-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Standardisation of the Slovenian version of the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog)
Standardizace slovinské verze škály Alzheimer’s Disease Assessment Scale – kognitivní subškála (ADAS-Cog)
Cíl: Počet pacientů s Alzheimerovou chorobou se na celém světě rychle zvyšuje. Škála Alzheimer`s Disease Assessment Scale – cognitive subscale (ADAS-Cog) je screeningový nástroj sestávající z jedenácti položek vyvinutý pro časnou detekci kognitivních změn v důsledku Alzheimerovy choroby. Na škále ADAS-Cog je možné získat 0–70 bodů, přičemž nižší číslo znamená lepší výsledek. Vyšší celkové skóre tedy koreluje se závažnější poruchou kognitivních funkcí. Cílem naší studie bylo škálu ADAS-Cog standardizovat pro slovinskou populaci. Metody: Do studie bylo zařazeno 84 osob bez poruchy kognitivních funkcí (57 % byly ženy), u nichž byla provedena administrace slovinské verze škály ADAS-Cog. Pro analýzu demografických údajů, celkových skóre ADAS-Cog a skóre jednotlivých úkolů byly použity metody deskriptivní statistiky. Střední hodnoty pro jednotlivé úkoly a celkových skóre byly porovnány mezi pohlavími a osobami mladšími či staršími 65 let. Na závěr byl vytvořen lineární model pro celkové skóre ADAS-Cog a pohlaví, věk a délku vzdělávání v letech. Výsledky: Rozmezí věku bylo 40–87 (střední věk byl 67,3 [SD 11,1]) let, rozmezí délky vzdělávání bylo 8–18 (střední délka vzdělávání byla 12,5 [SD 2,8]) let a střední celkové skóre bylo 7,4 (SD 2,3) bodů. Mezi pohlavími nebyly v demografických údajích nebo ve skóre ADAS-Cog rozdíly na úrovni jednotlivých úkolů nebo celkového skóre. Významný rozdíl byl zaznamenán v celkovém skóre mezi mladší a staršími účastníky (6,0 [SD 1,7] vs. 8,2 [SD 2,3] bodů). Pomocí lineární regrese bylo zjištěno, že celkové skóre bylo významně ovlivněno věkem (B = 0,138; p < 0,001) a délkou vzdělávání v letech (B = –0,236; p = 0,018). Závěr: Škála ADAS-Cog byla standardizována pro slovinskou populaci. Celkové skóre pro jedince bez poruchy kognitivních funkcí je relativně nízké a může být ovlivněno věkem a vzděláváním. U starších osob s nižším vzděláním může být dosaženo vyššího skóre než u zbytku populace.
Klíčová slova:
Alzheimerova choroba – mírná kognitivní porucha – skríningový test – standartizace – psychologické hodnocení
Authors: J. Ulbl; M. Rakusa
Authors place of work: Medical Centre Maribor, Slovenia ; Department of Neurology, University
Published in the journal: Cesk Slov Neurol N 2021; 84/117(4): 381-387
Category: Původní práce
doi: https://doi.org/10.48095/cccsnn2021381Summary
Aim: The number of patients with Alzheimer‘s disease is rapidly increasing worldwide. Alzheimer`s Disease Assessment Scale – cognitive subscale (ADAS-Cog) is an eleven-task screening tool developed for detecting early cognitive changes due to Alzheimer‘s disease. The ADAS-Cog score ranges from 0 to 70 points, and a lower number of points means a better result. Thus, a higher total score correlates with more significant cognitive impairment. Our study aim was to standardize ADAS-Cog for the Slovenian population. Methods: 84 cognitively unimpaired (CU) subjects were included (57% females). We tested them with the Slovenian version of ADAS-Cog. We used descriptive statistics to analyze the demographic data, ADAS-Cog total scores and individual task scores. The mean values for each task and the total score were compared between the sexes and between people younger and older than 65 years. At the end, we created a linear model between the ADAS-Cog total score and sex, age and years of education. Results: The age range was 40–87 (the mean age was 67.3 [SD 11.1]) years, the education range was 8–18 (mean years of education 12.5 [SD 2.8]) years and the mean total score was 7.4 (SD 2.3) points. There were no differences between the sexes in the demographic data or ADAS-Cog score at the individual task or in total. A significant difference was found in the total score between younger and older participants (6.0 [SD 1.7] vs. 8.2 [SD 2.3] points). Using linear regression, we found that the total score was significantly influenced by the age (B = 0.138; P < 0.001) and years of education (B = –0.236; P = 0.018). Conclusion: We standardized ADAS-Cog for the Slovenian population. The total score for CU people is relatively low and may be influenced by age and education. Thus, older subjects with a lower education may achieve higher scores than the rest of the population.
Keywords:
Alzheimer‘s disease – mild cognitive impairment – screening test – standardisation – psychological evaluation
Introduction
Alzheimer‘s disease (AD) is the most common cause of neurodegenerative dementia worldwide [1], followed by Lewy body/Parkinson‘s disease [2] and vascular dementia [3]. The number of people affected is increasing yearly. Until recently, disease-specific drugs were unavailable. With the approval of aducanumab for the treatment of AD, it is more crucial now than ever to identify people with AD in the mild stage of the disease [4].Cerebrospinal fluid (CSF) analysis is a widely used and well-accepted method to support the diagnosis of AD. CSF levels of beta-amyloid, total tau protein (t-tau) and phosphorylated tau protein (p-tau) may vary between laboratories and countries [5,6]. Despite different cut-offs, low levels of beta-amyloid and high t-tau and p-tau are characteristic of AD [7].
Another important tool is a cognitive test. There are several short screening tests that evaluate different cognitive domains: memory and visuospatial functions Clock Drawing Test [8], Coin in the Hand test for memory deficit [9], the test of gestures (TEGEST) for episodic memory [10], the Picture Naming and Immediate Recall test (PICNIR) [11], and The Amnesia Light and Brief Assessment (ALBA) test for memory and language [12,13]. The two most common tests which evaluate several cognitive domains are the Mini-Mental State Exam [14] and the Montreal Cognitive Assessment [15], which are validated in several different languages [16–21].
Although short screening tests may be practical for quick evaluation in the outpatient clinic, they may not be sensitive or specific enough to detect subtle cognitive impairment. A more elaborated tool is the Alzheimer‘s Disease Assessment Scale (ADAS). It was designed especially for screening for patients with AD. The initial version of the test was published in 1984 and was designed for evaluating cognitive (11 items) and behavioural impairment (10 items) in subjects with AD [22]. The non-cognitive subscale (ADAS-Noncog) is used to evaluate behaviour and mood impairment. With the cognitive subscale (ADAS-Cog), we assess memory, orientation, language and praxis. The maximum score is 70 points, where a higher score correlates with more significant cognitive impairment. The ADAS-Noncog is rarely used nowadays. However, the ADAS-Cog has become the gold standard for evaluating the progression of AD and for monitoring the efficacy of antidementia treatments [23].
ADAS-Cog has been adapted to and validated in many different countries (Slovakia [24], Spain [25], Portugal [26], Hungary [27], Greece [28], Turkey [29], Tunisia [30], China [31], South Korea [32] and Singapore – modified English version [33]). The scale has demonstrated high sensitivity (up to 97% [27]) and specificity (up to 98% [26]) for detecting AD. Furthermore, ADAS-Cog was also effective for detecting mild cognitive impairment, with lower sensitivity and specificity [26].
Although ADAS-cog is a valuable tool for detecting cognitive impairment, we should not copy it into another language, but adapt it to social, cultural and educational backgrounds that may differ between countries.
In our study, we aimed to standardize ADAS-Cog for the Slovenian population.
Methods
Population
Our study included 84 cognitively unimpaired (CU) subjects. All participants were native Slovenian speakers and had normal or corrected eyesight. Each participant was interviewed extensively. The demographic data and years of education were collected. All participants had a negative history regarding cognitive or cardio - or cerebrovascular disorders. They did not have any neurological impairments. No further neuroimaging was done.
Cognitive evaluation
All participants were tested with the Slovenian version of ADAS-Cog. It consists of eleven tasks with different score ranges: Word Recall (0–10 points), Naming Objects and Fingers (0–5 points), Commands (0–5 points), Constructional Praxis (0–5 points), Ideational Praxis (0–5 points), Orientation (0–8 points), Word Recognition (0–12 points), Remembering Test Instructions (0–5 points), Spoken Language Ability (0–5 points), Word Finding Difficulty (0–5 points), Comprehension of Spoken Language (0–5 points). The maximum score was 70 points and for each task the number of mistakes a participant made was assessed. Thus, a higher score meant greater cognitive impairment. For the Word Recall task, participants were shown one by one 10 words which were written on cards and asked to remember them. We used the following Slovenian words: maslo (Eng. butter), roka (Eng. arm), plaža (Eng. beach), pismo (Eng. letter), kralj (Eng. king), koča (Eng. cottage), os (Eng. axis), vstopnica (Eng. ticket), trava (Eng. grass), aparat (Eng. apparatus). Afterwards, the participants were asked to repeat the words in random order. There were three trials in this task and at the end the mean number of words which were not recalled across the trials was calculated.
In the Naming task, the participants had to name 12 randomly presented real objects (flower, bed, whistle, pencil, rattle, mask, scissors, comb, wallet, harmonica, stethoscope, funnel) as shown in Fig. 1. Participants also had to name the fingers on their dominant hand. For the Commands task, the participants were asked to carry out five separate commands (e. g., “Make a fist”, “Point to the ceiling and then to the floor”). The Constructional Praxis task demanded that the participants copy four geometric forms (circle, two overlapping rectangles, rhombus and cube). Ideational praxis was assessed with the task in which the participants had to pretend they were sending a letter by following these instructions: “I want you to pretend you have written yourself a letter. Take this sheet of paper, fold it so that it will fit into the envelope, and then put it into the envelope. Then seal the envelope, address the envelope to yourself and show me where the stamp goes.” For the Orientation task, participants were asked to provide the following pieces of information: person (their first name and surname), day of the week, date, month, year, season, time of day and place (where they currently are). For the Word Recognition task, participants had one trial to learn a list of 12 words; afterwards, they had to try to identify these 12 words, which were mixed in among 12 other distracter words. At the end, the participants‘ ability to remember the test instructions, language, word-finding difficulty and comprehension of the spoken language were assessed [23,34]. The estimated administration time of ADAS-Cog was around 15 min for each participant plus an additional 5 min for scoring.
Fig. 1. Twelve items for the “Naming task” (flower, bed, whistle, pencil, rattle, mask, scissors, comb, wallet, harmonica, stethoscope, funnel).
Obr. 1. Dvanáct předmětů pro subtest “Pojmenování” (květina, postel, píšťalka, tužka, chrastítko, maska, nůžky, hřeben, peněženka, harmonika, stetoskop, trychtýř).
Statistical analysis
SPSS (IBM SPSS Statistics for Windows, Version 27.0. IBM Corp, Armonk, NY, USA) and GraphPad Prism 9, (GraphPad Software, San Diego, USA) were used for statistical analysis.
The descriptive statistics for age, years of education, score on individual ADAS-Cog task and the ADAS-Cog total score were calculated. For the score of individual tasks and the total score, we calculated the One-Sample T-test. Then we correlated the scores of the individual tasks with the total score.
The mean values were compared between the sexes and between the participants aged 65 years or older and the participants younger than 65 years with an unpaired t-test.
At the end, a linear model for the ADAS-Cog total score was calculated. The independent variables were sex (males/females), age (years) and education (years). P < 0.05 was considered significant for all calculations.
Results
We included 36 CU males and 48 CU females. The mean age was 67.3 (SD 11.1) years, the mean duration of education 12.5 (SD 2.8) years and the mean total ADAS-Cog score 7.4 (SD 2.3) points (Tab. 1, Fig. 2). The scores on the tasks Word Recall, Constructional Praxis, Word Recognition, Remembering Test Instructions, Word Finding Difficulty and the total score were significantly different from 0 (Tab. 1).
Fig. 2. The histogram of total ADAS-Cog scores. Columns represent absolute number of subjects and percentage of the sample (N = 84).
ADAS-Coq – Alzheimer`s Disease Assessment Scale – cognitive subscale
Obr. 2. Histogram celkových skóre ASAD-Cog. Sloupce představují absolutní počet subjektů a procento vzorku (n = 84).
ADAS-Coq – Alzheimer`s Disease Assessment Scale – cognitive subscale
Tab. 1. Demographic data, mean scores on ADAS-cog, and correlation with the ADAS-Cog total score. 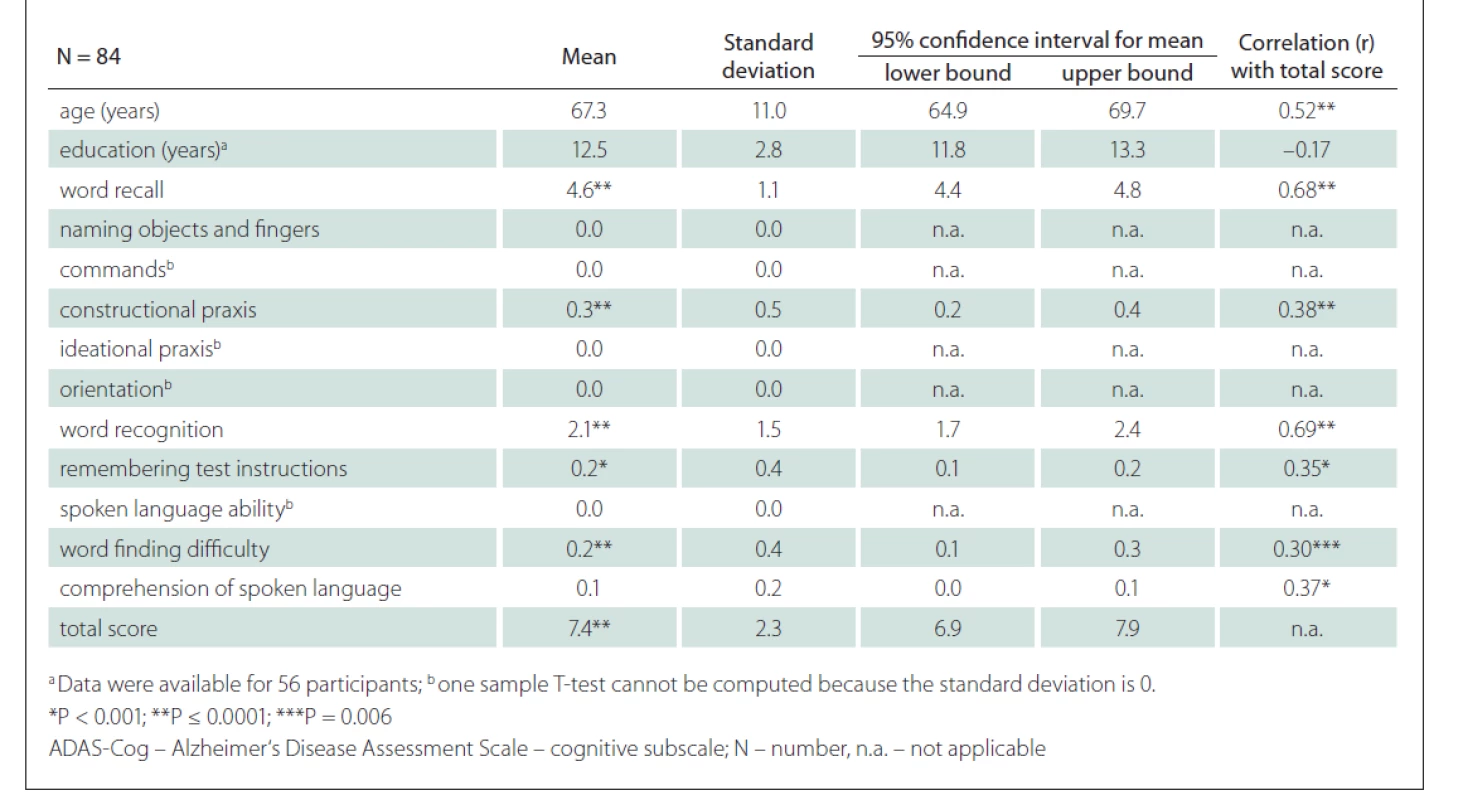
A strong positive correlation between the ADAS-Cog total score and the age of the participants was found (r = 0.52; P < 0.001) (Fig. 3). A strong correlation was also shown between the ADAS-Cog total score and the Word Recall task score and Word Recognition task score. A moderate correlation was determined between the ADAS-Cog total score and the scores on the Constructional Praxis, Remembering Test Instructions, Word Finding Difficulty and Comprehension of Spoken Language tasks (Tab. 1). The correlation between the ADAS-Cog total score and the years of education was weakly negative (r = –0.17), but statistically insignificant (Fig. 3). Other correlations were also insignificant. We did not find any differences between males and females in age, education, scores on individual ADAS-Cog tasks or ADAS-Cog total score (Tab. 2). Participants younger than 65 years had 1.5 years less formal education than participants aged 65 years or older and had significantly better results in the Word Recall task and the Word Recognition task and the total ADAS-Cog score (Tab. 3).
Fig. 3. A correlation between the age, education the and ADAS-Cog total score.
Correlation for age was significant (r = 0.52; P < 0.001). Correlation for education was not significant (r = –0.17). Data on education were available for 56 participants.
ADAS-Coq – Alzheimer`s Disease Assessment Scale – cognitive subscale
Obr. 3. Korelace mezi věkem, vzděláním a celkovým skóre ADAS-Cog.
Korelace pro věk byla signifikantní (r = 0,52; p < 0,001). Korelace pro vzdělání signifikantní nebyla (r = –0,17). Údaje o vzdělání byly k dispozici u 56 účastníků.
ADAS-Coq – Alzheimer`s Disease Assessment Scale – cognitive subscale
Tab. 2. Differences between males and females in ADAS-Cog. 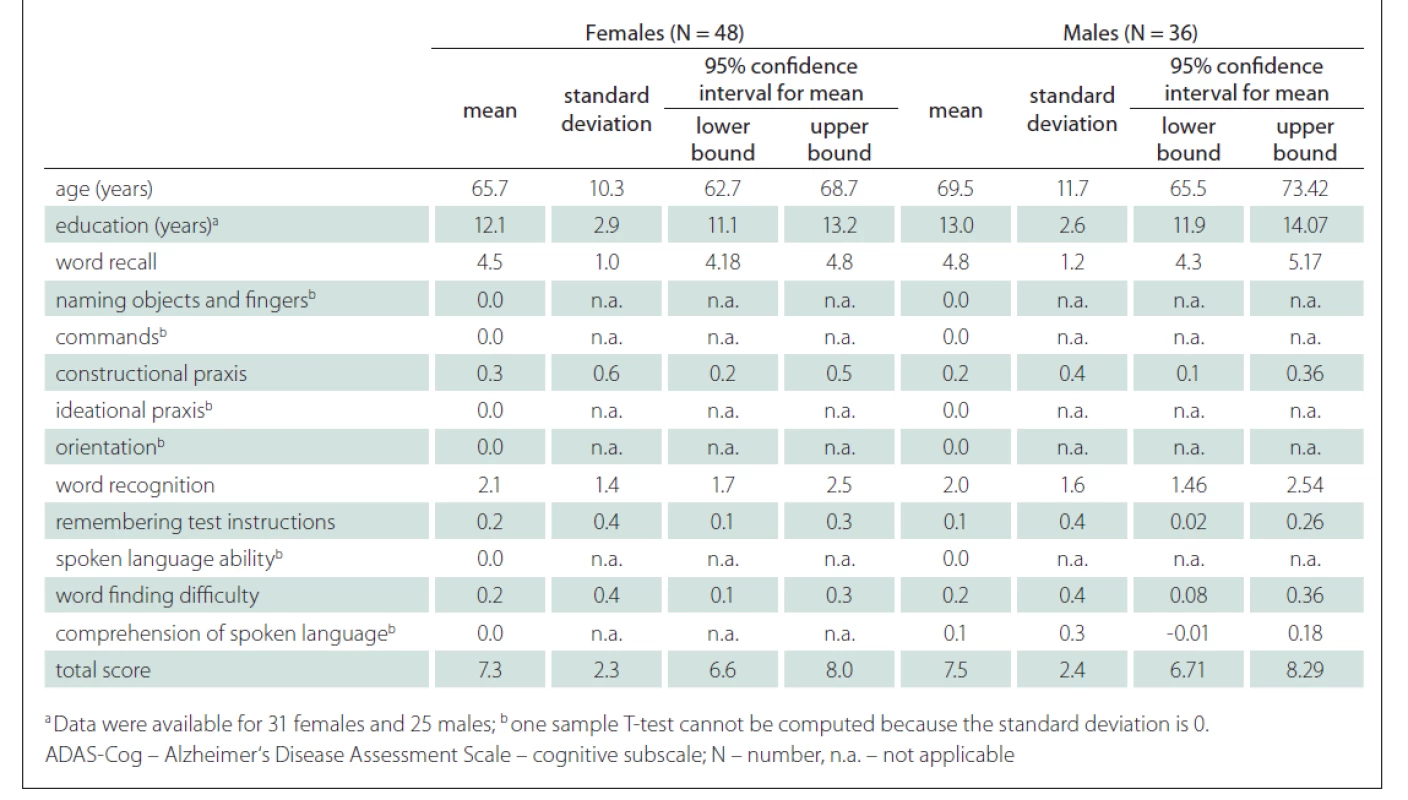
Tab. 3. Differences between cognitively unimpaired subjects aged 65 years or older and younger than 65 years on ADAS-Cog. 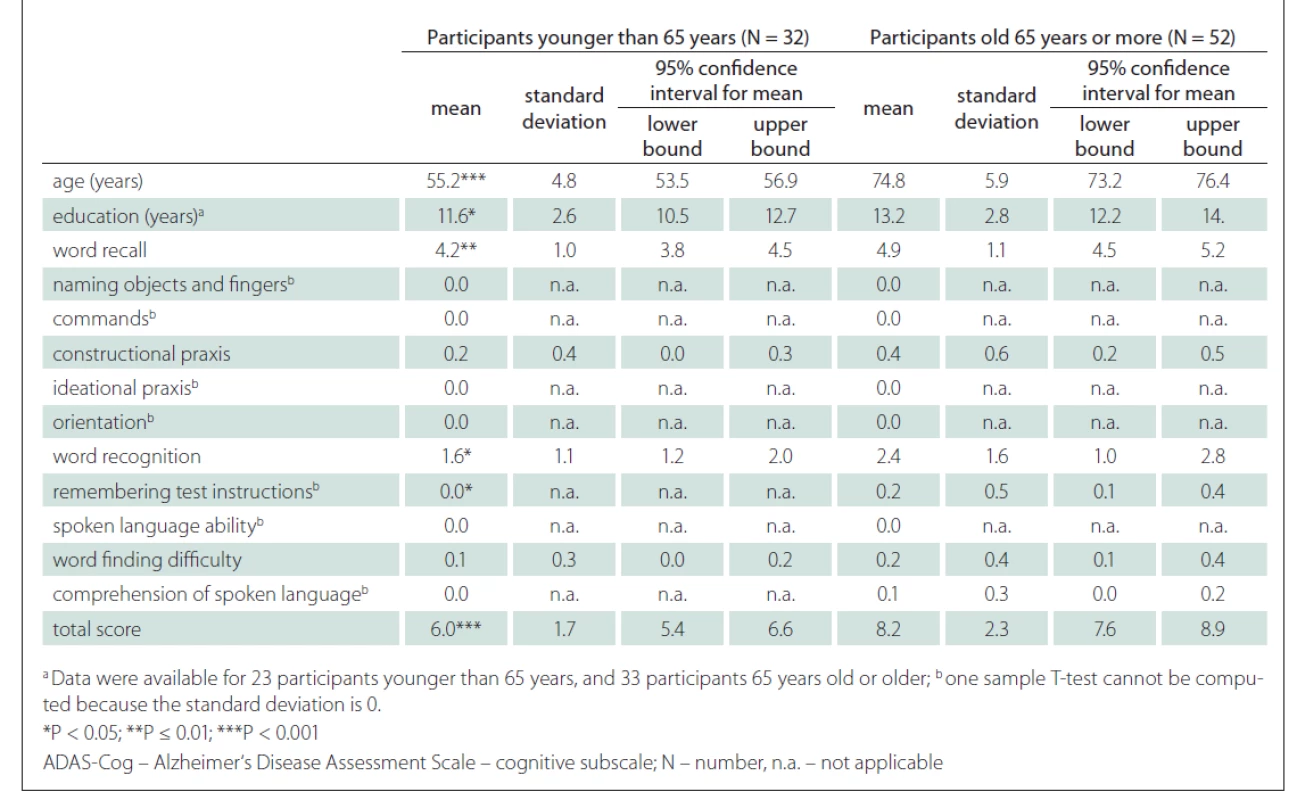
Using linear regression (R2 = 0.424, constant 0.688), we found a significant relationship (P < 0.001) between the ADAS-Cog total score and the age with a total score increase of 0.138 (standard error 0.23) for each year of age, and –0.236 (standard error 0.096) decrease for each year of education. There were no correlations between the total score and the sex of the participants.
Discussion
Our study aimed to standardize ADAS-Cog for the Slovenian population. The values for our sample obtained in our study are similar as in the following similar studies. In most studies, a mean ADAS-Cog total score of CU people is between 5 and 10 points [23,25–27,29–31,34,35]. Our study‘s mean total ADAS-Cog score (7.4 points) (Fig. 2) was very similar to the mean score from the study performed in Hungary (7.8 points) [27]. In comparison with their study, our sample was twice as large. However, our participants and theirs had nearly the same mean age and mean duration of education. The highest mean ADAS-Cog total score for the CU was reported from Korea (11.5) [32]. Such a high score can be ascribed to the specific characteristics of their sample. The participants in the Korean study were older than ours (mean age 72 vs. 67 years) and were significantly less educated (mean duration 4.2 vs. 12.5 years in our study). The lowest mean total score (4.0) was reported in the study from Singapore, where younger CU subjects were included, compared to our study (mean age 62 vs. 67 years). The duration of education did not differ significantly. [33]
The analysis of the individual tasks revealed that our participants made the most mistakes at the Word Recall and Word Recognition tasks. The same was shown in the Portuguese [26], Chinese [31] and Turkish [29] studies. These two tasks are believed to be the most difficult in the ADAS-Cog [23], where cognitively unimpaired individuals also tend to make a few mistakes. Furthermore, very few mistakes (mean score less than 0.5) were made at Constructional Praxis, Remembering Test Instructions and Word Finding Difficulty, similar to other studies [29,30,33]. In Constructional Praxis, mistakes were made when drawing a cube, which is the most complex geometrical object to copy in this task [34]. The ability to remember test instructions is assessed solely on the participant‘s performance in the Word Recognition task, which is among the most challenging tasks in ADAS-Cog. Scoring a single point on the Word Finding Difficulty task means that the participant was looking for a word once or twice, which is considered to be clinically irrelevant [23].
Effect of sex, age and education on the ADAS-Cog total score
In our sample, no differences were found between males and females (Tab. 2). Although our male participants were slightly older than female participants, they did not differ significantly in the duration of education, ADAS-Cog total scores or individual task scores. Sex was not a significant variable in the linear model. Our results are similar to the studies performed by Weyer et al [36], Graham et al [37], Liu et al [38] and Jemaa et al [30], who could not find differences in the performance between males and females on the ADAS-Cog.
As expected, we found a strong correlation (r = 0.52) between the ADAS-Cog total score and the age of participants (Fig. 3). A significant correlation between the total score and the age was previously shown by Graham et al [37], Jemaa et al [30], Pákáski et al [27] and Mavioglu et al [29]. Younger participants tend to make fewer mistakes and thus obtain a lower total ADAS-Cog score.
Age may not be the only factor that influences the total ADAS-Cog score. We found a weak significant correlation between the education and the ADAS-Cog total score (Fig. 3). The effect of education is not unified across the studies. For example, Mavioglu et al [29] noted that only age, but not education, influenced the total ADAS-Cog score. In another study, Graham et al [37] reported a significant correlation between the age and the total ADAS-Cog score and a very weak correlation (r = –0.04) between the education and the total ADAS-Cog score. One possible explanation for a weak correlation in the study by Graham et al is the low variability in education of the participants. When we compared our results with Graham et al [37] (Tab. 3), the results in our study were significantly higher than in the study of Graham et al (for younger than 65 years 6.0 vs 5.0 and for aged 65 years or more 8.2 vs 5.2) regardless of the age of the participants. One possible reason, besides cultural differences, may be that the duration of education in our study was shorter. The mean duration of education in our younger group was 11.6 vs. 15.3 years, while the mean duration of education in the older group was 13.2 vs. 15.6 years (Tab. 3).
On the other hand, several other studies found a significant correlation between the education and the total ADAS-cog score [8,11–15]. The results from some studies suggest that only low levels of education (e. g., 6 years or fewer) may influence the total ADAS-Cog scores [37–39]. In another study [39], statistically significant differences in the ADAS-Cog total scores were found only between high school dropouts and high school graduates. There were no statistically significant differences among better-educated groups (high school graduates, college graduates and post-graduates). The effect of education may be lost for people with ten or more years of schooling [37].
Limitations
The main limitation of our study is a relatively small sample size of very old and poorly educated participants. However, according to the statistical data, there are not many people with fewer than six years of education, since primary school that lasted eight years in the past (now nine years) is mandatory in Slovenia.
Another limitation is the intrinsic property of the ADAS-Cog. The results may be influenced by the age and the education of participant, as described previously. Jemaa et al [30] suggested a correction table for the ADAS-Cog total score to minimise the influence of age and education. The participants were divided into groups according to their education (primary, secondary, university) and age (50–59, 60–69, 70–79, 80+ years). In accordance with their age and education group, older and less educated participants had between 0 and 3 points subtracted from their total scores. Inversely, younger and more educated participants had between 0 and 3 points added to their total scores. Similarly, a simpler model may be used, where 6 points would be considered as a normal value for subjects younger than 65 years and 8 points as a normal value for subjects aged 65 years or older (Tab. 2).
We may also use our linear regression model for a more elaborate prediction of a normal ADAS-Cog score. The influence of education on the total score may not be obvious from the correlation. Still, a significant association may be found after creating a linear model considering both the education and the age. For example, for a 70-year-old CU subject with 14 years of education, an expected ADAS-Cog total score equals to 7 (0.688 + 0.138 × 70 – 0.236 × 14 = 7.044).
However, there are also limitations of our model. With the proposed formula, we can explain 42.4% of the sample, but we have to consider standard errors and may not make assumptions for subjects with an age or education out of our data range (Fig. 3).
There are also limitations related to the ADAS-Cog tasks themselves. For example, a demonstration of how to send a letter in the Ideational Praxis task may seem slightly outdated. However, our sample consisted of older participants who are probably still used to sending letters. For younger participants, a different task could be considered. Another drawback is that the last four tasks related to language are based on a subjective assessment of an examiner. Before solving the ADAS-Cog tasks and in between solving them, we had a short conversation with the participants from which we were able to assess their language abilities. Furthermore, each participant was evaluated by the same examiner.
Conclusion
In our study, we prepared data for the Slovenian population and a linear model for the total ADAS-Cog score. This may be of practical use for clinical work in countries with similar cultural, educational and social backgrounds as ours [40].
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). The study was approved by the Institutional Review Board, University Medical Centre Maribor (document number UKC-MB-KME-8/20). All participants signed informed consent forms.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
Acknowledgement
The authors would like to thank Mrs Katja Knez for proofreading the manuscript.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Martin Rakusa, MD, PhD
Department of Neurology
University Medical Centre Maribor
Ljubljanska ulica 5
2000 Maribor
Slovenia
e-mail: ris101@gmail.com
Accepted for review: 12. 6. 2021
Accepted for print: 29. 7. 2021
Zdroje
1. Rabinovici GD. Late-onset Alzheimer disease. Continuum (Minneap Minn) 2019; 25 (1): 14–33. doi: 10.1212/ CON.0000000000000700.
2. Žakelj N, Menih M, Rakuša M. Cognitive impairment and Parkinson’s disease dementia. Zdr Vestn 2020; 89 (9–10): 539–551. doi: 10.6016/ZdravVestn.3003.
3. Ovčar Štante K, Potočnik J, Rakuša M. Vascular cognitive impairment and vascular dementia. Zdr Vestn 2017; 86 (7–8): 330–344. doi: 10.6016/ZdravVestn.1543.
4. Cavazzoni P. FDA’s decision to approve new treatment for Alzheimer’s disease. [online]. Available from URL: https: //www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease.
5. Bartoš A, Smětáková M, Nosková L et al. Možnosti stanovení likvorového tripletu tau proteinů a b-amyloidu 42 metodami ELISA a orientační normativní vodítka. Cesk Slov Neurol N 2019; 82/115 (5): 533–540. doi: 10.14735/amcsnn2019533.
6. Rakuša M, Modric E. Validation and optimisation of cerebro-spinal fluid (CSF) biomarkers for Alzheimer’s disease. Neurodegener Dis 2017; 17 (Suppl 1): 998.
7. McKhann GM, Knopman DS, Chertkow H et al. The diag - nosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7 (3): 263–269. doi: 10.1016/j.jalz.2011.03.005.
8. Rakusa M, Jensterle J, Mlakar J. Clock drawing test: a simple scoring system for the accurate screening of cognitive impairment in patients with mild cognitive impairment and dementia. Dement Geriatr Cogn Disord 2018; 45 (5–6): 326–334. doi: 10.1159/000490381.
9. Bartoš A, Raisová M. Test mince v ruce k detekci předstírání oslabeného paměťového výkonu ve srovnání s mírnou kognitivní poruchou a s mírnou demencí u Alzheimerovy nemoci. Cesk Slov Neurol N 2019; 115 (3): 316–321. doi: 10.14735/amcsnn2019316.
10. Bartoš A. Test gest (TEGEST) k rychlému vyšetření epizodické paměti u mírné kognitivní poruchy. Cesk Slov Neurol N 2018; 81/114 (1): 37–44. doi: 10.14735/amcsnn201837.
11. Bartoš A, Polanská H. Správná a chybná pojmenování obrázků pro náročnější test písemného Pojmenování obrázků a jejich vybavení (dveřní POBAV). Cesk Slov Neurol N 2021; 84/117 (2): 151–163. doi: 10.48095/cccsnn2021151.
12. Bartoš A. Dvě původní české zkoušky k vyšetření paměti za tři minuty – Amensia Light and Brief Assessment (ALBA). Cesk Slov Neurol N 2019; 82/115 (4): 420–429. doi: 10.14735/amcsnn2019420.
13. Bartoš A, Diondet S. Test Amnesia Light and Brief Assessment (ALBA) – druhá verze a opakovaná vyšetření.Cesk Slov Neurol N 2020; 83/116 (5): 535–543. doi: 10.14735/amcsnn2020535.
14. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res 1975; 12 : 189–198. doi: 10.1016/0022-3956 (75) 90026-6.
15. Nasreddine Z, Phillips N, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53 (4): 695–699. doi: 10.1111/j.1532-5415.2005.53 221.x.
16. Bartos A, Fayette D. Validation of the Czech Montreal cognitive assessment for mild cognitive impairment due to Alzheimer disease and Czech norms in 1,552 elderly persons. Dement Geriatr Cogn Disord 2018; 46 (5–6): 335–345. doi: 10.1159/000494489.
17. Bartos A, Raisova M. The mini-mental state examination: Czech norms and cutoffs for mild dementia and mild cognitive impairment due to Alzheimer’s disease. Dement Geriatr Cogn Disord 2016; 42 (1–2): 50–57. doi: 10.1159/000446426.
18. Rakuša M, Granda G, Kogoj A et al. Mini-mental state examination: standardisation and validation for the elderly Slovenian population. Eur J Neurol 2006; 13 (2): 141–145. doi: 10.1111/j.1468-1331.2006.01185.x.
19. Potocnik J, Ovcar Stante K, Rakusa M. The validity of the Montreal cognitive assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke. Acta Neurol Belg 2020; 120 (3): 681–685. doi: 10.1007/s13760-020-01330-5.
20. Gil L, Ruiz de Sánchez C, Gil F et al. Validation of the Montreal cognitive assessment (MoCA) in Spanish as a screening tool for mild cognitive impairment and mild dementia in patients over 65 years old in Bogotá, Colombia. Int J Geriatr Psychiatry 2015; 30 (6): 655–662. doi: 10.1002/gps.4199.
21. Lifshitz M, Dwolatzky T, Press Y. Validation of the Hebrew version of the MoCA test as a screening instrument for the early detection of mild cognitive impairment in elderly individuals. J Geriatr Psychiatry Neurol 2012; 25 (3): 155–161. doi: 10.1177/0891988712457047.
22. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984; 141 (11): 1356–1364. doi: 10.1176/ajp.141.11.1356.
23. Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer’s disease assessment scale-cognitive subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. A narrative review. J Alzheimers Dis 2018; 63 (2): 423–444. doi: 10.3233/JAD-170 991.
24. Kolibáš E, Kořínková V, Novotný V et al. ADAS-cog (Alzheimer’s disease assessment scale-cognitive subscale) – validation of the Slovak version. Bratisl Lek Listy 2000; 101 (11): 598–602. doi: 10.1016/S0924-9338 (00) 94850-8.
25. Monllau A, Pena-Casanova J, Blesa R et al. Diagnostic value and functional correlations of the ADAS-Cog scale in Alzheimer’s disease: data on NORMACODEM project. Neurologia 2007; 22 (8): 493–501.
26. Nogueira J, Freitas S, Duro D et al. Validation study of the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-Cog) for the Portuguese patients with mild cognitive impairment and Alzheimer‘s disease. Clin Neuropsychol 2018; 32 (Suppl1): 46–59. doi: 10.1080/13854046.2018.1454511.
27. Pákáski M, Drótos G, Janka Z et al. Validation of the Hungarian version of Alzheimer’s disease assessment scale-cognitive subscale. Orv Hetil 2012; 153 (12): 461–466. doi: 10.1556/OH.2012.29332.
28. Tsolaki M, Fountoulakis K, Nakopoulou E et al. Alzheimer’s disease assessment scale: the validation of the scale in Greece in elderly demented patients and normal subjects. Dement Geriatr Cogn Disord 1997; 8 (5): 273–280. doi: 10.1159/000106644.
29. Mavioglu H, Gedizlioglu M, Akyel S et al. The validity and reliability of the Turkish version of Alzheimer’s disease assessment scale-cognitive subscale (ADAS-Cog) in patients with mild and moderate Alzheimer’s disease and normal subjects. Int J Geriatr Psychiatry 2006; 21 (3): 259–265. doi: 10.1002/gps.1457.
30. Ben Jemaa S, Attia Romdhane N, Bahri-Mrabet A et al. An Arabic version of the cognitive subscale of the Alzheimer’s disease assessment scale (ADAS-Cog): reliability, validity, and normative data. J Alzheimers Dis 2017; 60 : 11–21. doi: 10.3233/JAD-170222.
31. Yang H, Cheng Z, Li Z et al. Validation study of the Alzheimer’s disease assessment scale-cognitive subscale for people with mild cognitive impairment and Alzheimer’s disease in Chinese communities. Int J Geriatr Psychiatry 2019; 34 (11): 1658–1666. doi: 10.1002/gps.5179.
32. Youn JC, Lee DY, Kim KW et al. Development of the Korean version of Alzheimer’s disease assessment scale (ADAS-K). Int J Geriatr Psychiatry 2002; 17 (9): 797–803. doi: 10.1002/gps.699.
33. Zainal NH, Silva E, Lim LLH et al. Psychometric properties of Alzheimer’s disease assessment scale-cognitive subscale for mild cognitive impairment and mild Alzheimer’s disease patients in an Asian context. Ann Acad Med Singap 2016; 45 (7): 273–283.
34. Schafer K, Aisen P, Böhm P et al. Administration and scoring manual Alzheimer’s disease assessment scale-cognitive (ADAS-cog). New York: The Mount Sinai School of Medicine 2012.
35. Zec RF, Landreth ES, Vicari SK et al. Alzheimer disease assessment scale. Alzheimer Dis Assoc Disord 1992; 6 (3): 164–181. doi: 10.1097/00002093-199206030-00004.
36. Weyer G, Erzigkeit H, Kanowski S et al. Alzheimer’s disease assessment scale: reliability and validity in a multicenter clinical trial. Int Psychogeriatrics 1997; 9 (2): 123–138. doi: 10.1017/S1041610297004298.
37. Graham DP, Cully JA, Snow AL et al. The Alzheimer’s disease assessment scale-cognitive subscale: normative data for older adult controls. Alzheimer Dis Assoc Disord 2004; 18 (4): 236–240.
38. Liu H-C, Lee Teng E, Chuang Y-Y et al. The Alzheimer’s disease assessment scale: findings from a low-education population. Dement Geriatr Cogn Disord 2002; 13 (1): 21–26. doi: 10.1159/000048629.
39. Doraiswamy PM, Krishen A, Stallone F et al. Cognitive performance on the Alzheimer’s disease assessment scale: effect of education. Neurology 1995; 45 (11): 1980–1984. doi: 10.1212/WNL.45.11.1980.
40. Ulbl J, Rakusa M. Validation of Slovenian version of ADAS-Cog for patients with mild cognitive impairment and Alzheimer’s disease. Acta Neurol Belg 2021 [ahead of print]. doi: 10.1007/s13760-021-01780-5.
Štítky
Detská neurológia Neurochirurgia Neurológia
Článok vyšiel v časopiseČeská a slovenská neurologie a neurochirurgie
Najčítanejšie tento týždeň
2021 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Naděje budí časná diagnostika Parkinsonovy choroby založená na pachu kůže
- Neuromultivit v terapii neuropatií, neuritid a neuralgií u dospělých pacientů
-
Všetky články tohto čísla
- Editorial
- Proč se dráhy kříží? Základní principy uspořádání mozku obratlovců
- Role mikroRNA v patogenezi spinální muskulární atrofie
- Nové možnosti laboratórnej diagnostiky ochorení spojených s tvorbou amyloidov
- Využití rohovkové konfokální mikroskopie u neurologických onemocnění
- Poruchy čichu po COVID-19 – diagnostika, význam a léčba
- Kappa free light chains in multiple sclerosis – diagnostic accuracy and comparison with other markers
- Studijní protokol – robotická terapie chůze pomocí přístroje Lokomat Pro FreeD u pacientů v subakutní fázi ischemické cévní mozkové příhody
- Validace dotazníku pro poruchy chůze u pacientů s roztroušenou sklerózou – česká verze MSWS-12
- Characterization of swallowing disorders in myasthenia gravis through a fibre-optic endoscopic evaluation
- Standardisation of the Slovenian version of the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog)
- The frequency of silent brain infarcts in polycythaemia vera and essential thrombocytosis
- COVID-19 u nemocných s myasthenia gravis
- CANVAS – nově identifikovaná genetická příčina ataxie s pozdním nástupem. Popis prvních diagnostikovaných pacientů v České republice
- Benefits of 18F-FET PET in preoperative assessment of glioma heterogeneity demonstrated in two case reports
- COVID-19 asociovaná myelitida – kazuistika vzácné komplikace závažné SARS-CoV-2 infekce
- Intramedulární absces
- Successful usage of rituximab in a patient with overlapping myelin oligodendrocyte glycoprotein encephalomyelitis and systemic lupus erythematosus
- Informace vedoucího redaktora
- Prof. MUDr. Hana Krejčová, DrSc. 90letá
- Aktualita z kongresu EAN 2021
- Česká a slovenská neurologie a neurochirurgie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Poruchy čichu po COVID-19 – diagnostika, význam a léčba
- CANVAS – nově identifikovaná genetická příčina ataxie s pozdním nástupem. Popis prvních diagnostikovaných pacientů v České republice
- Proč se dráhy kříží? Základní principy uspořádání mozku obratlovců
- COVID-19 asociovaná myelitida – kazuistika vzácné komplikace závažné SARS-CoV-2 infekce
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



