change in the respiratory muscles, which can lead to the dependence on ventilation; group IV – late MG, in which the disease becomes generalized in the first or the second year after the onset of the condition.
Swallowing was assessed by means of FEES; before the beginning of the examinations, all patients remained at rest for 30 min. The FEES was performed by a physician and a speech pathologist with experiece in FEES using a fibre endoscope (Welch-Allym, Auburn, NY, USA) with a microcamera Toshiba (Minato, Japan) CCD (charge-coupled device) IK-M41A. The exams were viewed on a videomonitor (Sony KV-1311 CR [Sony, Minato, Japan]) and recorded in digital media (digital video disc recorder – Sony NS67P DVD) to be evaluated by 2 independent physicians with experience in FEES and the result was achieved by consensus. The patient was asked to remain seated, with the cervical region in the position of slight ventroflexion, simulating their position during a normal meal. A flexible fibre-optic nasopharyngolaryngoscope was introduced through the widest nasal cavity, without the use of topical anaesthetic so as not to interfere in pharynx-larynx sensitivity. The patient was offered 5 mL and 20 mL of orange juice in a coffee cup (50 mL) in the following consistencies: liquid, nectar (one measuring spoon of thickener per 100 mL of juice), honey (two measuring spoons of thickener per 100 mL of juice), purée (three measuring spoons per 100 mL of juice) in a spoon and a solid (biscuit) put in the patient’s hand. After receiving each consistency, the patient was asked to perform complete swallowing as many times as needed. Each foodstuff was coloured with blue liquid aniline (Arcólor®, São Paulo, Brazil). The consistencies used were obtained with thickener (Resource® ThickenUP Clear, Nestlé Health Sciences, Vevey, Switzerland).
During the test, the presence of pharyngeal contraction was assessed by asking the patient to utter the vowel ‘i’ in an acute tone and by observing the movement of the musculature, it was classified into two variables: adequate and reduced. Laryngeal sensitivity was investigated by gently touching the aryepiglottic folds with the tip of the fiberscope and the reaction with adduction reflex was characterized as present or reduced.
The items assessed in the swallowing protocol were: premature food spillage, penetration, aspiration, presence of residues (pooling) at the base of the tongue, vallecula, posterior pharynx wall and piryform sinus. To quantify and characterize the degree of stasis, the modified Rosenbek et al scale [21] by Jung et al [22] was used and to determine the severity of pharyngeal residues, the scale of 4 points of gravity of pharyngeal residue was used [22]. The analysis was performed from swallowing saliva and from each of the offered consistencies. In the case patient had any restriction regarding a texture, this limitation was respected.
Results
The demographics, clinical data and classification of the disease severity for our study groups are shown in Tab. 1. The distribution classification was as follows: 6 patients classified as group I, 7 patients as group IIA, 17 as group IIB and 7 as group III participated in our study; no patients we classified as group IV. Among the patients in our study group, 94.6% were using cholinesterase inhibitors and many of these (36%) were using immunosuppressant drugs. Most of the patients were undergoing treatment for anxiety and/or depression.
Tab. 1. Patients’ distribution according to clinical and demographic variables (N = 37). 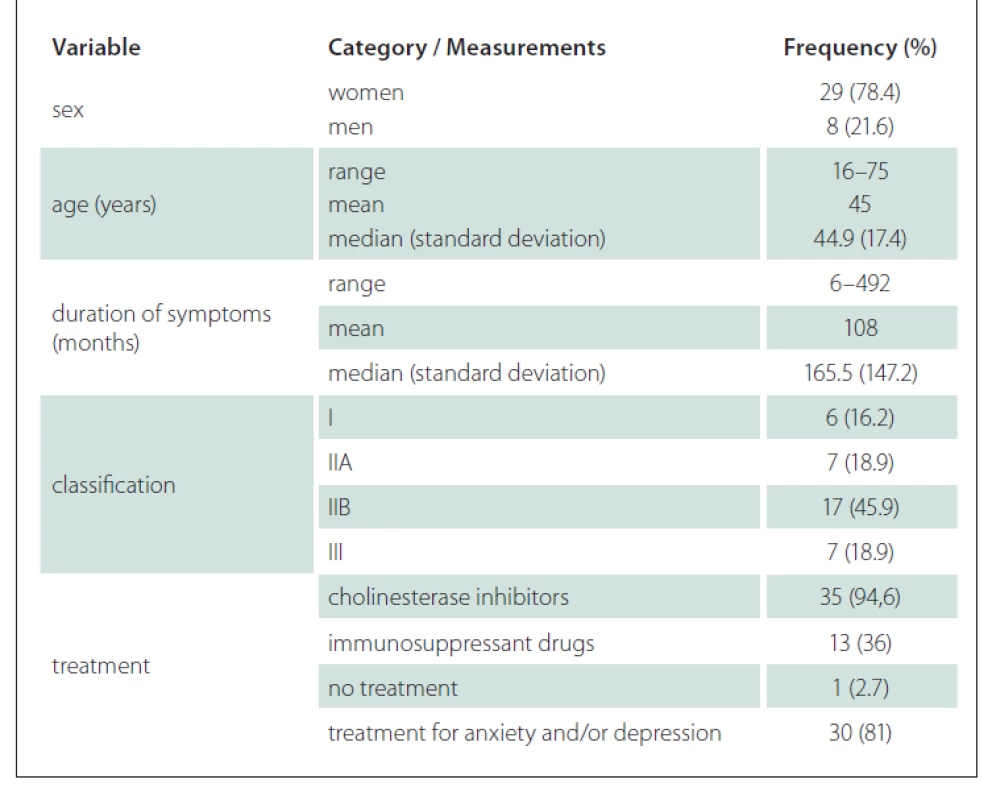
According to the FEES test, pharyngeal contraction was preserved in the majority of patients (86.5%), whereas laryngeal sensitivity was reduced in 40.5% of patients. Premature spillage was identified mainly in the solid consistency (32.4%), followed by liquid (16.2%), honey (13.5%), nectar and purée (10.8%) and finally saliva (5.4%).
The test also identified one patient who presented with penetration for all consistency variables with greater difficulty for the liquid (level 4). Aspiration was not identified; these results are shown in Tab. 2.
Tab. 2. Frequency and percentage of pentration/aspiration according to the FEES variables. 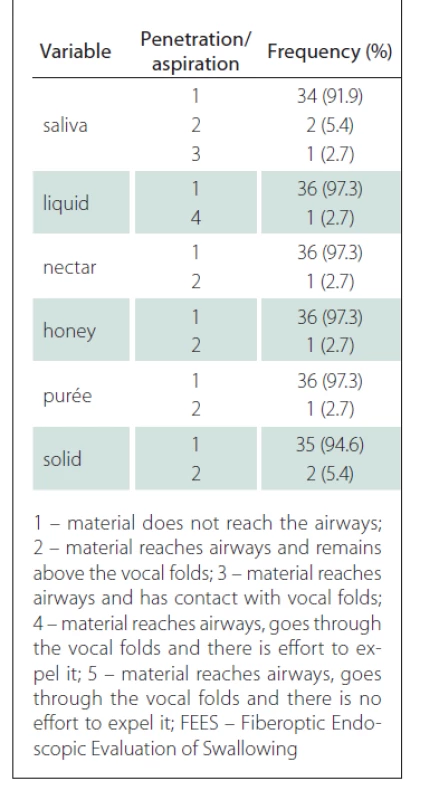
A few patients presented with stasis and, when present, it was in a mild to moderate degree. The test also showed worse performance for thicker consistencies, with stasis at the base of the tongue at a greater percentage for purée (10.8%) and solid (24.3%) (Tab. 3).
Tab. 3. Frequency and percentage of stasis degree according to the FEES variables. 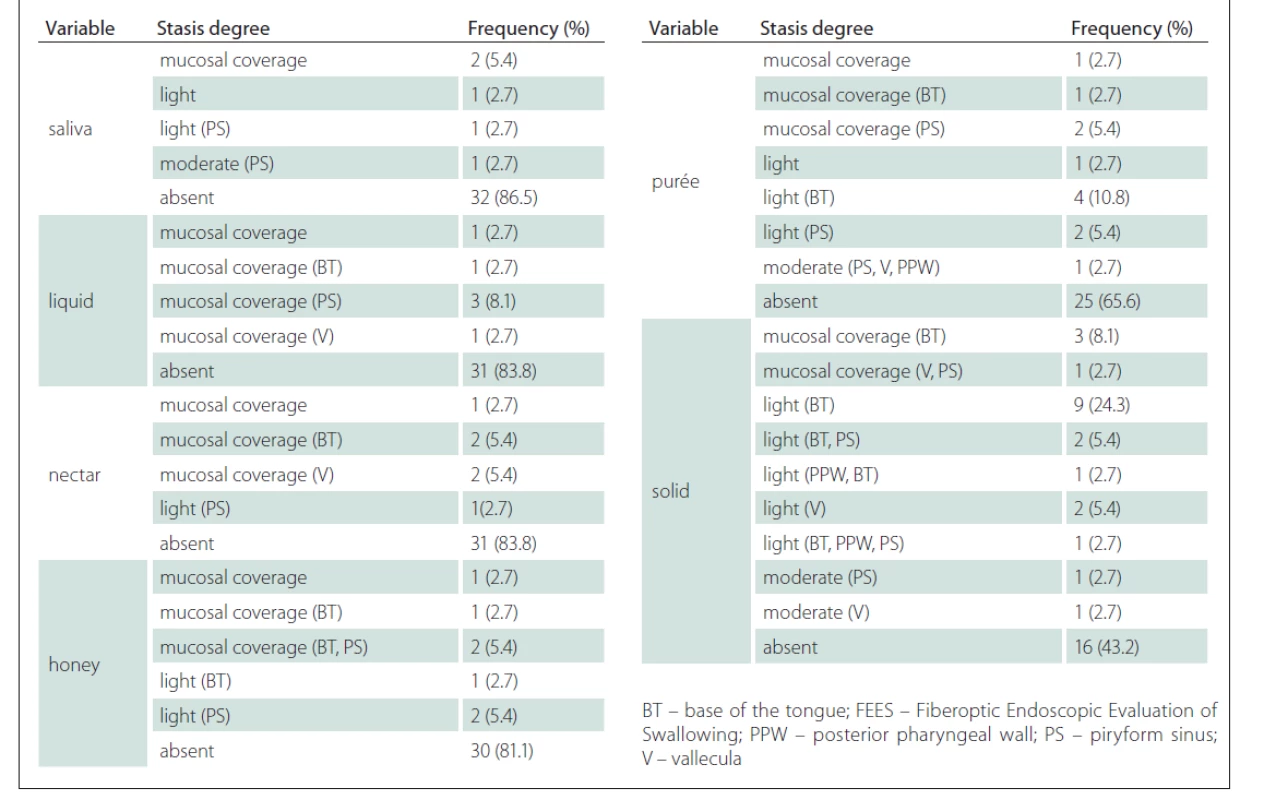
Table 4 shows that when comparing the degree of the disease with the variables of pharynx contraction and laryngeal sensitivity in the FEES, it is possible to observe that the greatest occurrence of their reduction in patients with the disease at a more advanced stage and with bulbar damage.
Tab. 4. Frequency and percentage of reduced pharyngeal contraction and reduced laryngeal sensitivity according to the degree of the disease. 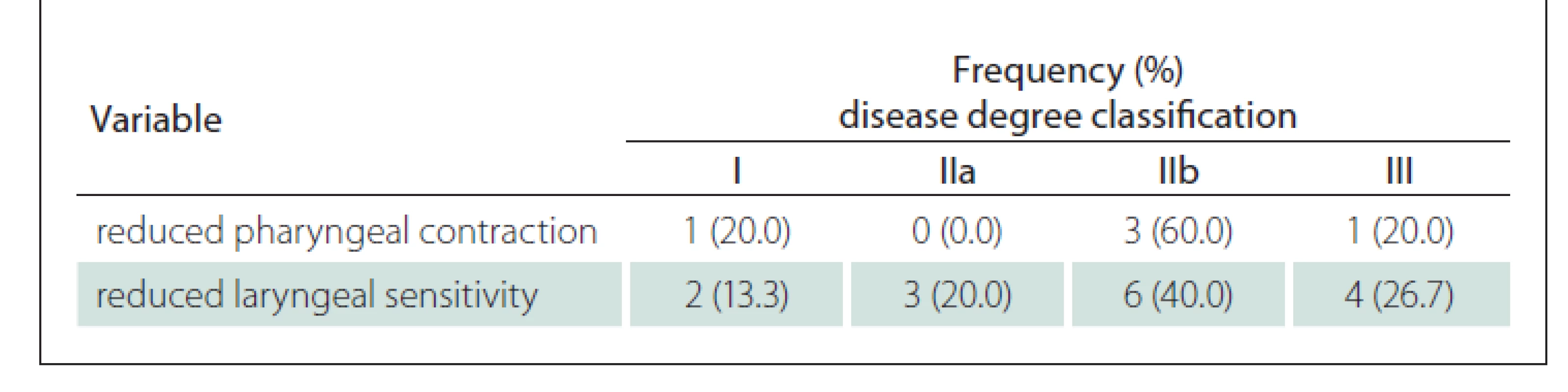
The vast majority of premature spillage occurrences were identified in more advanced cases of the disease with bulbar damage (IIb and III). However, for the solid group, this occurrence was detected from the very early stages of the disease. Similarly, stasis for the purée and solid consistencies was also noticed from the initial phases of the disease as shown in Tab. 5.
Tab. 5. Frequency and percentage of stasis according to the Fiberoptic Endoscopic Evaluation of Swallowing in relation to the degree of the disease. 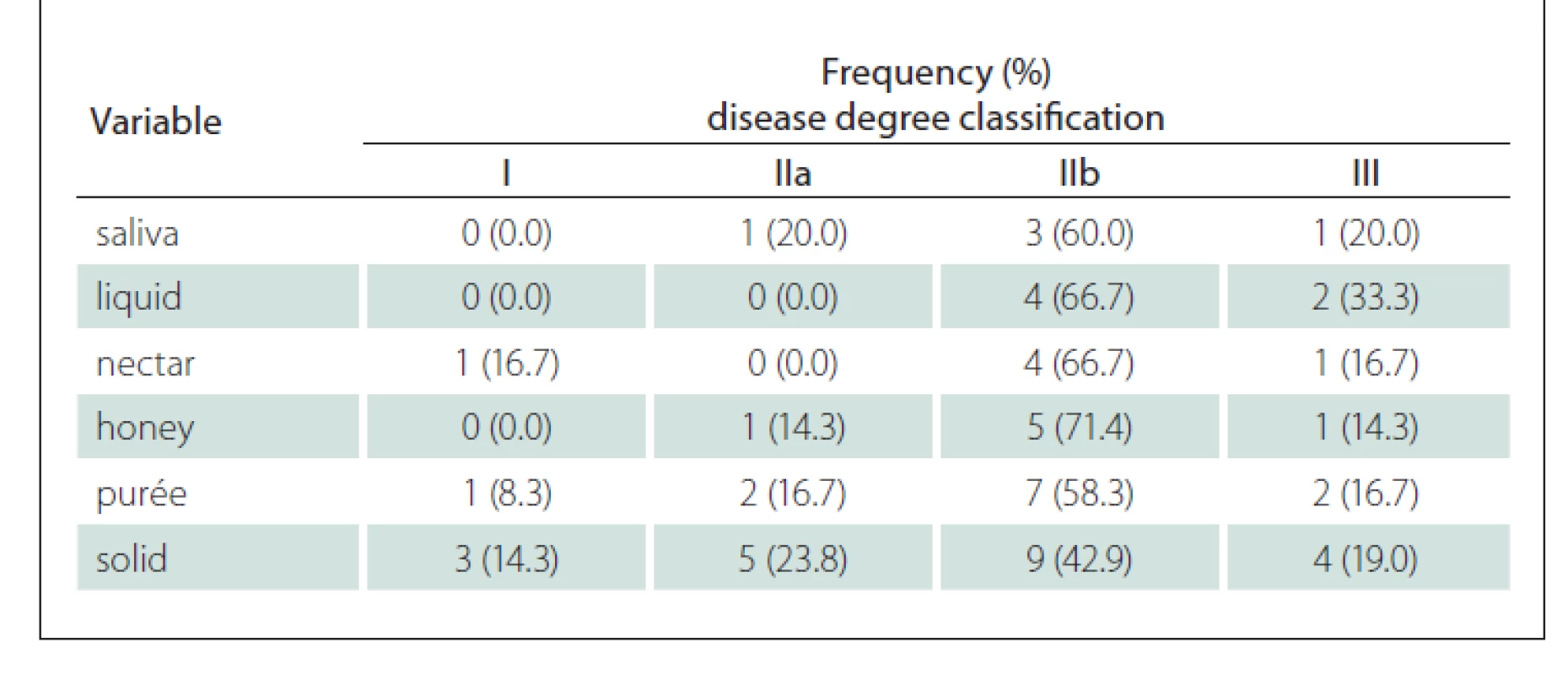
The association between the presence of laryngeal sensitivity and stasis showed that patients with sensitivity reduction had stasis for saliva, liquid and especially for the purée (P = 0.025) and solid (P < 0.001) with statistical significance (Tab. 6).
Tab. 6. Correlation between the presence of stasis and laryngeal sensitivity. 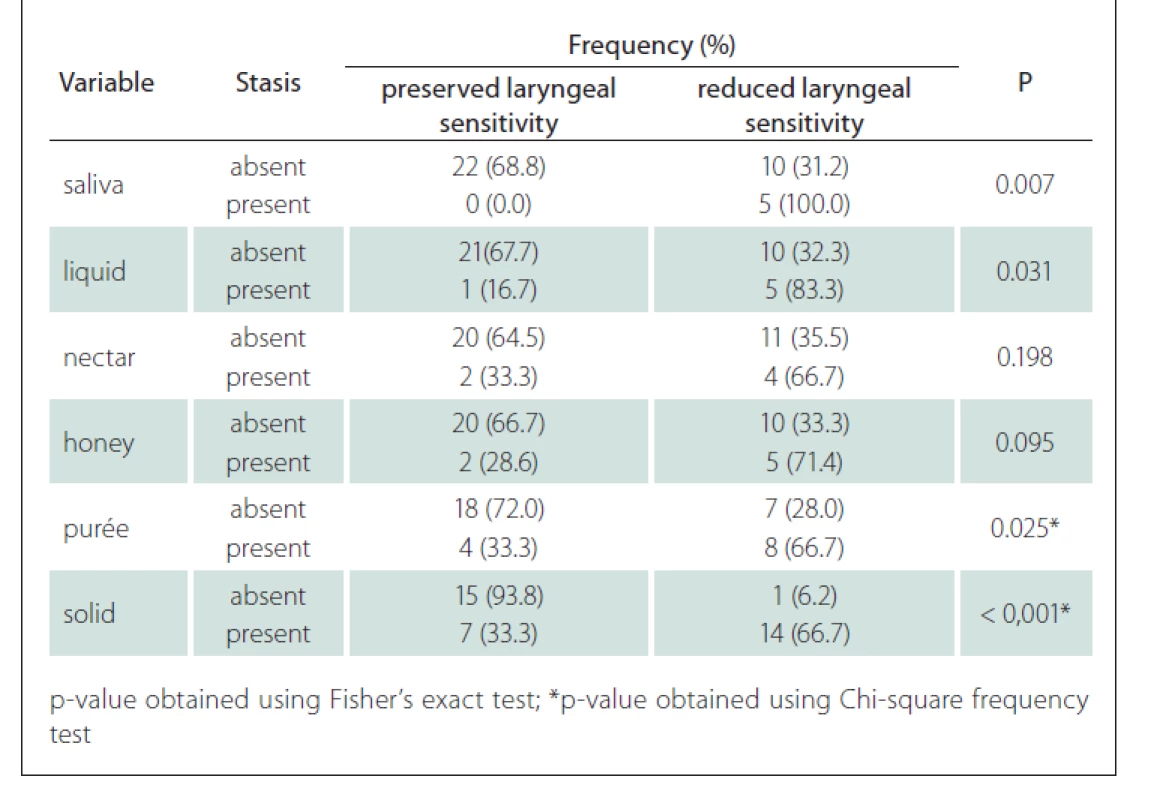
Discussion
The swallowing mechanism can be divided into 3 phases: oral, pharyngeal and oesophageal. Any disruption in the swallowing process, which includes difficulty in transporting (or a lack of transporting) a food or liquid bolus from the mouth through the pharynx and oesophagus into the stomach can be considered dysphagia [23]. Dysphagia is a symptom of the disease reflecting structural or neuromuscular disorders of the oropharynx or oesophagus. The correct diagnosis evaluation includes taking the patient’s history thoroughly and performing a physical examination. Koopman et al evaluated the ability of 4 clinical tools to predict aspiration in 20 patients with MG, as they considered the need for the risk of dysphagia and aspiration important for the management of MG. The patients completed a self-directed questionnaire, underwent clinical neurological assessment and a bedside speech pathology assessment, and were assessed with quantitative myasthenia gravis (QMG) score [24].
Radiological oesophagogram, videofluoroscopy, FEES, manometry, ultrasound examination, scintigraphy, electromyography and 24-h pH monitoring are the main diagnostic procedures of dysphagia [25]. In our study, FEES was used as a diagnostic procedure to detect small changes, such as salivation stasis and more severe dysphagia with more significant penetration and aspiration.
Although FEES is not considered the “gold standard” method for studying swallowing, it has been proposed in recent years as a useful tool for this purpose due to the important advantages it offers: easy to use, well tolerated, radiation-free, possibility of bedside examination, less costly and can also be used for sensory testing [18]. A study performed by Labeit et al used FEES to evaluate pharyngeal hypoesthesia. The conclusion was that FEES was one of the most common and well tolerated methods to objectively assess dysphagia [26]. Dziewas et al confirmed that FEES, even when performed by less experienced clinicians was a safe and well tolerated procedure and had a significant impact on the patients’ clinical course [27].
Additionally, the literature shows that the results obtained with videofluoroscopy and videoendoscopy correlate well in the detection of some pathological aspects, such as aspiration of the bolus into the airways and the presence of bolus residue in the pharynx and pharyngeal-laryngeal area [15,16]. In line with this, a systematic literature review was conducted by Warnecke et al, searching MEDLINE from the beginning to May 2020 for FEES findings in neurological diseases of interest. Based on a retrospective analysis of FEES videos in neurological diseases and considering the results of the review, fatigable swallowing weakness was the main phenotype in MG and was not seen in other disorders as the main mechanism. Thus, MG manifests in the oropharynx similarly to other systemic symptoms and is characterized by exertion-related muscle weakness with an increase of residue with repetitive swallowing trials [28].
In agreement with Buchholz et al [29], symptoms of a bulbar nature – dysarthria, dysphagia, dysphonia and dyspnea – were described by the patients as clinical findings from the earliest manifestations of MG and have always been attributed to weakness in the structures involved, with the typical characteristic of the fluctuation of symptoms.
Khan et al [30] reported 4 patients who had undergone exhaustive investigations for dysphagia by gastroenterologists and otolaryngologists before a MG diagnosis. In our study, the time between the onset of symptoms and an MG diagnosis varied between 6 and 480 months.
Oda et al [31] tested the regional sensitivity in the pharynx and supraglottis with a touch of the fibroscope and this was considered adequate (reflex of present cough) in 90.9% of the cases. In our study, the test detected a reduction of laryngeal sensitivity in 40.5% of the patients and premature spillage mainly for solid and liquid consistencies (32.4%). Although MG is a disease that affects the striated musculature and consequently without alterations of sensitivity, this difference is probably due to the fact that our patients had a longer time of disease evolution. The presence of alimentary residues after 3 swallows in the rhinopharynx, base of the tongue, valleculae, piriform recess, posterior wall of the pharynx and glottis were observed in the nasofibrolaryngoscopic analysis in patients with MG [31]. In our study, the stasis was mostly of a mild degree, with a worsening of performance as the consistency was thickened, as in the purée and solid consistencies.
Weakness in the oropharyngeal musculature is an important aspect which can compromise the entire swallowing performance, making oral contention of the bolus and its preparation difficult. This, in turn, leads to a difficulty in mastication and grinding of heavier consistencies such as solids and also in liquid retention, which requires more sophisticated oral motor control and provokes premature spillage [32–34]. With the increase of weakness, more interference in the progression of the bolus in the pharynx occurs. These factors, combined with sensory deficit can aggravate the condition since the stasis, even of thicker consistencies, may not be perceived. This may explain the fact that in our group, premature spillage occurred in a higher percentage in patients with more advanced stages of the disease with bulbar damage (IIb and III). Nevertheless, even in patients in earlier stages of the disease, premature spillage and stasis occurred for purée and solid consistencies.
Weakness of the bulbar muscles is common in MG and can appear in the oral phase to a greater or lesser degree, as well as in the pharyngeal phase. The possibility of changes even when not directly implicated in aspiration can be observed in the initial stages of MG. The risk may increase as the difficulty in managing the stasis in the pharynx develops and the degree of penetration and its management, such as the ability for pharyngeal cleaning by continuous swallowing, phlegm or cough deteriorate. In our study, patients classified with generalized involvement and greater MG development, according to the classification of Osserman et al [20] presented with premature spillage, diagnosed by swallowing videoendoscopy.
In our series, patients presenting with bulbar damage had some reduction of both pharyngeal contraction and laryngeal sensitivity. This aspect had an intrinsic relationship with the increase in the occurrence of stasis mainly for purées (P = 0.025) and solids (P < 0.001).
Unlike other studies, aspirations were not detected in our series, however, penetrations of a discreet degree were observed in a few patients. In the studied group, the average age was 44.9 years. In another study [35], the average age of one-half of the patients was 65, which can be explained by the interference of the ageing process with the reduction of the functional reserve and the ability to compensate for what could be a slight or subclinical dysfunction [36,37].
In order to be diagnosed with dysphagia, a patient does not necessarily need to present with bronchoaspiration. Some symptoms like nasal reflux, coughing during or after swallowing and the sensation of food stuck in the throat, if neither recognized nor treated, may lead patients to malnutrition, dehydration and respiratory complications. Swallowing in some patients with MG is likely to be characterized much more by stasis and discreet penetrations rather than by aspiration [32] as shown in the study. Umay et al suggest that all patients with MG should be checked for dysphagia at certain intervals through clinical examinations or procedures that focus on oropharyngeal phase disorders and using methods that evaluate repetitive swallowing activities and the oesophageal phase [38].
Another relevant factor is that the patients in our group had an average of 13 years of treatment; and though all of them had a history of significant crises with global worsening of the motor aspect including swallowing, at the time of the study all of them had been medicated with very well optimized doses. Therefore, the weakness was not as evident. The majority of patients presented with a good standard of response to anticholinesterase treatment and were using immunosuppressive drugs, thus favoring the stabilization of the disease. Considering that even patients with the compensated disease may present with dysphagia characterized by laryngeal sensory changes, stasis and discreet penetrations, it is true that the need to follow them up throughout the course of the disease, assessing more subtle aspects, can actually help in the prevention of more severe complications related to bronchoaspiration.
In addition, anxiety-induced exhaustion, as a result of continuous muscle contraction, complicates vital functions, breathing and swallowing, causing an increase of anxiety [39]. However, a vicious circle settles in, if patients learn to relax and to do less, they can stop wasting energy; this will result in a clear increase in functions and a positive change in psychological and emotional behaviour without the need to increase or change the medication [37]. Positive results of speech therapy will be prolonged if the patients receive dietary advice, concerning food that can be swallowed easily, if medication is attuned to mealtimes and if physical activity is limited prior to eating [40]. In our study, 81% of patients were treated for depression and/or anxiety related to the diagnosis and all patients received guidance and referrals for multidisciplinary treatment.
Conclusion
MG patients present swallowing disorders. Patients classified with generalized involvement and greater progression of MG presented changes in pharynx contraction and reduction of laryngeal sensitivity, which were diagnosed in the FEES and led to premature spillage and saliva stasis, from purées, liquids, nectars and solids.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). This research was approved by the Institutional Review Board of the Medical School of the Department of Head and Neck Surgery, São Paulo, number 719.846, on July 16, 2014.
Conflict of interest
The authors declare that they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Dr. Andrea de C. A. F. de Castro
Department of Head and Neck
Surgery
São Paulo Medical School
University of São Paulo
Rua Dr. Luis de Faria 109 apto 94
CEP: 11060-481
Santos, São Paulo
Brazil (55-13) 981942000
e-mail: deanacleto@hotmail.com
Accepted for review: 31. 7. 2020
Accepted for print: 29. 7. 2021
Zdroje
1. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009; 8 (5): 475–490. doi: 10.1016/S1474-4422 (09) 70063-8.
2. Hoch W, McConville J, Helms S et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001; 7 (3): 365–368. doi: 10.1038/85 520.
3. Higuchi O, Hamuro J, Motomura M et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 2011; 69 (2): 418–422. doi: 10.1002/ana.22312.
4. Zhang B, Shen C, Bealmear B et al. Autoantibodies to agrin in myasthenia gravis patients. PLoS One 2014; 9 (3): e91816. doi: 10.1371/journal.pone.0091816.
5. Oliveira EF, Valério BCO, Cavalcante V et al. Quantitative myasthenia gravis score: a Brazilian multicenter study for translation, cultural adaptation and validation. Arq Neuropsiquiatr 2017; 75 (7): 457–463. doi: 10.1590/0004-282X20170075.
6. Grob D, Arsura EL, Brunner NG et al. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci 1987; 505 : 472–499. doi: 10.1111/j.1749-6632.1987.tb51317.x.
7. Carpenter RC, Mc Donald TJ, Howard EM. The otolaryngologic presentation of myasthenia gravis. Laryngoscope 1979; 89 (6 Pt 1): 922–928. doi: 10.1288/00005537-197906000-00008.
8. Donner MW, Silbiger ML. Cinefluorographic analysis of pharyngeal swallowing in neuromuscular disorders. Am J Med Sci 1966; 251 (5): 600–616. doi: 10.1097/00 000441-196605000-00013.
9. Khan OA, Campbell WW. Myasthenia gravis presenting as dysphagia: clinical considerations. Am J Gastroenterol 1994; 89 (7): 1083–1085.
11. Gwathmey KG, Burns TM. Myasthenia gravis. Semin Neurol 2015; 35 (4): 327–339. doi: 10.1055/s-0035-1558975.
12. American Speech Language Hearing Association. Special interest division 13. Swallowing and swallowing disorders. Atlanta, GA, USA 1998.
13. Logemann JA. Swallowing physiology and pathophysiology. Otolaryngol Clin North Am 1988; 21 : 613–623.
14. Logemann JA. Dysphagia: evaluation and treatment. Folia Phoniatr Logop 1995; 47 (3): 140–164. doi: 10.1159/000266348.
15. Bastian RW. Videoendoscopic evaluation of patients with dysphagia: an adjunct to the modified barium swallow. Otolaryngol Head Neck Surg 1991; 104 (3): 339–350. doi: 10.1177/019459989110400309.
16. Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia 1988; 2 (4): 216–219. doi: 10.1007/BF02414429.
17. Aviv JE, Kaplan ST, Thomson E et al. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia 2000; 15 (1): 39–44. doi: 10.1007/s004559910008.
18. Aviv JE, Kim T, Thomson JE et al. Fiberoptic endoscopic evaluation of swallowing with sensory testing (FEESST) in healthy controls. Dysphagia 1998; 13 (2): 87–92. doi: 10.1007/PL00009561.
19. Sebastian S, Nair GP, Thomas P et al. Oropharyngeal dysphagia: neurogenic etiology and manifestation. Indian J Otolaryngol Head Neck Surg 2015; 67 (Suppl 1): 119–123. doi: 10.1007/s12070-014-07 94-3.
20. Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med 1971; 38 (6): 497–537.
21. Rosenbek JC, Robbins JA, Roecker EB et al. A penetration – aspiration scale. Dysphagia 1996; 11 (2): 93–98. doi: 10.1007/BF00417897.
22. Jung SH, Kim J, Jeong H et al. Effect of the order of test diets on the accuracy and safety of swallowing studies. Ann Rehabil Med 2014; 38 (3): 304–309. doi: 10.5535/arm.2014.38.3.304.
23. Olszewski J. Causes, diagnosis and treatment of neurogenic dysphagia as an interdisciplinary clinical problem. Otolaryngol Pol 2006; 60 (4): 491–500.
24. Koopman WJ, Wiebe S, Colton-Hudson A et al. Prediction of aspiration in myasthenia gravis. Muscle Nerve 2004; 29 (2): 256–260. doi: 10.1002/mus.10538.
25. Halama AR. Clinical approach to the dysphagic patient. Acta Otorhinolaryngol Belg 1994; 48 (2): 119–126.
26. Labeit B, Muhle P, Ogawa M et al. FEES-based assessment of pharyngeal hypesthesia: proposal and validation of a new test procedure. Neurogastroenterol Motil 2019; 31 (11): e13690. doi: 10.1111/nmo.13 690.
27. Dziewas R, auf dem Brinke M, Birkmann U et al. Safety and clinical impact of FEES: results of the FEES-registry. Neurol Res Pract 2019; 1 : 16. doi: 10.1186/s42466-019-0021-5.
28. Warnecke T, Labeit B, Schroeder J et al. Neurogenic dysphagia: systematic review and proposal of a classification system. Neurology 2021; 96 (6): e876–e889. doi: 10.1212/WNL.0000000000011350.
29. Buchholz DW, Robbins J. Neurologic diseases affecting oropharyngeal swallowing. In: Pearlman AL, Schulze-Delrieu K (eds). Deglutition and its disorders: anatomy, physiology, clinical diagnosis and management. New York: Singular 1997.
30. Khan OA, Campbell WW. Myasthenia gravis presenting as dysphagia: clinical considerations. Am J Gastroenterol 1994; 89 (7): 1083–1085.
31. Oda AL, Chiappetta ALML, Annes M et al. Clinical, endoscopical and manometric evaluation of swallowing in patients with acquired autoimmune myasthenia gravis. Arq Neuropsiquiatr 2002; 60 (4): 986–995. doi: 10.1590/s0004-282x2002000600019.
32. St Guily JL, Périé S, Willig TN et al. Swallowing disorders in muscular diseases functional assessment and indications of cricopharyngeal myotomy. Ear Nose Throat J 1994; 73 (1): 34–40.
33. Manrique D. Avaliação da deglutição em crianças com paralisia cerebral tetraespástica: análise nasofibrolaringoscópica. São Paulo: Universidade Federal de São Paulo 1998.
34. Colton-Hudson A, Koopmann WJ, Moosa T et al. A prospective assessment of the characteristics of dysphagia in myasthenia gravis. Dysphagia 2001; 17 (2): 147–151. doi: 10.1007/s00455-001-0114-4.
35. Robbins J. Normal swallowing and aging. Semin Neurol 1996; 16 (4): 309–317. doi: 10.1055/s-2008-1040 989.
36. Logeman JA. Effects of aging on swallowing mechanism. Otolaryngol Clin North Am 1990; 23 (6): 1045–1056.
37. Swart BJM, Padberg GW, Engelen BGM. Less is more: treatment of aggravating behaviour in myasthenia gravis patients with dysphagia. Eur J Neurol 2002; 9 (6): 687–702. doi: 10.1046/j.1468-1331.2002.00447_3.x.
38. Umay EK, Karaahmet F, Gurcay E et al. Dysphagia in myasthenia gravis: the tip of the Iceberg. Acta Neurol Belg 2018; 118 (2): 259–266. doi: 10.1007/s13760-018-0884-1.
39. Logemann JA, Rademaker AW, Pauloski BR et al. Normal swallowing physiology as viewed by videofluoroscopy and videoendoscopy. Folia Phoniatr Logop 1998; 50 (6): 311–319. doi: 10.1159/000021473.
40. Buchholz DW. Neurologic disorders of swallowing. In: Groher ME (eds). Dysphagia: diagnosis and management. Boston: Butterworth Heinemann 1997; 53–54.
Štítky
Detská neurológia Neurochirurgia Neurológia
Článok vyšiel v časopiseČeská a slovenská neurologie a neurochirurgie
Najčítanejšie tento týždeň
2021 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Neuromultivit v terapii neuropatií, neuritid a neuralgií u dospělých pacientů
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
-
Všetky články tohto čísla
- Editorial
- Proč se dráhy kříží? Základní principy uspořádání mozku obratlovců
- Role mikroRNA v patogenezi spinální muskulární atrofie
- Nové možnosti laboratórnej diagnostiky ochorení spojených s tvorbou amyloidov
- Využití rohovkové konfokální mikroskopie u neurologických onemocnění
- Poruchy čichu po COVID-19 – diagnostika, význam a léčba
- Kappa free light chains in multiple sclerosis – diagnostic accuracy and comparison with other markers
- Studijní protokol – robotická terapie chůze pomocí přístroje Lokomat Pro FreeD u pacientů v subakutní fázi ischemické cévní mozkové příhody
- Validace dotazníku pro poruchy chůze u pacientů s roztroušenou sklerózou – česká verze MSWS-12
- Characterization of swallowing disorders in myasthenia gravis through a fibre-optic endoscopic evaluation
- Standardisation of the Slovenian version of the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog)
- The frequency of silent brain infarcts in polycythaemia vera and essential thrombocytosis
- COVID-19 u nemocných s myasthenia gravis
- CANVAS – nově identifikovaná genetická příčina ataxie s pozdním nástupem. Popis prvních diagnostikovaných pacientů v České republice
- Benefits of 18F-FET PET in preoperative assessment of glioma heterogeneity demonstrated in two case reports
- COVID-19 asociovaná myelitida – kazuistika vzácné komplikace závažné SARS-CoV-2 infekce
- Intramedulární absces
- Successful usage of rituximab in a patient with overlapping myelin oligodendrocyte glycoprotein encephalomyelitis and systemic lupus erythematosus
- Informace vedoucího redaktora
- Prof. MUDr. Hana Krejčová, DrSc. 90letá
- Aktualita z kongresu EAN 2021
- Česká a slovenská neurologie a neurochirurgie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Poruchy čichu po COVID-19 – diagnostika, význam a léčba
- CANVAS – nově identifikovaná genetická příčina ataxie s pozdním nástupem. Popis prvních diagnostikovaných pacientů v České republice
- Proč se dráhy kříží? Základní principy uspořádání mozku obratlovců
- COVID-19 asociovaná myelitida – kazuistika vzácné komplikace závažné SARS-CoV-2 infekce
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



