-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Prevalence of infectious complications in burn patients requiring intensive care: data from a pan-European study
Prevalence infekčních komplikací u popálených pacientů vyžadujících intenzivní péči: data z panevropské studie
Cíl studie:
Stanovit prevalenci infekčních komplikací u popálených pacientů vyžadujících intenzivní péči v rámci jednodenní multicentrické studie za účasti řady popáleninových center z různých zemí Evropy.Materiál a metodika:
Celkem bylo v databázi EBA vybráno 87 center jak v západní, tak i ve východní Evropě. Pro diagnostiku jednotlivých infekčních komplikací u pacientů s termickým traumatem, byla použita doporučení ABA (Americká popáleninová asociace). Získaná data byla statisticky zpracována.Výsledky:
Celkem bylo do studie zařazeno 134 pacientů (44 en) z 27 popáleninových center v Evropě. Průměrný věk pacientů v souboru byl 40,39 roku (SD ± 22,17). Průměrné ABSI bylo 7,5 (SD ± 2,54), průměrný rozsah popálené plochy byl 30,49 % TBSA (SD ± 20,14). Průměrná délka hospitalizace v den provedení studie byla 24,32 dní (SD ± 30,64). Infekční komplikace byly zjištěny u 92 pacientů (68,7 %). Celkem 76 pacientů (56,7 %) splňovalo kritéria pro infekci popálené plochy, 26 pacientů (19,4 %) pro infekci krevního řečiště, 21 pacientů (15,7 %) pro pneumonii, 13 pacientů (9,7 %) pro infekci močového systému. U 29 pacientů (21,6 %) byla zjištěna multifokální infekce.
U 67 pacientů (50,0 %) byl zjištěn jako potenciálně patogenní mikroorganismus grampozitivní kmen bakterie, u 73 pacientů (54,5 %) gramnegativní kmen bakterie a u 18 pacientů (13,4 %) byla zjištěna kvasinka. U žádného z pacientů v souboru nebyla izolována vláknitá houba.Závěr:
Jedním ze základních pilířů budoucích standardů v jednotlivých popáleninových centrech je monitorování výskytu infekčních komplikací u popálených pacientů, prevence šíření těchto komplikací a hlášení rezistentních patogenů v Evropě. Předkládaná práce je důležitým projektem právě v této oblasti.Klíčová slova:
popáleniny – infekční komplikace – bakterie – inhalační trauma – Evropa
Authors: B. Lipový 1,2; P. Brychta 1,2; H. Řihová 1; M. Hanslianová 3; A. Loskotová 1; Jiří Jarkovský 4
; Y. Kaloudová 1; I. Suchánek 1
Authors place of work: Department of Burns and Reconstructive Surgery, University Hospital Brno, Czech Repub 1; Faculty of Medicine, Masaryk University Brno, Czech Republic 2; Department of Medical Microbiology, University Hospital Brno, Czech Republic 3; Institute of Biostatistics and Analyses, Masaryk University Brno, Czech Republic 4
Published in the journal: Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 1, s. 25-32
Category: Původní práce
Summary
The objective of this study was to determine the prevalence of infectious complications in burn patients requiring intensive care in a one-day multicenter study encompassing burn centers in various European countries.
Material and methods:
The European Burns Association database identified 87 centers in Western and Eastern Europe, 27 of which agreed to cooperate. American Burn Association recommendations were used for diagnosis of various infectious complications in patients with thermal trauma.Results:
From those centers, we randomly assigned 134 patients (44 women) to the analysis. Mean age of the group was 40.39 ± 22.17(SD) years. Mean abbreviated burn severity index was 7.5±2.54, mean size of burned area was 30.49 ± 20.14% of total body surface area. Mean length of hospitalization to date was 24.32 ± 30.64 days. Infectious complications were observed in 92 patients (68.7%), 76 (56.7%) of whom met the criteria for infection of the burned area, 26 patients (19.4%) for bloodstream infection, 21 (15.7%) for pneumonia, and 13 (9.7%) for urinary system infection. Multifocal infections were found in 29 patients (21.6%). Gram-positive bacterial strains as potentially pathogenic microorganisms were identified in 67 patients (50.0%), Gram-negative bacterial strains in 73 (54.5%), and yeasts in 18 (13.4%) patients. Filamentous fungi were not isolated from any patient in the group.Conclusion:
Cornerstone future standards in individual burn centers should be to monitor the occurrence of infectious complications in burn patients, prevent the spread of these complications, and report resistant pathogens. This work constitutes an important project in this area.Keywords:
burns – infectious complications – bacteria – inhalation injury – EuropeINTRODUCTION
Infectious complications today constitute a significant medical and economic problem both for severely burned patients and in patients requiring intensive care generally. Severely burned patients are confronted daily with numerous potentially pathogenic microorganisms (PPMs). Thus, prevention and treatment of infectious complications during the treatment of thermal trauma is the greatest challenge facing all burn teams around the world. From the perspective of developing infectious complications, patients with serious or critical burn injuries are among the most vulnerable on the whole. Necrotic tissue resulting from burns provides an excellent growth medium not only for bacteria but also for yeasts and fungi [1].
Patients with severe burns often develop a specific form of immunodeficiency or immunosuppression, which is determined especially by the severity of the trauma [2].
MATERIALS AND METHODS
It was first necessary to discuss this project with the European Burns Association (EBA). After the necessary documentation had been prepared and presented, the project was unanimously approved by the EBA’s International Scientific Council on 19 March 2011. Thereafter, individual burn centers in Europe providing intensive care for patients with thermal trauma were identified and contacted utilizing the EBA database. A query of the database returned 87 relevant centers in Western and Eastern Europe. The study’s design was prepared in detail and carried out by questionnaire. During the first phase, all centers returned by the database query were sent an initial letter. In accordance with promises for active participation, the next step was to establish clear objectives and criteria while setting the exact date of the 1-day prevalence study and then to send out the questionnaire. Due to the absence of clear recommendations from the EBA for the diagnosis of individual infectious complications in patients with thermal trauma, the recommendations formulated by the American Burn Association were used [3]. To evaluate the severity of burn trauma, the abbreviated burn severity index (ABSI) score was used [4].
Statistical processing
Categorical variables were described by the absolute and relative frequencies of the categories and continuous variables were described by the mean and standard deviation (SD) as well as the median and 5-to-95 percentile range. Due to the asymmetric distribution of a number of continuous variables, mean values should be interpreted as an overall characterization of the analyzed population; medians can then be interpreted as characteristic of a typical patient within the group.
The non-parametric Mann–Whitney U test was used to test the differences in continuous variables between groups of patients. In the case of categorical variables, the chi-squared test of maximum plausibility was used. Logistic regression was used for analyzing risk factors for patient death.
Statistical significance for all tests was set at the level α = 0.05. Statistical analyses were made in IBM SPSS Statistics software version 20.0.0 (Armonk, New York, USA).
RESULTS
Basic demographic data
The study included a total of 27 burn centers from 12 European countries. Of the 134 patients included in the dataset, 44 were women (32.8%). Thus, the ratio of men to women was 2.65 : 1. The mean age (± SD) of the group was 40.39 ± 22.17 years. The youngest patient was one year old, the oldest 83 years old. The study included 20 children (14.9%) 0–19 years of age. Fig. 1. shows the patient population’s age distribution.
Figure 1. Age distribution of patients in the group 
The mean extent of the burned area for patients within the group was 30.49 ± 20.14% of total body surface area (TBSA). The largest number of patients was hospitalized with burned areas within a range of 11–20% of TBSA (43 patients, or 32.1%) followed by those within range 21–30% of TBSA (25 patients, or 18.7%). The distribution of patient numbers according to the extent of the burned area is shown in Fig. 2.
Figure 2. Distribution of patients according to extent of burned area 
The mean length of hospitalization to date as of the day of microbiological investigation was 24.32 ± 30.64 days. The largest numbers of patients had been hospitalized for 10 days or less since being admitted due to the burn injury (45 patients, 33.6%) and the second largest group had been in the hospital already for 11–20 days (43 patients, 32.1%). Fig. 3. shows the distribution of patient numbers according to length of hospitalization to date.
Figure 3. Distribution of patients according to length of hospitalization to date 
The mean ABSI value for patients within the cohort was 7.5 ± 2.54. The highest number of patients was recorded in the category ≤ 5 (37 patients, 27.6%) and then in the categories 6–7 and 8–9 (32 patients each, 23.9%). The category ABSI ≥ 12 contained only 7 patients (5.2%). The highest ABSI value recorded for patients in the cohort was 15.
What is the risk of developing infectious complications in severely burned patients? What is the most common infection localization in severely burned patients?
Of the total 134 patients, 92 patients (68.7%) had at least one infectious complication in the screening day. A total of 29 patients (21.6%) had multifocal infections. The most common infectious complication was infection of the burned area, which was recorded in 76 patients (56.7%). Table 1 describes the frequency of infectious complications in burned patients included in the group.
Tab. 1. Representation of individual infectious complications in patients within the group (N = 134) 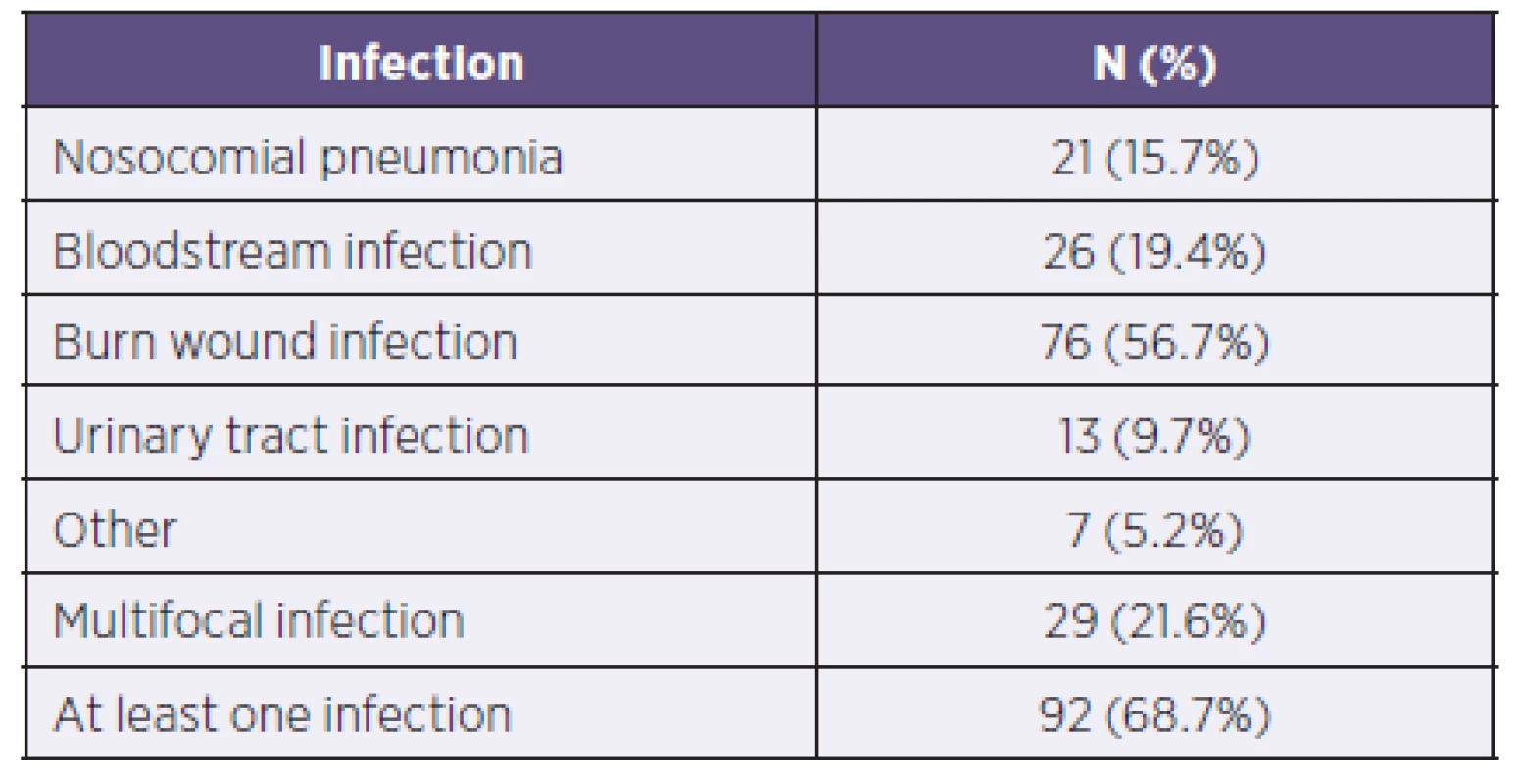
How does the risk of developing infectious complications correspond to the ABSI value?
In the group with ABSI values ≤ 5, infectious complications were observed in 17 patients (45.9%). All these patients had infections of the burned area and only one patient had also a diagnosed retropharyngeal abscess. In the category ABSI 6–10, already 55 patients (72.4%) had verified infectious complications. In the group with ABSI values ≥ 11 totaling 21 patients, 20 (95.2%) of them had specified infectious complications. Table 2 provides a complete overview of the risk of developing infectious complications according to the ABSI values, including characterization of individual organ systems.
Tab. 2. Representation of individual potentially pathogenic microorganisms in individual organ systems of patients in the study 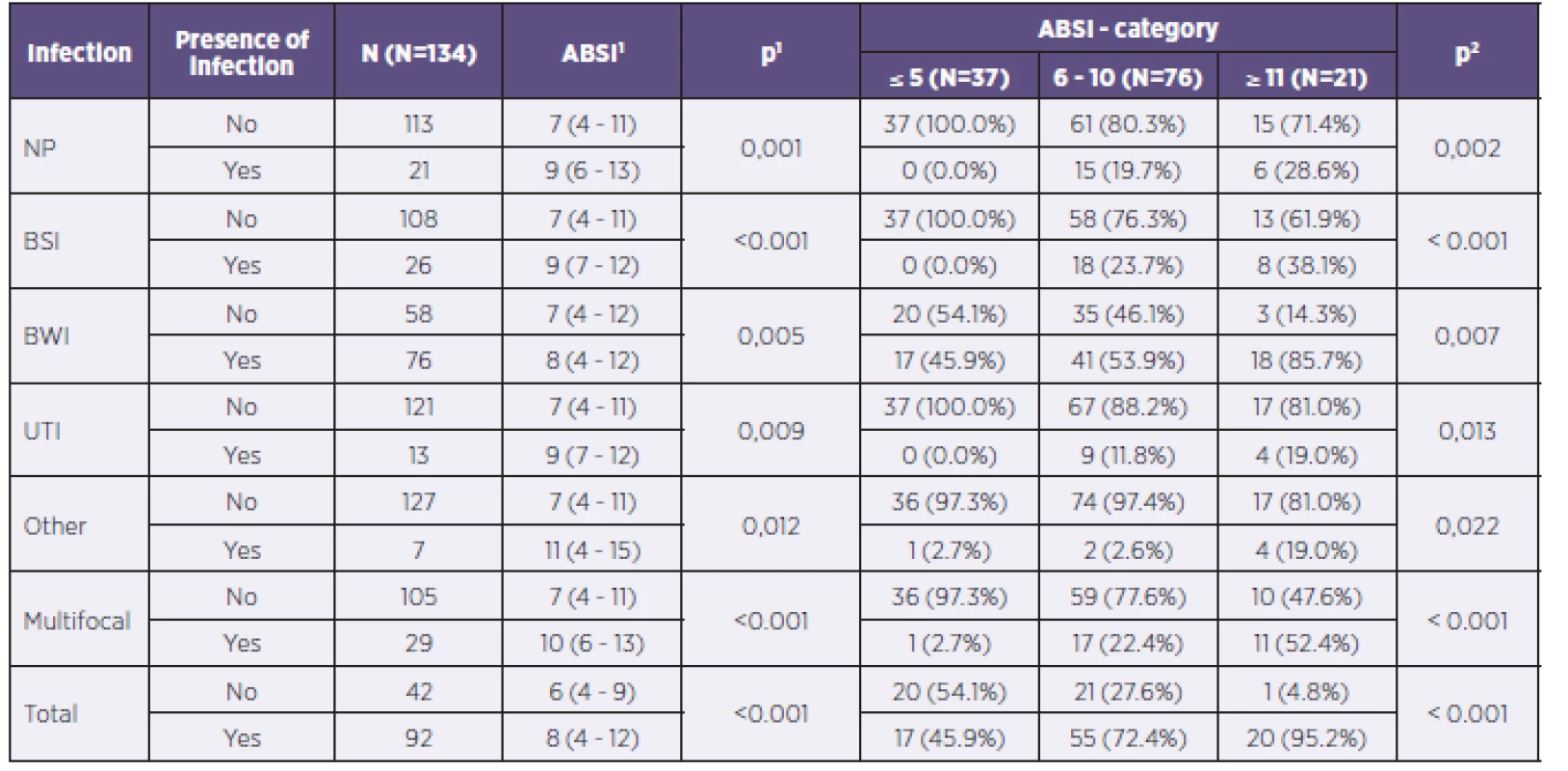
NP = nosocomial pneumonia BSI = bloodstream infection BWI = burn wound infection UTI = urinary tract infection 1ABSI score is described using the median and 5–95% percentile. Tested using the Mann–Whitney U test. 2Tested using the chi-squared test of maximum plausibility. What are the age and gender proportions for developing infection? How does the length of hospitalization correlate with the risk of developing infectious complications?
The studied group included 44 women and 90 men. In 28 women (63.6%) and 64 men (71.1%), at least one infectious complication was diagnosed. Statistically significant differences by gender were not observed in any of the fields, including that of multifocal infectious complications.
Mean age of patients with infectious complications was 43.5 years, and that of patients without infection was 40.0 years. Across all subperiods for length of hospitalization to date, there occurred an increase in the number of patients with diagnosed infections in relation to the duration of that hospitalization. While in the period up to 10 days from the start of hospitalization infectious complications were diagnosed in 25 out of a total of 45 patients (55.6%), in the period longer than 41 days of hospitalization infectious complications were observed in 14 out of 15 patients (93.3%).
A complete evaluation of the impact of age, gender, and length of hospitalization to date on the risk of developing infectious complications overall and by individual organ systems in patients within the group is shown in Table 3.
Tab. 3. Complete evaluation of the influence of age, length of hospitalization to date, and gender on the risk of developing infectious complications overall and by individual organ systems in the patient population 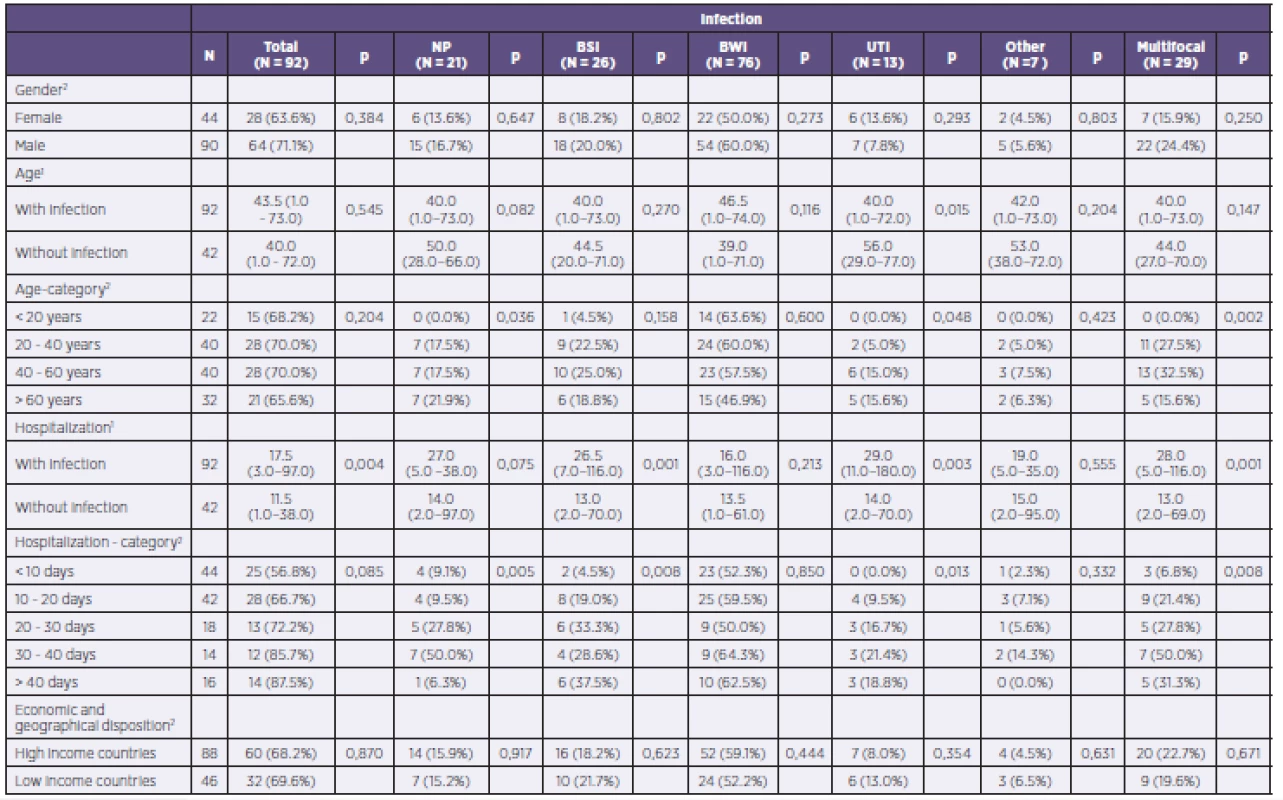
NP = nosocomial pneumonia BSI = bloodstream infection BWI = burn wound infection UTI = urinary tract infection 1Continuous variables described using the median and 5–95% percentile. Tested using the Mann–Whitney U test 2Categorical variables described using absolute and relative frequencies. Tested using the chi-squared test of maximum plausibility. What is the spectrum of major pathogens in severely burned patients? How does the PPM spectrum causing infection differ during the treatment of thermal trauma?
A total of 168 PPMs were isolated from patients in the study. Bacteria had the highest proportion, with a total of 67 isolates of Gram-positive and 73 of Gram-negative bacteria. In 10 cases, the specific PPMs responsible for infection were not detected on the day of the study.
Gram-negative bacteria dominated as the etiological agent in the development of nosocomial pneumonia (52.4% of cases) while Gram-positive bacteria were more frequently isolated as agents developing bloodstream infections (53.8%). Representation of individual PPMs in the different organ systems is shown in Table 4.
Tab. 4. Representation of individual potentially pathogenic microorganisms in individual organ systems of patients in the study 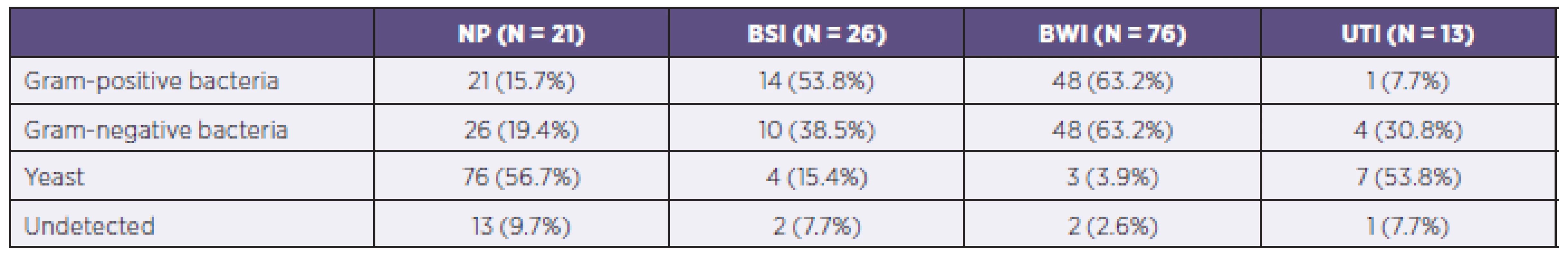
NP = nosocomial pneumonia BSI = bloodstream infection BWI = burn wound infection UTI = urinary tract infection The most common PPM in the studied group was Pseudomonas aeruginosa, which was isolated in 42 cases and caused 47.4%of infections of the burned area and 19.0% of cases of nosocomial pneumonia. Staphylococcus aureus was identified as the causative agent in the burned area in 38.2% of such infections and 19.0% of cases of nosocomial pneumonia. Unlike strains of Staphylococcus aureus, coagulase-negative Staphylococcus species were isolated only in 16 cases of infectious complications. Almost every fourth PPM causing bloodstream infections was a coagulase-negative Staphylococcus species. Detailed analysis of all PPM determined inpatients within the group is shown in Table 5.
Tab. 5. All isolated and identified potentially pathogenic microorganisms according to infections of individual organ systems 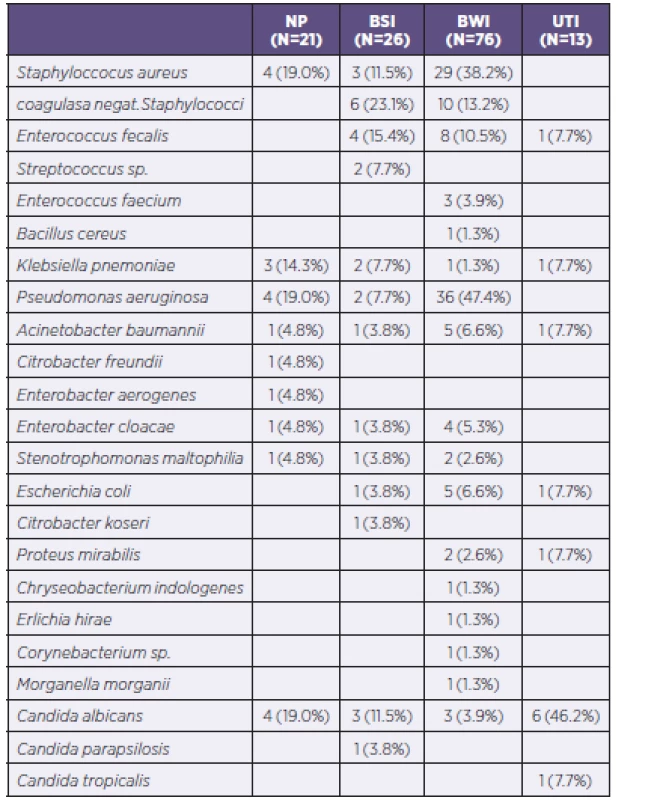
NP = nosocomial pneumonia BSI = bloodstream infection BWI = burn wound infection UTI = urinary tract infection During hospitalization, there occurred an increasing number of PPMs from gram-negative spectrum in monitored patients. While during the first 10 days of hospitalization the G+/G - ratio was 1.63 : 1, in the period 11 to 20 days the G+/G - ratio was 1.23 : 1, in the period of 21–30 days the G+/G - ratio was 1 : 2, and in the period of 31–40 days the G+/G - ratio was already 1 : 3.4. The ratio in the period > 40 days of hospitalization was already 1 : 1.
DISCUSSION
The study included 134 patients from 27 burn centers in 12 European countries. Although the total number of patients is not sufficient for an accurate assessment of the situation in Europe, almost regular distribution of individual burn centers in different parts of Europe, allows at least partial orientation in the issue. A total of 92 patients (68.7%) had at least one infectious complication on the day of microbiological investigation. Infection of the burned area was the most common infectious complication in burn patients within the study. All important studies on the topic of infectious complications have reached the same conclusion. Although since the 1970s therapeutic treatment of the burned area has become quite aggressive, even today infection of the burned area is the most common of all infectious complications. Compared to bloodstream infections or infections of the lower respiratory tract, infection of the burned area does not carry with it such high risk of death or of developing other complications.
There are many epidemiological studies today that deal with infection of the burned area. Lari and Alaghehbandan described in their work the representation of PPMs affecting the burned area during the first week following the beginning of hospitalization in Teheran [5]. During a period of 18 months, a total of 1,410 imprints or smears from the burned area were collected from 582 hospitalized patients. What is surprising about that study is the fact that on the first day of hospitalization the most common cause of infection of the burned area was identified as Pseudomonas aeruginosa (35.5%) and its representation increased to 87.2% during the first week. Conversely, the representation of other PPMs declined during this period (e.g., Staphylococcus aureus was 34.4% on the first day of hospitalization and 9.2% on day 7 of hospitalization while Acinetobacter baumannii infection rate was 22.2% on day one of hospitalization and 3.5% on day 7). Unfortunately, no data is available in this work that would explain why this was the case. We do not know, for example, if it was due to delayed hospitalization, the quality of pre-hospital care, or something else. Even though that study was from the turn of the millennium, a generally inadvisable precaution surprisingly was taken as a matter of everyday practice at that burn center according to this work: all patients with moderate or severe burns were administered a prophylactic parenteral antibiotic combination (cephalothin and amikacin) and topical silver–sulfadiazine cream. We will never know whether this precaution reduced the incidence of PPMs in burn patients or led to the selection of more resistant strains of bacteria. Despite this fact, the prophylactic use of antibiotics in burn patients has not been and currently is not in compliance with the recommendations of many professional societies.
Keen et al. described the bacteriological profile in the burned area in patients who had suffered thermal trauma in the context of armed conflict while also comparing these data with those from burned civilians [6]. It was assumed that the burned area of war victims would be generally much more greatly contaminated than those of patients who suffer burns in peaceful conditions. In comparison with the cases of burned civilians, patients with thermal trauma from war were especially more likely to be infected by Acinetobacter baumannii and Pseudomonas aeruginosa strains. Dominance was expressed mainly quantitatively (according to the timing of primary detection of these Gram-negative bacteria). The median first isolation of A. baumannii and P. aeruginosa in burned soldiers was 2 days and 13 days after the injury, respectively, while in burnt civilians it was 9 days and 18 days, respectively.
Taneja et al. compared the use of antibiotics in groups of patients with proven infection (of the burned area and bloodstream) and in those without evidence of infection (7). From 33 patients with proven infection of the burned area, there were isolated 23 strains of S.aureus, 15 strains of P. aeruginosa, and 12 strains of Streptococcus β hemolyticus. In the study, there were 14 patients with documented infections who received third-generation cephalosporins. There would be nothing special about this if 9 patients without evidence of infection had not also received third-generation cephalosporins. Similarly striking is the administration of aminoglycosides in this group of patients (24 patients with confirmed infection and 10 patients without infection). Although this is not discussed in the article, it can be expected that the administration of aminoglycosides may occur in patients without the evidence of infection only as a prophylactic measure. No accepted scheme exists which would systematically assign prophylactic administration of third-generation cephalosporins, mainly due to their high potential for selecting multidrug-resistant strains of bacteria.
Bloodstream infection is one of the most feared complications during the treatment of burn injuries. This means the penetration of microorganisms into the bloodstream in association with more generalized infection. The basic division of bloodstream infection is into primary, secondary, infective endocarditis, and so-called catheter sepsis or catheter-related bloodstream infection. An Israeli group led by Raz-Pasteur published results of a retrospective collection of data regarding the incidence of early - and late-onset bloodstream infection [8]. As the early form of bloodstream infection was therein defined, infections developed during the first week after the beginning of hospitalization. Bloodstream infection occurring after the first week of hospitalization was defined as late-onset infection. During 2001–2009, 159 severely burned patients were identified. Of these, 74 patients (46.5%) had at least one episode of bloodstream infection. Also observed was change in the qualitative composition of the PPM causing the bloodstream infection. In late-onset infections, there was an increase in the number of Gram-negative bacteria and generally selection of more resistant clones of bacteria (e. g., Klebsiella pneumoniae producing carbapenemase, methicillin-resistant S. aureus, imipenem-resistant P. aeruginosa). In contrast to late-onset infections, no yeast was identified as the originator of bloodstream infection in early-onset cases.
Soares de Macedo and Santos presented findings related to infectious complications in patients with thermal trauma in 2006 [9]. Theirs was a monocentric and prospective collection of data from the burn center of Da Asa Norte in Brazil. In this study, it is surprising that bloodstream infection was the most common infectious complication in 278 patients hospitalized at the center during 2004. Its etiology was dominated by such Gram--positive cocci as coagulase-negative Staphylococcus species (19 cases out of 72) and S.aureus (14 cases).
Recent work by a group of Australian authors led by Patel dealt comprehensively with the issue of bloodstream infections [10]. They evaluated data retrospectively obtained in the course of 11 years. The total number of patients hospitalized in the burn center was 2,364. Of this number, bloodstream infection was identified in 99 patients (212 episodes). In 20 patients, the source of the bloodstream infection was infection of the lower respiratory tract and only in 10 patients was it infection of the burned area. In 30 patients, however, the source of bloodstream infection (meaning here the primary bloodstream infection) was not determined. The most common primary pathogen cultured and identified as the originator of bloodstream infection was P. aeruginosa (30 patients), followed by strains of methicillin-resistant Staphylococcus species (17 patients), coagulase-negative Staphylococcus species (14 patient), and A. baumannii (10 patients). Mortality among patients with bloodstream infections differed by type of bacteria. From the total of 99 patients, 19 patients died. For 6 patients, the cause of bloodstream infection was P. aeruginosa, for three patients it was A. baumannii, and for three patients it was strains of Staphylococcus. These results suggest a higher mortality in patients with bloodstream infections caused by Gram-negative bacilli.
Burn patients also constitute a high-risk group in terms of lower respiratory tract infection, especially if it is complicating the diagnosis of inhalation injury. The study by Cotte et al. notes the fact that intubation in prehospital care in patients with burns localized in the head and neck area is an independent risk factor for early-onset pneumonia [11]. The fact that the incidence of ventilator-associated pneumonia in patients with inhalation injuries is high was also noted by Brusselaers et al. [12]. Of the 70 recorded episodes of ventilator-acquired pneumonia, a total of 23 episodes were caused by multidrug-resistant pathogens, in particular P. aeruginosa. Treatment of such infections is often very complicated. Benefits from using antibiotics in a nebulized form in patients with lower respiratory tract infections and inhalation injuries have been reported by Ackerman et al. [13].
According to several studies, the most important agents of urinary infections in burn patients are representatives of the Enterobacteriaceae, especially Escherichia coli [14–16]. Thus, unlike in other areas, urogenital tract infections and perianogenital area infections are far more frequently caused by Gram-negative bacilli than they are by Gram-positive cocci. This is true throughout the entire treatment course for affected patients. In our study, a total of 13 PPMs were isolated from the urinary systems of patients in the group. In only one patient was this a Gram-positive strain (Enterococcus faecalis) while the other 4 strains were from the Gram-negative part of the spectrum. It is nevertheless surprising that bacteria were not the dominating PPMs in the development of urinary tract infection in the patient population, as 7 examples of Candida species were also isolated. Candida albicans was isolated 6 times and in only one case was a non-albicans Candida species isolated (Candida tropicalis).
In their work, Kim et al. described a dramatic increase in the number of yeast infections in the urinary system, especially during the second week after the establishment of a permanent urethral catheter [17]. These observations were dominated by non-albicans Candida species, with the largest representation being Candida tropicalis (60.2%). This finding is in contrast to the situation we examined, because in our group of patients Candida albicans dominated.
The presence of a foreign material (permanent urinary catheter) leads to an increased risk for developing infectious complications in the urinary system (18). On the other hand, patients with less severe burns do not require the establishment of a permanent urinary catheter, and thus the incidence of infectious complications in this area will be lower compared with the group with established permanent urinary catheters.
We can say that the risk of developing infectious complications in the urinary system, as well as in other areas, is caused by more factors than simply the presence or absence of a permanent urinary catheter.
CONCLUSION
In cooperation with many burn centers in Europe, we succeeded in this study to determine the highest-risk PPMs in burn patients requiring intensive care. Regular monitoring of nosocomial infections and accurate reporting of particularly dangerous PPMs may lead to improvements in the quality of care provided to these patients.
Acknowledgment: We would like to acknowledge all European burn centers participating in this study and the European Burns Association for allowing the study design.
Do redakce došlo dne 7. 7. 2015.
Adresa pro korespondenci:
MUDr. Břetislav Lipový, Ph.D.
Jihlavská 20
625 00 Brno
e-mail: b.lipovy@seznam.cz
Zdroje
1. Lipový B, Řihová H, Hanslianová M, et al. Unsuccessful therapy of combined mycotic infection in a severely burned patient: A case study. Acta Chir Plast, 2009;51(3-4):83–84.
2. Heideman M, Bengtsson A. The immunologic response to thermal injury. World J Surg, 1992;16(1):53–56.
3. Greenhalgh DG, Saffle JR, Holmes JH 4th, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res, 2007;28(6):776–790.
4. Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med, 1982;11(5):260–262.
5. Lari AR, Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns, 2000;26(8):737–740.
6. Keen EF 3rd, Robinson BJ, Hospenthal DR, et al. Incidence and bacteriology of burn infections at a military burn center. Burns, 2010;36(4):461–468.
7. Taneja N, Emmanuel R, Chari PS, et al. A prospective study of hospital-acquired infections in burn patients at a tertiary care referral centre in North India. Burns, 2004;30(7):665–669.
8. Raz-Pasteur A, Hussein K, Finkelstein R, et al. Blood stream infections (BSI) in severe burn patients – early and late BSI: a 9-year study. Burns, 2013;39(4):636–642.
9. Soares de Macedo JL, Santos JB. Nosocomial infections in a Brazilian Burn Unit. Burns, 2006;32(4):477–481.
10. Patel BM, Paratz JD, Mallet A, et al. Characteristics of bloodstream infections in burn patients: An 11-year retrospective study. Burns, 2012;38(5):685–690.
11. Cotte J, Prunet B, Esnault P, et al. Early onset pneumonia in patients with severely burned face and neck: A 5-year retrospective study. Burns, 2013:pii: S0305–4179(12)00395-6. doi: 10.1016/j.burns.2012.12.003.
12. Brusselaers N, Labeau S, Vogelaers D, et al. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med, 2013;39(3):365–375. doi: 10.1007/s00134-012-2759-x.
13. Ackerman BH, Haith LR Jr, Patton ML, et al. A comparison of clinical and microbiological efficacy of antibiotic regimens against Acinetobacter baumannii. J Burn Care Res, 2013;34(4):403–412. doi: 10.1097/BCR.0b013e318270003a.
14. Lesseva MI, Hadjiski OG. Analysis of bacteriuria in patients with burns. Burns, 1995;21(1):3–6.
15. Bonadio M, Meini M, Spitaleri P, et al. Current microbiological and clinical aspects of urinary tract infections. Eur Urol, 2001;40(4):439–444.
16. Askarian M, Hosseini RS, Kheirandish P, et al. Incidence of urinary tract and bloodstream infections in Ghotbeddin Burn Center, Shiraz 2000–2001. Burns, 2003;29(5):455–459.
17. Kim J, Kim DS, Lee YS, et al. Fungal urinary tract infection in burn patients with long-term foley catheterization. Korean J Urol, 2011;52(9):626–631.
18. Rosanova MT, Stamboulian D, Lede R. Infections in burned children: epidemiological analysis and risk factors. Arch Argent Pediatr, 2013;111(4):303–308. doi: 10.1590/S0325-00752013000400008.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Mikrobiológia
Článok vyšiel v časopiseEpidemiologie, mikrobiologie, imunologie
Najčítanejšie tento týždeň
2016 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Antibiotic treatment of clostridial colitis
- Prevalence of infectious complications in burn patients requiring intensive care: data from a pan-European study
- Úvodník
- The prevalence of Candida pelliculosa, Candida utilis, and Candida fabianii in the Olomouc University Hospital: epidemiological study
- The benefit from mumps virus IgG antibody avidity testing in the population with high vaccine coverage in the context of other serological methods for laboratory diagnosis of mumps and the current epidemiological
- Hepatitidis E virus
- Influenza in the pediatric population in Istanbul: a one center experience 2009–2014
- Assessment of invalidity as a result of infectious diseases
- Infectious and other somatic comorbidity in problem drug users – results of a cross-sectional study with medical examination
- XXV. Tomáškovy dny mladých mikrobiologů
- PhMr. Jaroslav Švec
- Epidemiologie, mikrobiologie, imunologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Antibiotic treatment of clostridial colitis
- Infectious and other somatic comorbidity in problem drug users – results of a cross-sectional study with medical examination
- Hepatitidis E virus
- Assessment of invalidity as a result of infectious diseases
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



