-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Estimated glomerular filtration rate in diabetic patients
Odhad glomerulární filtrace u pacientů s diabetem
Cíl studie:
Cílem studie je porovnat odhadovanou glomerulární filtraci (eGFR) ze sérového kreatininu (eGFRcreatinine) a cystatinu C (eGFRcystatin C) a studovat dopad těchto odhadů na detekci a klasifikaci chronického onemocnění ledvin (CKD) u pacientů s diabetes mellitus.Typ studie:
retrospektivní průřezováNázev a sídlo pracoviště:
Oddělení klinické biochemie, Krajská nemocnice T. Bati a. s. Havlíčkovo nábřeží 600, Zlín 762 75Materiál a metody:
Studovanou populaci tvořilo 565 po sobě jdoucích diabetiků z diabetické ambulance Krajské nemocnice Tomáše Bati ve Zlíně. Sérový kreatinin a cystatin C jsme měřili standardizovanými metodami a eGFR byla počítána podle Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) rovnic, které byly vytvořeny v roce 2012. CKD je definováno jako GFR pod 1,0 ml/s/1,73m2.Výsledky:
Průměrná eGFRcreatinine (1,443 ± 0,014) ml/s/1,73m2 byla nižší než eGFRcystatin C(1,512 ± 0,017) ml/s/1,73m2, (p < 0,002). Našli jsme malou shodu v detekci CKD mezi eGFRcreatinine a eGFRcystatin C. Rozdílná detekce byla u 38 pacientů.Závěr:
Průměrná eGFRcystatin C byla významně vyšší než eGFRcreatinine. eGFRcystatin C dává větší průměrné hodnoty než eGFRcreatinine, hlavně v oblasti eGFR nad 1,5 ml/s/1,73m2. Naše výsledky podporují společné používání eGFRcystatin C a eGFRcreatinine+cystatin C u pacientů s diabetes mellitus bez albuminurie nebo jiného markeru poškození ledvin ve stadiu GFR 2 a 3a podle eGFRcreatinine.Klíčová slova:
kreatinin, cystatin C, glomerulární filtrace, chronické onemocnění ledvin, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)
Authors: T. Šálek 1; P. Ponížil 2,3
Authors place of work: Department of Clinical Biochemistry, Tomas Bata Regional Hospital in Zlín a. s., Havlíčkovo nábřeží 600, 76 75 Zlín, Czech Republic 1; Centre of Polymer Systems, Polymer Centre, Tomas Bata University in Zlín, Náměstí T. G. M. 5555, 760 05 Zlín, Czech Republic 2; Department of Physics and Materials Engineering, Faculty of Technology, Tomas Bata University at Zlín, Náměstí T. G. M. 275, 762 72 Zlín, Czech Republic 3
Published in the journal: Klin. Biochem. Metab., 22 (43), 2014, No. 1, p. 4-7
Summary
Objective:
The aim of the study is to compare estimated glomerular filtration rate (eGFR) from serum creatinine (eGFRcreatinine) and cystatine C (eGFRcystatin C) and to study the impact of these estimations on detection and staging of chronic kidney disease (CKD) in diabetic patients.Design:
retrospective cross section design.Settings:
Department of clinical biochemistry, Tomas Bata Hospital Inc., Zlín, Czech Republic.Materials and methods:
The study population consisted of 565 consecutive diabetic patients from the outpatient diabetic clinic of Tomas Bata Hospital in Zlin in the Czech Republic. Serum creatinine and cystatin C were measured by newly standardized methods and eGFR was calculated according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations, which were established in 2012. CKD is defined as GFR below 1.0 ml/s/1.73m2.Results:
The mean eGFRcreatinine (1.443 ± 0.014) ml/s/1.73m2 was lower than eGFRcystatin C (1.512 ± 0.017) ml/s/1.73m2, (p < 0.002). We found poor accordance to identify CKD. The discrepancy was found in 38 patients.Conclusion:
Mean eGFRcystatin C was significantly higher than eGFRcreatinine. eGFRcystatin C gives higher values than eGFRcreatinine mainly at eGFR over 1.5 ml/s/1.73m2. Our results support the use of both eGFRcystatin C and eGFRcreatinine +cystatin C in patients with diabetes mellitus without albuminuria or another marker of kidney damage at GFR stages 2 and 3a according to eGFRcreatinine.Keywords:
creatinine, cystatin C, glomerular filtration rate, chronic kidney disease, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)Introduction
Diabetic patients are routinely screened for chronic kidney disease (CKD). It is included in diabetes mellitus medical clinical practice guidelines [1]. Decreased glomerular filtration rate (GFR) is a part of the definition of CKD. CKD is divided into 6 stages accor-ding to GFR: G1 ≥1.5 ml/s/1.73m2, G2 1.0 – 1.49 ml/s/1.73m2, G3a 0.75 – 0.99 ml/s/1.73m2, G3b 0.5 – 0.74 ml/s/1.73m2, G4 0.25 – 0.49 ml/s/1.73m2, G5 < 0.25 ml/s/1.73m2. The knowledge of GFR is important for the diagnosis and staging of CKD. GFR is the best overall index of kidney function [2]. Drug do-sing also depends on GFR [3]. Renal function should be considered in patients with acute kidney failure and CKD [4]. Reference methods with exogenous filtration marker for measurement of GFR (mGFR) are time consuming and are available only in specialized centres. GFR is estimated from endogenous serum markers in clinical practice. eGFR is better than repor-ting the concentration of these endogenous markers without estimation of GFR [5-7]. The GFR is most routinely estimated from serum creatinine, serum cystatin C and by combined equation (eGFRcreatinine+cystatin C). eGFRcreatinine is recommended for initial assessment of kidney function. Decreased eGFRcreatinine below 1.0 ml/s/1.73m2 should be confirmed by eGFRcystatin C in patients without albuminuria or other marker of kidney damage2. We can measure both serum creatinine and cystatin C by standardized methods. Serum creatinine level depends on muscle mass. Serum cystatin C level does not have this limitation [8]. Serum cystatin C level may be falsely changed in patients with thyroid dysfunction [9] and corticosteroid administration [10]. Increased cystatin C level is regarded as cardiovascular disease risk factor [11].
The aim of this study is to compare eGFRcreatinine and eGFRcystatin C in diabetic patients and to compare our results with previous studies. We studied the impact of these two equations on the CKD staging, which is important for clinical practice, mainly for treatment. We looked at the proportion of patients who are reclassified by cystatin C based equations. The first certified reference material for cystatin C was announced in 2010 [12].One or two years later it entered routine clinical practice. New equations were developed in 2012 and we still do not have enough information on clinical utility of these equations. The biological variation of serum markers and the uncertainty of measurement are rarely taken into account. This is the reason why we performed this study.
Materials and methods
Patients
The cross sectional retrospective study included 565 consecutive diabetic patients from the outpatient diabetic clinic of Tomas Bata hospital in the town of Zlin in the Czech Republic. Patients with gestational diabetes were not included. The age of participants ranged from 19 to 86 years. There were 268 females with mean age of 59±14 years and 297 males with mean age of 57±13 years.
Laboratory methods
We measured serum creatinine by standardized photometric enzymatic method on Abbott Architect analyzer. The calibration is traceable to NIST SRM 967 reference material. Enzymatic traceable methods have lower bias than nonspecific Jaffé method [13]. Estimation of GFR from serum creatinine was calculated according to the Chronic Kidney Disease Epidemio-logy Collaboration (CKD-EPI) equation [14]. Cystatin C was determined by standardized immunoturbidimetric technique on Abbott Architect analyzer. The calibration was traceable to DA ERM 471 reference material [12]. We used CKD-EPI equations for estimation from serum cystatin C and combined estimation from serum creatinine + cystatin C [15].
Statistical tests
A Bland–Altman plot [16] was used to compare esti-mations of eGFR based on creatinine and/or cystatin. The mean of the (eGFRcystatin C + eGFRcreatinine)/2 was assigned as the abscissa (x-axis) value, and the diffe-rence (eGFRcystatin C - eGFRcreatinine) as the ordinate (y-axis) value. Student’s t-Test was used for comparison of means. Paired t-Test was used for testing of the differences.
The study was approved by The Ethical Committee of Tomas Bata Hospital.
Results
The mean eGFRcreatinine (1.443 ± 0.014) ml/s/1.73m2 was lower than eGFRcystatin C (1.512 ± 0.017) ml/s/1.73m2, (p < 0.002). It is showed at Bland-Altman plot (Fig. 1). The average difference for GFR higher than 1.5 ml/s/1.73m2 (eGFRcystatin C - eGFRcreatinine) is (0.156 ± 0.011) ml/s/1.73m2. It is evident that, at values of GFR < 1.0 ml/s/1.73m2, the GFR values estimated from cystatin C are lower than values estimated from creatinine. The average difference for GFR lower than 1.0 ml/s/1.73m2 (eGFRcystatin C - eGFRcreatinine) is - (0,067 ± 0,019) ml/s/1.73m2 (p = 0,0007).
Fig. 1. Bland-Altman plot: eGFR<sub>cystatin C</sub> and eGFR<sub>creatinine</sub> 
From a total amount of 565 patients, 42 of them had both eGFRcystatin C and eGFRcreatinine lower than 1 ml/s/1.73m2. The total of 11 patients had eGFRcystatin C ≥1 ml/s/1.73m2 and eGFRcreatinine <1 ml/s/1.73m2. 27 patients has eGFRcystatin C <1 ml/s/1.73m2 and eGFRcreatinine ≥1 ml/s/1.73m2. The discrepancy was found in 38 patients. Numbers of patients according to GFR stages are given in Table 1.
Tab. 1. Number of patients in assigned into GFR stages by cystatin C or creatinine only and both cystatin C and creatinine. 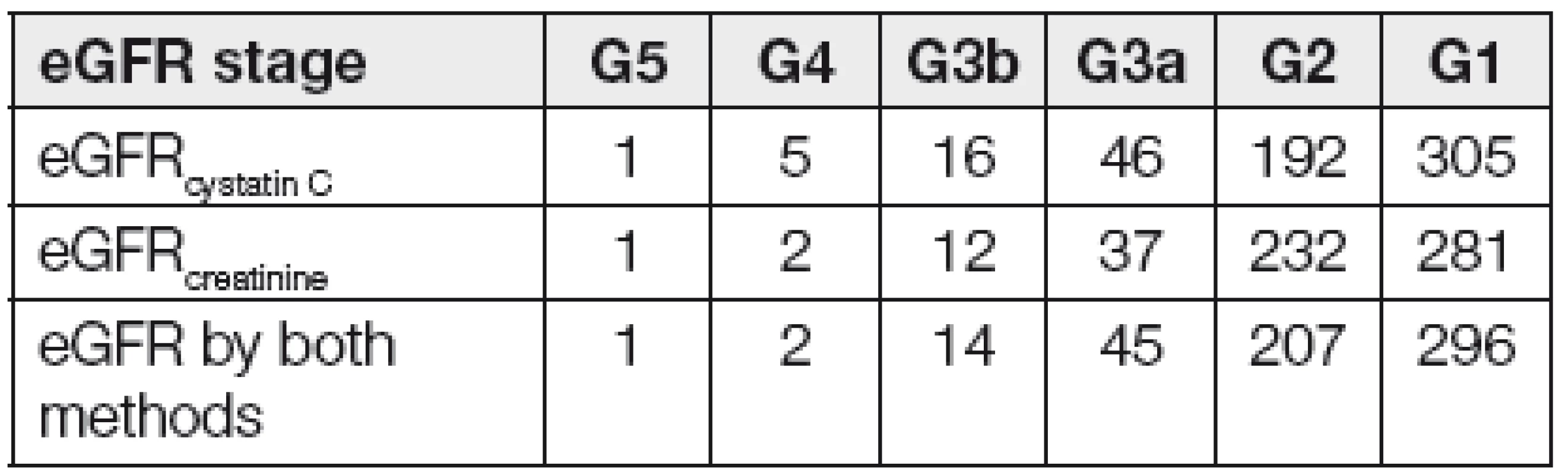
Discussion
We found similar results of CKD-EPI eGFRcreatinine equation in the work by Sebastjan Bevc [17]. Authors used gold standard mGFR by 51CrEDTA clearance method and found high negative bias for the CKD-EPI eGFRcreatinine equation. Creatinine was not determined by standardized enzymatic reaction, but less specific Jaffé reaction was used. eGFR from cystatin C traceable to reference method was also used in this study. eGFR from cystatin C gave systematically higher values compared to CKD-EPI eGFRcreatinine. The results are similar as in our study, but the equation for eGFRcystatin C was different than in our study. Work by Silverio also found underestimation of GFR estimated from serum creatinine by the CKD-EPI equation [18]. The reference method in this study was 51Cr-EDTA clearance. Creatinine in this study was determined by Jaffé method on Roche Modular P analyzer. The third work with same direction of bias between inulin clearance mGFR and CKD-EPI eGFRcreatinine was demonstrated by Nicolas Rognant’s work [19]. Unspecific Jaffé reaction was also employed in this study. The negative bias of the CKD-EPI eGFRcreatinine is greater in diabetic patients than in healthy individuals [20]. Creatinine in this study was determined by Jaffé reaction.
The main advantage of our study is the fact that both our laboratory methods are standardized. The standardization of measurement of creatinine and cystatin C is the key point for obtaining true results. Standardized cystatin C has only been available in clinical practice since 2011. If we use standardized method we get comparable results at different time and place. New CKD-EPI equations, which we use, have been available since 2012 [15].
The most important decision point of GFR is 1.0 ml/s/1.73m2. Patients with GFR bellow 1.0 ml/s/1.73m2 are designated as having CKD. It means that the most important task of eGFR is the identification of the stage 3a. When we look at the ability of eGFRcreatinine and eGFRcystatin C to identify the stages 2 and 3a, we can see that there are a significant number of patients who are identified only by one method. It may be useful to perform also eGFRcystatinC in patients with eGFRcreatinine at stages 2 and 3a. We do not need second marker of GFR for confirmation of CKD in patients with increased albuminuria or other markers of kidney damage, because these patients fulfill the definition of CKD. Albuminuria usually precedes the decrease of kidney function [21].
The overlap is also between GFR stages 1 and 2, but it is not so clinically important.
The need for the use of two markers near the 1.0 ml/s/1.73m2 as an important decision point may be sup-ported by the fact that each serum marker has its own biological variability [22] and an uncertainty of measurement exists [23]. Analytical performance characteristics of creatinine and their impact on eGFR are described in the work from Mayo Clinic. Small analytic changes in serum creatinine create major shifts in distribution of eGFR [24]. The issue of cystatin C is the same.
Creatinine and cystatin C are two makers of GFR in clinical practice. It is important to consider which of the markers has higher prognostic importance.
The eGFRcystatin C is of higher prognostic importance than eGFRcreatinine. The prognostic importance for cardiovascular and overall mortality is supported by prospective The Atherosclerosis Risk in Communities Study. Any degree of decreased eGFRcystatin C or any degree of albuminuria is associated with increased risk of all-cause mortality, incident coronary heart disease and incident heart failure hospitalization [25]. Results of eGFRcystatin C in this study were re-expressed accor-ding to International Federation of Clinical Chemistry and Laboratory Medicine ERM DA 471 reference material. CKD-EPI equation was used for estimation of GFR in this study. This standardization enables us to use the outcomes of risk evaluation from the study also for participants of our study.
The cohort of 1153 diabetic patients was derived from the prospective ESTHER study and investigators assessed the ability of eGFRcreatinine and eGFRcystatin C to predict cardiovascular events. Authors concluded that only the cystatin C based CKD definition was an independent risk predictor for cardiovascular events in diabetic study cohort [26].
When we take into account that only low proportion of patients at CKD stage 3a is identified by both eGFRcreatinine and eGFRcystatin C, biological variability of creatinine and cystatin C, the uncertainty of measurement of these markers and at the end better prognostic value of cystatin C, we can support the use of eGFRcystatin C and eGFRcreatinine+cystatin C in diabetic patients without marker of kidney damage at the CKD stages 2 and 3a according to eGFRcreatinine.
The major limitation of our study is the lack of GFR measurement by reference method with exogenous GFR marker, but the same situation is in real clinical practice.
External quality assessment systems play important role in interlaboratory comparability of kidney function tests [27].
Conclusion
Mean eGFRcystatin C was significantly higher than eGFRcreatinine. eGFRcystatin C gives higher values than eGFRcreatinine mainly at eGFR over 1.5 ml/s/1.73m2. Our results support the use of both eGFRcystatin C and eGFRcreatinine+cystatin C in patients with diabetes mellitus without albuminuria or another marker of kidney damage at GFR stages 2 and 3a according to eGFRcreatinine.
Do redakce došlo 14. 11. 2013
Adresa pro korespondenci:
MUDr. Tomáš Šálek
Záhumení 789
687 22 Ostrožská Nová Ves
e-mail: tsalek@seznam.cz
Zdroje
1. American Diabetes Association. Standards of Medical Care in Diabetes – 2013. Diabetes Care, 2012, 36(S1), p. S11–S66.
2. International Society of Nephrology. Definition and Classification of CKD. Kidney Int. Suppl., 2013, 3(1), p. 19–62.
3. Jones, G. R. D. Estimating Renal Function for Drug Dosing Decisions. Clin. Biochem. Rev., 2011, 32(2), p. 81–8.
4. Matzke, G. R., Aronoff, G. R., Atkinson, A. J. Jr. et al. Drug Dosing Consideration in Patients with Acute and Chronic Kidney Disease – a Clinical Update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int., 2011, 80(11), p. 1122–1137.
5. Hostetter, T. H., Levey, A. S., Stevens, L. A. Clinical Impact of Reporting Estimated Glomerular Filtration Rates. Clin. Chem., 2010, 56(9), p. 1381–3.
6. Hemmelgarn, B. R., Zhang, J., Manns, B. J. et al. Nephrology Visits and Health Care Resource Use before and after Reporting Estimated Glomerular Filtration Rate. JAMA, 2010, 303(12), p. 1151–8.
7. Friedecký, B. Kreatinin a odhad glomerulární filtrace. Kli-nická biochemie a metabolismus, 2007,15(3), p. 164–7.
8. Baxmann, A. C., Ahmed, M. S., Marques, N. C. et al. Influence of Muscle Mass and Physical Activity on Serum and Urinary Creatinine and Serum Cystatin C. Clin. J. Am. Soc. Nephrol., 2008, 3(2), p. 348–54.
9. Den Hollander, J. G., Wulkan, R. W., Mantel, M. J., Berghout, A. Is Cystatin C a Marker of Glomerular Filtration Rate in Thyroid Dysfunction? Clin. Chem., 2003, 49(9), p. 1558–9.
10. Shigemura, M., Konno, S., Nasuhara, Y., Shimizu, C., Matsuno, K., Nishimura, M. Impact of Asthmatic Control Status on Serum Cystatin C Concentrations. Clin. Chem. Lab. Med., 2012, 50(8), p. 1367–71.
11. Taglieri, N., Koenig, W., Kaski, J. C. Cystatin C and Cardiovascular Risk. Clin. Chem., 2009, 55(11), p. 1932–43.
12. Grubb, A., Blirup-Jensen, S., Lindström, V. et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem. Lab. Med., 2010, 48(11), p. 1619-21.
13. Drion, I., Cobbaert, C., Groenier, K. H. et al. Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol., 2012, 13(1), p. 133.
14. Levey, A. S., Stevens, L. A., Schmid, C. H. et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Inter. Med., 2009, 150(9), p. 604–12.
15. Inker, L. A., Schmid, C. H., Tighiouart, H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl. J Med., 2012, 367(1), p. 20–9.
16. Altman, D. G., Bland, J. M. Measurement in Medicine: The Analysis of Method Comparison Studies. The Statistician, 1983, 32, p. 307–17.
17. Bevc, S., Hojs, R., Ekart, R., Završnik, M., Gorenjak, M., Puklavec, L. Simple Cystatin C Formula for Estimation of Glomerular Filtration Rate in Overweight Patients with Diabetes Mellitus Type 2 and Chronic Kidney Disease. Exp. Diabetes Res., 2012, Sep 12. [Epub] doi:10.1155/2012/179849.
18. Silveiro, S. P., Araújo, G. N., Ferreira, M. N., Souza, F. D., Yamaguchi, H. M., Camargo, E. G. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation Pronouncedly Underestimates Glomerular Filtration Rate in Type 2 Diabetes. Diabetes Care, 2011, 34(11), p. 2353–5.
19. Rognant, N., Lemoine, S., Laville, M., Hadj-Aïssa, A., Dubourg, L. Performance of the Chronic Kidney Disease Epidemiology Collaboration Equation to Estimate Glomerular Filtration Rate in Diabetic Patients. Diabetes Care, 2011, 34(6), p. 1320–2.
20. Camargo, E. G., Soares, A. A., Detanico, A. B. et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation Is Less Accurate in Patients with Type 2 Diabetes When Compared with Healthy Individuals. Diabet. Med., 2011, 28(1), p. 90–5.
21. Llinares-Tello, F. Rational use of laboratory tests: albuminuria. Clin. Chem. and Lab. Med., 2013, 51(4), e55–e56.
22. Selvin, E., Juraschek, S. P., Eckfeldt, J., Levey, A. S., Inker, L. A., Coresh, J. Within-person Variability in Kidney Measures. Am. J. Kidney Dis., 2013, 61(5), p. 716–22.
23. Dimech, W., Francis, B., Kox, J., Roberts, G. Serology Uncertainty of Measurement Working Party. Calculating Uncertainty of Measurement for Serology Assays by Use of Precision and Bias. Clin. Chem., 2006, 52(3), p. 526–9.
24. Klee, G. G., Schryver, P. G., Saenger, A. K., Larson, T. S. Effects of Analytic Variations in Creatinine Measurements on the Classification of Renal Disease Using Estimated Glomerular Filtration Rate (eGFR). Clin. Chem. Lab. Med., 2007, 45(6), p. 737–41.
25. Waheed, S., Matsushita, K., Sang, Y. et al. Combined Association of Albuminuria and Cystatin C-based Estimated GFR with Mortality, Coronary Heart Disease, and Heart Failure Outcomes: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J Kidney Dis., 2012, 60(2), 207–16.
26. Schöttker, B., Herder, C., Müller, H., Brenner, H., Rothenbacher, D. Clinical Utility of Creatinine - and Cystatin C-Based Definition of Renal Function for Risk Prediction of Primary Cardiovascular Events in Patients With Diabetes. Diabetes Care, 2012, 35(4), p. 879–86.
27. Friedecký, B. Program zlepšování kvality měření sérového kreatininu. Klinická biochemie a metabolismus, 2006, 14(35), p. 173–6.
Štítky
Biochémia Nukleárna medicína Nutričný terapeut
Článok vyšiel v časopiseKlinická biochemie a metabolismus
Najčítanejšie tento týždeň
2014 Číslo 1
-
Všetky články tohto čísla
- Possible pitfalls in laboratory examination of patient with a hematological disease
- Possibility of prediction of acute pancreatitis severity by determination of adipokines (adiponectin, FGF-21 and A-FABP) during hospitalization
- Doporučení k využití nádorových markerů v klinické praxi
- Nová doporučení k diagnóze a klasifikaci chronických ledvinových onemocnění
- Estimated glomerular filtration rate in diabetic patients
- Provádění všeobecného prenatálního screeningu vrozených vývojových vad
- prof. MUDr. Miroslav Engliš, DrSc.
-
Estimated glomerular filtration rate and problems in the interpretation of CKD-EPI equations.
(Short communication - data expansion and comments to the article „Estimated glomerular filtration rate in diabetic patients“ published by Šálek, T. and Ponížil, P.)
- Klinická biochemie a metabolismus
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Doporučení k využití nádorových markerů v klinické praxi
-
Estimated glomerular filtration rate and problems in the interpretation of CKD-EPI equations.
(Short communication - data expansion and comments to the article „Estimated glomerular filtration rate in diabetic patients“ published by Šálek, T. and Ponížil, P.) - Possibility of prediction of acute pancreatitis severity by determination of adipokines (adiponectin, FGF-21 and A-FABP) during hospitalization
- Provádění všeobecného prenatálního screeningu vrozených vývojových vad
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



