-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Targeted Therapies in the Treatment of Advanced Non Small Cell Lung Cancer: Update
Cílené terapie při léčbě pokročilého nemalobuněčného karcinomu plic: nové poznatky
Po několika letech klinických studií pokročilého nemalobuněčného karcinomu plic (non small cell lung cancer – NSCLC), které byly charakteristické nedostatečnou účinností chemoterapie v porovnání s nejlepší podpůrnou péčí, jsme v poslední době zaznamenali významný klinický přínos vybraných cílených terapií. Během neustálého vývoje těchto nových protinádorových terapií byla prokázána lepší prognóza přežití nejen v prvotní léčbě, ale v poslední době dokonce u pacientů s recidivou po předchozí jedné nebo dvou neúspěšných chemoterapiích. Prvními látkami v této rozsáhlé skupině, u kterých byla prokázána klinická účinnost, byly tyrozinkinázové (TK) inhibitory receptorů epidermálního růstového faktoru (epidermal growth factor receptor – EGFR). Nejlepšími zástupci nových léčiv pro kontrolu a zmírnění nádoru jsou erlotinib (typ EGFR) a bevacizumab (receptor vaskulárního endotelového růstového faktoru – vascular endothelial growth factor receptor – VEGFR). Tento článek uvádí přehled nejslibnějších nově cílených látek včetně těch, které již byly schváleny a v současné době jsou v lékařské praxi používány.
Klíčová slova:
nemalobuněčný karcinom plic – tyrozin kináza – EGFR – VEGFR
Authors: L. Mendoza
Authors place of work: INC Research, Prague, Czech Republic
Published in the journal: Klin Onkol 2009; 22(4): 131-138
Category: Přehledy
Summary
After several years of clinical trials in the setting of advanced non small cell lung cancer (NSCLC) that were characterized by a lack of efficacy of chemotherapy over best supportive care, we have more recently seen meaningful clinical benefits realized from selected targeted therapies. In their constant development, the survival advantage of these new anti cancer therapies has been demonstrated not only in the first line setting, but, lately, even in patients with recurrent disease after failure of one or two previous chemotherapy lines. The first agents in this broad class to demonstrate clinical efficacy were the epidermal growth factor receptor (EGFR) tyrosine kinase (TK) inhibitors. Erlotinib, an EGFR, and bevacizumab, a vascular endothelial growth factor receptor (VEGFR), are the best representative new drugs for tumour control and palliation. This article reviews the most promising new targeted agents including those that have already been approved and are currently used in the medical practice.
Key words:
non small cell lung carcinoma – tyrosine kinase – EGFR – VEGFRIntroduction
Despite aggressive surgical and chemotherapeutic interventions, NSCLC is the leading cause of cancer related death in men and women in North America and Europe with overall cure rates of less than 15%. About 75 to 80% of all lung cancers are NSCLC. Most patients with advanced NSCLC present with metastatic disease and, if left untreated, have a median survival after diagnosis of 4–5 months and a 1-year survival of less than 10% [1]. After several years of clinical trials in the setting of advanced NSCLC, the use of combined chemotherapy (CT) has resulted in a modest increase in survival at the cost of significant toxicity to these patients [2]. Consequently, there is substantial interest in identifying potentially exploitable new entities against lung cancer. Major advances in the understanding of cancer biology have identified many potential targets for rationally designed novel therapies (tab. 1). Most of these targets are components of signalling pathways or metabolic processes contributing to one of the hallmarks of the cancer phenotype. Drugs directed at these targets should prove more specific for cancer cells than the traditional cytotoxic agents, which target all proliferating tissues. The advent of new molecular-targeted drugs has therefore generated much optimism in the improvement of patient’s survival and quality of life. The first agents in this broad class to demonstrate clinical activity were gefitinib (Iressa; AstraZeneca) and erlotinib (Tarceva; OSI Pharmaceuticals, Roche, Genetech). Gefitinib and erlotinib are two small molecule drugs that specifically target the TK activity of EGFR. Within the group of new agents against EGFR we should also include monoclonal antibodies (mAbs). Cetuximab (Erbitux; Bristol Myers-Squibb and ImClone) and panitumumab (Vectibix; Amgen) are drugs that have been studied most extensively in NSCLC to date. More recently, the antiangiogenic agent bevacizumab (Avastin; Roche and Genetech), which is a recombinant humanized mAb against VEGF, has also demonstrated encouraging activity against NSCLC. On the basis of the positive results, FDA and the European Medicines Agency (EMEA) approved it for the first line therapy in the subset of untreated patients with advanced NSCLC. Another kind of molecules discovered, representing multitargeted inhibitors of EGFR and VEGFR such as ZD6474 (Vandetanib; AstraZeneca), and agents such as sunitinib (Sutent; Pfizer) and sorafenib (Nexavar; Bayer), are also being tested in clinical trials. In this article, we review current data on the role of the most promising new targeted agents that are currently in a more advanced phase of development for the treatment of NSCLC.
Tab. 1. Current status of the most developed targeted therapies for NSCLC. 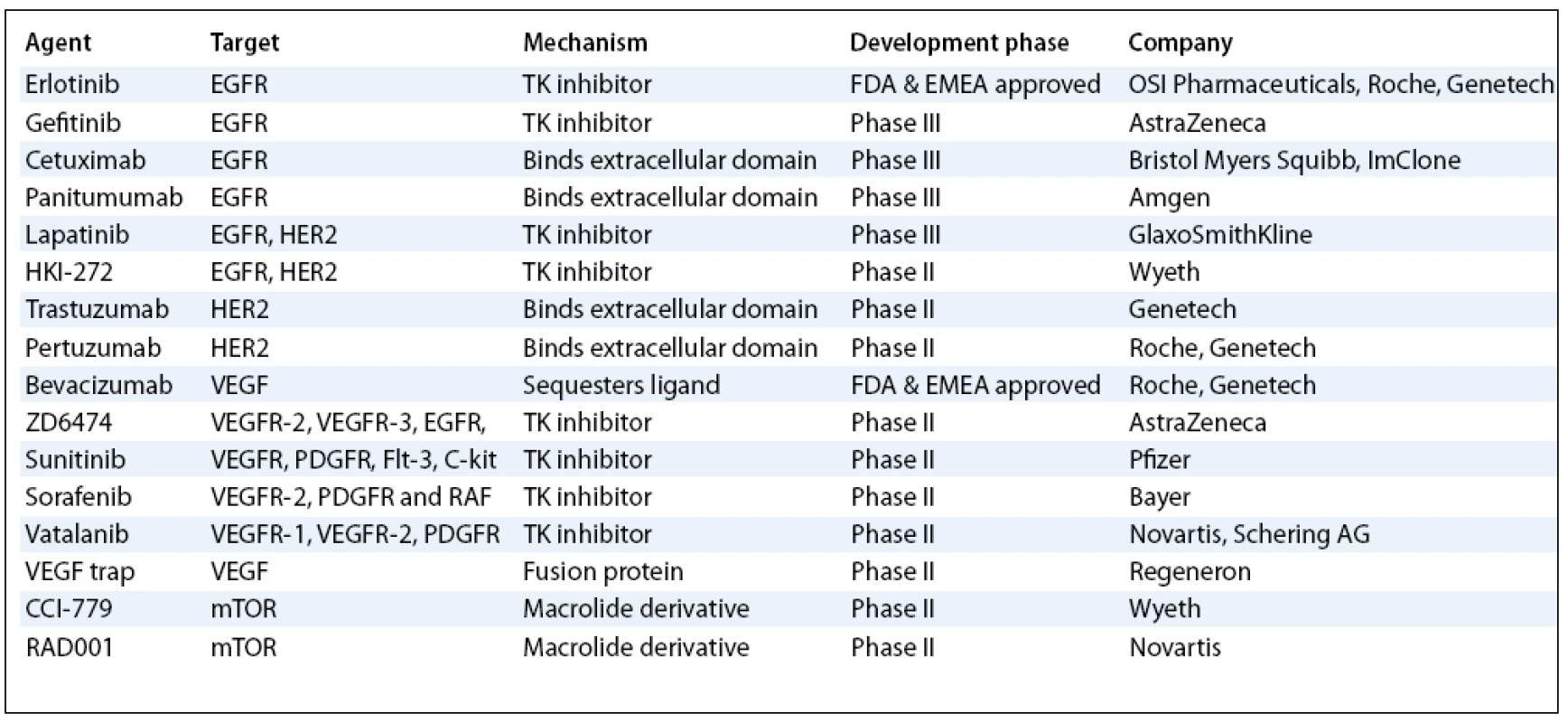
EGFR – Epidermal Growth Factor Receptor; VEGF – Vascular Endotheli al Growth Factor; VEGFR – Vascular Endotheli al Growth Factor Receptor; TK – Tyrosine Kinase; PDGFR – Platelet- Derived Growth Factor Receptor Inhibitors of EGFR TK Activity
The rationale for EGFR inhibition as a target for cancer therapy was proposed nearly 25 years ago, when it was noted that EGFR is frequently overexpressed in human tumours. EGFR belongs to the TK super-family of cell-surface receptors, which is composed of ERBB1 (also known as HER1), ERBB2 (also known as HER2), ERBB3 (also known as HER 3), and ERBB4 (also known as HER4) receptors. Recent retrospective analyses have reported EGFR expression in 62% of NSCLC cases, and its expression is correlated with a poor prognosis [3–5]. EGFR expression is more common in squamous cell carcinoma of the lung than in adenocarcinoma [6,7], in nonmucinous variants of bronchioalveolar carcinoma (BAC) than in mucinous variants [8], and in cavitating than in noncavitating tumours [6]. In a meta analysis, EGFR overexpression was noted in 58% of lung squamous cell carcinomas, 39% of adenocarcinomas, and 38% of large-cell carcinomas; it did not correlate significantly with survival [9]. Various strategies have been developed to target the family of EGFR. The most studied include small molecules of the intracellular TK domain and mAbs, which either bind the ligand or compete with the ligand for the extracellular domain.
Small Molecule Inhibitors of EGFR
Gefitinib and erlotinib are small molecules that reversibly target EGFR TK by competing with ATP for access to TK’s intracellular ATP binding pocket [10]. These TK inhibitors can be administered orally, have a rapid onset of action, and potentially have better tumour penetration than anti EGFR mAbs. Gefitinib, an anilinoquinazoline, was the first TK inhibitor selective for EGFR agent tested broadly in advanced NSCLC. Two randomized, phase II, multicentre trials (Iressa Dose Evaluation in Advanced Lung Cancer, IDEAL 1 and 2) [11,12] that compared two dosages (250 and 500mg/day) of gefitinib conducted in more than 400 patients with advanced NSCLC, pre treated with platinum based CT, demonstrated activity with a response rate (RR) between 12–18% and an approximately 40% improvement of the lung cancer symptoms. Side effects were generally mild, consisting of acneiform rash, dry skin and diarrhoea, but were significantly more common and severe at the higher dosage. Unfortunately, subsequent trials of untreated NSCLC patients [13,14], conducted after gefitinib was provisionally approved by FDA in May 2003, showed no improvement in efficacy with the addition of gefitinib compared with CT alone. Notably, in the multicentre study 709, Iressa Survival Evaluation in Lung Cancer (ISEL) [15], 1,692 previously treated patients with advanced NSCLC were randomized to receive gefitinib 250mg/day or placebo. A difference was observed between gefitinib and placebo in terms of survival (5.6 vs 5.1 months), although not reaching statistical significance (P = 0.087) in the overall survival of the entire population studied. However, gefitinib in a defined subset of never-smokers and patients of Asian origin demonstrated significantly better survival. On the basis of these negatives results, the FDA restricted the approval for gefitinib outside its use in the subset of patients where the drug demonstrated efficacy, and the new drug application for approval in Europe was withdrawn [16]. More recently, a randomized clinical trial (S0023) that was sponsored by the National Cancer Institute (NCI) and conducted by the Southwest Oncology Group (SWOG) and AstraZeneca was closed after interim analysis because no survival benefit was found compared with a placebo following chemotherapy and radiation for patients with NSCLC that had spread only to nearby tissues or lymph nodes [17]. In addition, the results of second line therapy suggested that gefitinib is likely to be comparable to docetaxel in terms of median survival, but is better tolerated [18]. Due to that, FDA is not considering market withdrawal of gefitinib at present. New clinical trials are being developed and other ongoing trials are being completed. These will determine the future role of gefitinib treatment.
Similarly as gefitinib, erlotinib is an orally active, EGFR-specific quinazoline TK inhibitor that demonstrated antitumour activity in xenograft models. Several phase II and III clinical trials with 150mg erlotinib testing its efficacy in advanced NSCLC patients have been performed. In 57 previously treated patients with EGFR-expressing NSCLC, the response rate (RR) to erlotinib was 12.3%. Responses were seen regardless of the number of prior chemotherapy regimens [19]. The median survival was 8.4 months, and the 1-year survival was 40% [19]. In chemonaive patients, in two different clinical trials, erlotinib achieved a RR from 10% to 23%, a response plus stable disease of 51% to 53%, and median overall survival (OS) of 11 to 13 months [20,21]. Patients subsequently treated with CT after erlotinib experienced a RR to chemotherapy (CT) of 6%, suggesting that there may be some degree of cross resistance [21]. From the results obtained in two large phase III studies [22,23], which included 1,172 and 1,079 non pre treated advanced NSCLC patients, respectively, demonstrated that the addition of erlotinib to CT resulted in no increase of the duration of survival compared with patients treated with CT alone. However, longer survival and progression free survival (PFS) was seen with the addition of erlotinib to CT in the subset of patients who had never smoked [22,23]. Among pre treated patients, the randomized phase III, placebo-controlled, double-blind trial (BR.21) demonstrated that erlotinib can prolong survival in patients with advanced NSCLC after first - or second line CT [24]. The OR was 6.7 months and 4.7 months, respectively (P < 0.001), in favour of erlotinib compared with recipients of placebo. On the basis of this trial, erlotinib tablets as monotherapy for the treatment of locally advanced and metastatic NSCLC after failure of at least one prior CT regimen was approved by the FDA in November 2004 and by EMEA in June 2005.
In multivariate analysis, prognostic factors that have emerged as being independent clinical predictors of survival in various trials of EGFR TK inhibitors (tab. 2) have included female sex [25], good performance status [25–27], adenocarcinoma histology [25], history of no prior chemotherapy [26], Asian race [27], never having smoked [27], less than 5% weight loss [27], no prior cisplatin [27], and a longer than 12-month interval from time of diagnosis to enrollment in the trial [27]. As mentioned above, never-smoker advanced NSCLC patients have demonstrated consistent and profound improvement in survival when they were treated with gefitinib or erlotinib. Results from pharmacokinetic studies have shown that erlotinib activity is significantly different in smokers than in non smoking healthy volunteers and the erlotinib clearance is 24% faster in current smokers than in former smokers or never-smokers [28]. The results suggest that the PK relationships that differ by smoking status may be more important than clinical or biological characteristics, and that higher doses of erlotinib may be required for current smokers.
Tab. 2. Prognostic factors associated with enhanced survival of NSCLC patients in EGFR KI inhibitor clinical trials. 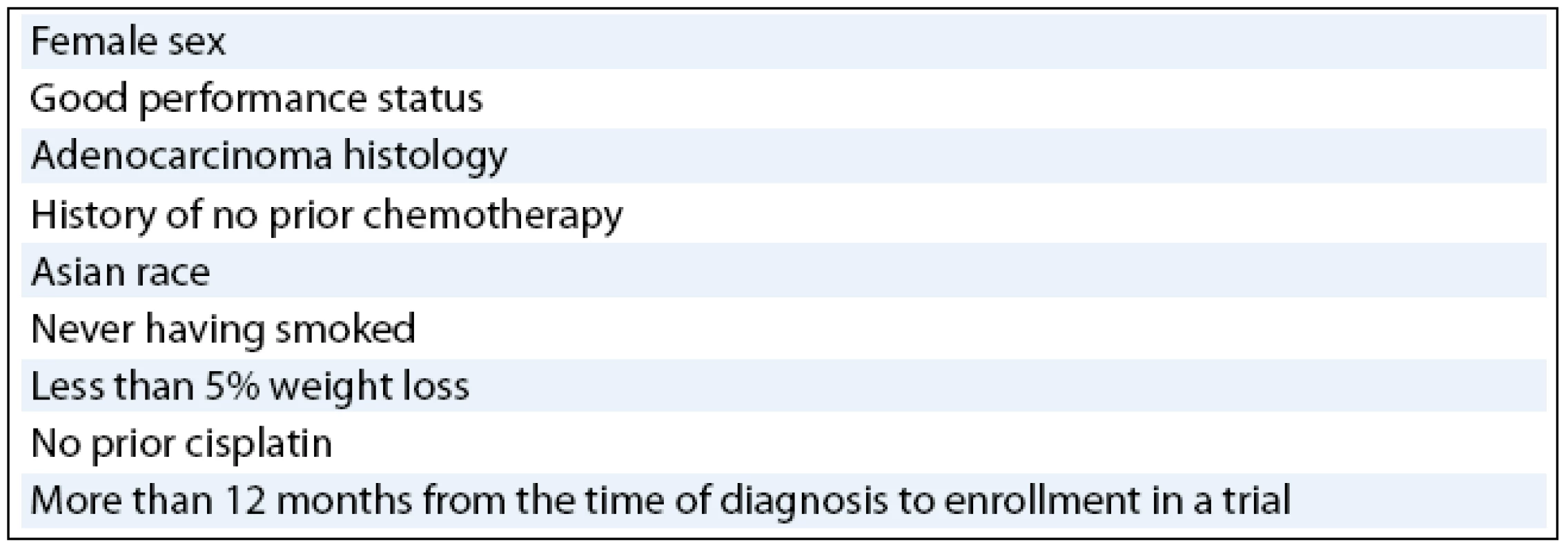
Other patients who may particularly benefit form small molecule inhibitor therapies include those with molecular features such as EGFR mutations or EGFR gene amplification detected by fluorescence in situ hybridization (FISH). EGFR mutations are found in tumours of approximately 7% to 12% of North American or European NSCLC patients [29–33], whereas they are seen in 19% to 59% of NSCLC Asian patients [31,34–36]. Prospective trials were thus undertaken to evaluate the activity of TK inhibitors in patients with TK mutations. In a study conducted by the West Japan Thoracic Oncology Group Trial [37], single agent gefitinib was administered at 250mg to 28 patients with advanced NSCLC and had an OR rate of 77%. Similarly, in a study performed in Spanish patients with advanced NSCLC treated with erlotinib, mutations were detected in approximately 15% of cases and the overall response (OR) rate reached in such population was 80% [38]. Moreover, an OR rate of 86% was reported with erlotinib, with four complete responses (CR) observed only in never-smoker patients, in a trial with no previously treated stage IV NSCLC patients carrying mutations in the TK domain of the EGFR [37]. Compilation of these data reveals that EGFR mutations are identified in approximately 80% of responders to EGFR small molecule TK inhibitors.
In addition, the role of EGFR gene copy number as a predictor of response to EGFR TK inhibitors has also been studied extensively. The findings suggest that the high EGFR gene copy number identified by FISH may be an effective predictor for the drug efficacy [39]. A prospective phase II study [40] was designed to assess the clinical activity of gefitinib in NSCLC patients whose tumours demonstrate high EGFR gene copy numbers. Of 42 patients enrolled, 36 patients were never-smokers and 19 were EGFR positive. The OR was 48%, whereas one subject reached a CR and 19 subjects reached partial response (PR). In patients with high EGFR gene copy numbers the observed response was 68%. Thus, it appears that there may be a number of molecular markers that are associated with response or outcome in NSCLC patients treated with EGFR TK inhibitors.
Monoclonal Antibodies against EGFR
Several monoclonal antibodies that target the EGFR are under development. At present, cetuximab is approved for head and neck cancer as a second line therapy, as well as for the treatment of metastatic colorectal cancer. Cetuximab, a chimeric human-murine IgG1 mAb, blocks ligand binding to EGFR, thereby diminishing receptor dimerization and autophosphorylation, and induces EGFR receptor downregulation, reducing the number of receptors on the cell surface. The immunoglobulin IgG1 isotype of cetuximab may also engage host immune functions, such as antibody-dependent cellular cytotoxicity (ADCC). The single agent activity of cetuximab against advanced NSCLC was studied in a phase II study. Patients with advanced NSCLC who progress following treatment with a platinum based regimen were eligible. At ASCO (American Society of Clinical Oncology) 2004, Lynch and colleagues reported the interim analysis results from 29 EGFR-positive patients showing a PR rate of 7% and a stable disease (SD) rate of 17% [41]. Interestingly, both of the responders did not harbour mutations in the EGFR ATP binding domain. It is possible that the presence of EGFR mutation is not critical in predicting response to mAbs against the EGFR. In another clinical trial, Rosell and colleagues conducted a randomized phase II study to evaluate the activity of cetuximab in patients with previously untreated advanced NSCLC [42]. Patients with EGFR-positive tumours were randomized to treatment with cisplatin plus vinorelbine alone or in combination with cetuximab. The study included 86 patients with 43 in each arm. The response rate was 35% for patients in the cetuximab arm, compared to 28% with CT alone. The median survival was 8.3 months with combination therapy and 7 months with CT alone. The 1-year survival and disease stabilization rates also favoured the cetuximab arm. Data from the randomized phase III FLEX (First line in Lung Cancer with Erbitux) trial presented at ASCO 2008 Annual Meeting have shown that the EGFR-positive patients with advanced NSCLC, treated first line with a combination of cetuximab, cisplatin and vinorelbine, live significantly longer than patients treated with the platinum based chemotherapy alone [43]. An overall survival difference of 1.2 months (11.3 vs 10.1 months) favoured the addition of cetuximab to chemotherapy (P = 0.004). The review and approval of these results by the regulatory authorities is expected in order to grant the use of cetuximab in combination with CT as first line treatment in patients suffering from advanced EGFR-positive NSCLC. Other studies are underway combining cetuximab with chemotherapy plus bevacizumab as frontline therapy for advanced NSCLC and combining it with chemoradiotherapy for stage III disease.
Panitumumab (Vectibix; Amgen), also known as ABX-EGF, is a fully humanized IgG2 mAb that targets EGFR and, unlike cetuximab, mediates its effect through mechanisms other than ADCC. Crawford and colleagues reported the results of a phase I study that evaluated panitumumab in combination with carboplatin and paclitaxel (standard CT regimen used in the United States for NSCLC) [44]. The study enrolled 21 patients with previously untreated advanced NSCLC. Three dose levels of panitumumab were studied. The recommended dose for phase II studies was 2.5mg/kg administered on a weekly basis in combination with the standard doses of carboplatin and paclitaxel. For the 19 evaluable patients, the median survival was an impressive 17 months, with a median time-to-progression (TTP) of 7 months. A two part, randomized phase II clinical trial to evaluate the safety and activity of panitumumab in combination with carboplatin and paclitaxel as first (N = 194) and second (N = 50) line of treatment of advanced NSCLC have been completed and the results are eagerly awaited.
Approximately 2% to 23% of patients with NSCLC have a high erbB2 gene copy number [45,46], and erbB2 protein expression by immunohistochemistry (IHC) is positive in tumour samples from 7% to 50% of NSCLC patients [45,47]. In a phase II trial of the anti erbB2 antibody trastuzumab (Herceptin; Genetech) combined with paclitacxel plus carboplatin in NSCLC patients with tumours positive for erbB2 on IHC, 24.5% responded and the median OS was 10.1 months [47]. However, the overall efficacy was similar to that expected with chemotherapy alone. In a phase II trial of another humanized anti erbB2 mAb, pertuzumab (also called 2C4, formerly known as Omnitarg; Genetech), from 43 patients treated neither CR nor PR responses were seen. Eighteen of 43 (41.9%) and nine of 43 (20.9%) patients had stable disease at 6 and 12 weeks, respectively [48]. Further clinical development of pertuzumab should focus on rational combinations of pertuzumab with other drugs active in NSCLC.
Angiogenesis Inhibitors in NSCLC Treatment
Several angiogenesis inhibitors have been studied in NSCLC. They include mAbs to VEGF and VEGFR and inhibitors of VEGFR TK [49]. The best-studied angiogenesis inhibitor is bevacizumab, an anti VEGFR antibody. In a randomized, controlled trial involving 99 patients with previously untreated advanced NSCLC, bevacizumab added to paclitaxel plus carboplatin improved OR rate compared with paclitaxel plus carboplatin alone. The median TTP was significantly higher for patients receiving a high dose (15mg/kg) bevacizumab regimen than for those receiving a low dose (7.5mg/kg) bevacizumab regimen (7.4 vs 4.2 months; P = 0.023) [50]. In addition, 19 patients who progressed on CT alone were crossed over to single agent bevacizumab, and five of them reached SD [50]. Subsequently, the Eastern Cooperative Oncology Group (ECOG) E 4599 study, which enrolled 878 patients who had not received prior CT, compared paclitaxel plus carboplatin with bevacizumab and without bevacizumab in advanced NSCLC [51]. This was the first phase III trial to demonstrate a survival advantage obtained from a first line treatment combining a targeted biological agent with CT alone, reporting a prolongation of PFS (6.2 vs 4.5 months) and OS (12.3 vs 10.3 months) [51]. One-year survival was 51% for patients receiving CT plus bevacizumab versus 44% for patients receiving CT alone. Two-year survival was 23 vs 15%, respectively. FDA approved the novel combination for first line treatment of patients with unresectable, locally advanced, recurrent or metastaic non squamous NSCLC in October 2006.
A second large multicentre phase III study, AVAIL (BO17704), adding the angiogenesis inhibitor bevacizumab to the combination of gemcitabine plus cisplatin, a regimen which is popular in Europe, improved progression free survival (PFS) by 20 to 30% and the RR was increased by up to 70% compared with CT alone in patients with advanced NSCLC. The duration of tumour response was increased from 4.7 to 6.1 months compared with CT alone. These results, which were presented at the ASCO 2007 Annual Meeting [52], support the findings from the earlier ECOG 4,599 study and show a significantly longer PF independent of the CT regimen used. Based on these data, EMEA has granted marketing authorization for bevacizumab in addition to any platinum based CT for the first line treatment of advanced NSCLC other than with predominantly squamous histology. Bevacizumab appears to be generally well tolerated. However, some bevacizumab associated adverse effects warrant special attention, including hypertension, proteinuria, and haemorrhage. Most cases of haemorrhage with bevacizumab have been mild, but some serious pulmonary haemorrhages have occurred. There are several other antiangiogenic agents, with several distinct mechanisms of action under clinical development, tested for their safety and efficacy in NSCLC patients. Cediranib (RecentinTM, AZD2171; AstraZeneca) is a highly potent and selective VEGF signalling inhibitor that targets all three VEGF receptors. Cediranib has demonstrated safety and encouraging activity in a phase I combination trial with carboplatin plus paclitaxel [53]. Other antiangiogenic agents, including soluble VEGFR inhibitors, are in earlier stages of clinical trial of lung cancer treatment. At the present time, it is not clear whether the haemorrhagic complications identified in the bevacizumab trial are specific to this compound or not.
Multitargeted Kinase Inhibitors
In addition to manipulating components of EGFR, complementary molecular therapeutic approaches that involve simultaneously targeting distinct signalling pathways have potential benefit. Although most of these approaches are empirical by nature, a rationale does exist for targeting both the tumour and its vasculature. One of the agents that exemplifies this strategy is ZD6474, an orally available TK that can inhibit both VEGF and EGFR [54]. ZD6474 has shown efficacy in tumour xenografts that are resistant to cetixumab and gefitinib [55,56]. In a phase I study of ZD6474, four of nine patients with refractory NSCLC achieved PR [57]. Phase II clinical trials of ZD6474 in combination with standard chemotherapies in first - and second line settings for advanced or metastatic NSCLC are ongoing. ZD6474 combined with carboplatin plus paclitaxel as a frontline therapy in NSCLC (IIIB–IV) demonstrated a 39% PR rate in a randomized, double-blind trial [58]. In the randomized phase II study, the activity of ZD6474 plus docetaxel was assessed in patients with previously treated NSCLC. The median PFS was 18.7 weeks for ZD6474 100mg plus docetaxel; 17 weeks for ZD6474 300mg plus docetaxel; and 12 weeks for docetaxel [59]. Based on the favourable results of the latter trial, AstraZeneca has commenced a prospective phase III study comparing docetaxel alone with docetaxel plus 100mg ZD6474 or placebo in the setting of second line treatment for patients with advanced NSCLC after failure of the first line treatment. In a randomized phase II trial involving 168 patients with locally advanced or metastatic NSCLC who had progressed despite first - or second line platinum based therapy, patients received either ZD6474 (300mg once daily) or gefitinib (250mg once daily) until disease progression or limiting toxicity, with PFS as the primary end point. There was a statistically significant improvement in median PFS with ZD6474 compared with gefitinib (11 vs 8.1 weeks; P = 0.025) [60]. On progression, patients had the option to cross over to the alternative therapy (part B). In part B, stable disease for > 8 weeks was achieved in 16 of 37 patients (43%) who switched from gefitinib to ZD6474 and in seven of 29 (24%) who switched from ZD6474 to gefitinib. Based on these results, an international randomized phase III trial is planned to compare ZD6474 with erlotinib as second - or third line therapy for patients with advanced NSCLC. Another international randomized phase III trial will be opening to accrual in the near future and will evaluate whether ZD6474 offers a survival advantage over best supportive care in patients with disease that has progressed on an EGFR TKI and for whom no standard treatment options are available.
Sorafenib is an oral multikinase inhibitor that inhibits the RAF/MEK/ERK cancer pathway in various cancer cell lines and tumour xenografts and exhibited potent oral antitumour activity in a broad spectrum of human tumour xenograft models [61]. Sorafenib also targets the VEGFR 2 and 3, and platelet-derived growth factor receptor (PDGFR) family and KIT receptor TK [62]. Together, these data suggest that sorafenib may inhibit tumour growth by a dual mechanism, acting either directly on the tumour (through inhibition of RAF and KIT signalling) and/or on tumour angiogenesis (through inhibition of VEGFR and PDGFR signalling). Sorafenib combined with agents used to treat NSCLC has demonstrated to be efficient in controlling the growth of NSCLC tumours in preclinical models [63]. A pilot trial with sorafenib administered at 400mg twice daily continuously in a 28-day cycle to patients with recurrent NSCLC was performed [64]. In this trial, the efficacy of sorafenib was assessed by means of dynamic contrast-enhanced magnetic resonance imaging combined with tumour biopsy. Out of five patients evaluable for response, two patients reached PR; two achieved stable disease and one patient progressive disease. Concomitantly, sorafenib was also tested as a single agent (400mg twice daily, continuous) in a phase II trial. In this trial, from the 52 patients who received sorafenib (400mg twice daily), 51 were evaluable for efficacy. The drug showed promising efficacy in patients with advanced, progressive NSCLC, with approximately 60% of patients achieving disease stabilization with a median PFS of 23.7 weeks [65]. Overall, the 51 evaluable patients had a median OS of 29.3 weeks. Based on these observations, the NCI recently sponsored a trial of a single agent sorafenib in patients previously treated with NSCLC.
Sunitinib is a novel small molecule, an orally selective multitargeted TK inhibitor that exhibits direct antitumour activity against tumour cells dependent upon signalling through PDGFR, KIT, FLT-3 (fms like TK), or VEGFR for proliferation and survival in addition to antiangiogenic activity through its potent inhibition of VEGFR and PDGFR signalling [66]. Socinski et al [67] reported the data of an open-label, two stage, multicentre phase II trial evaluating the single agent activity of sunitinib (4/2 schedule; four weeks on treatment followed by two weeks off) in refractory NSCLC. Eligibility criteria included confirmed diagnosis of NSCLC patients previously treated with one to two CT regimens. Patients received sunitinib at 50mg per day for four weeks followed by two weeks off treatment (six weeks considered as a cycle). Of the 63 patients treated with sunitinib, seven patients had confirmed partial responses, yielding an OR rate of 11.1%. An additional 18 patients (28.6%) experienced stable disease of at least eight weeks in duration. Median PFS was 12.0 weeks, and median OS was 23.4 weeks. The authors concluded that sunitinib has provocative single agent activity and is well tolerated in previously treated patients with recurrent and advanced NSCLC, with the level of activity similar to currently approved agents. The trial is being extended to explore a continuous dosing strategy of sunitinib at 37.5mg/day p.o. due to three haemorrhage related deaths reported in this study. Data from a phase II study demonstrated the antitumour activity of continuous dosing schedule of sunitinib in 47 previously-treated patients with advanced, recurrent NSCLC, who had received one to two prior CT regimens. One patient (2%) had a confirmed PR and eight patients (17%) had stable disease > 3 months. Median PFS was 12.1 weeks and observed median OS 37.1 weeks [68]. The trials with sunitinib patients to demonstrate its efficacy as single or combined agent in NSCLC are ongoing.
Conclusions
Management of patients with lung cancer has changed considerably in recent years. Nowadays, the most effective targeted therapies as single agents are being integrated into a standard CT or combined with other targeted therapy drugs in current clinical trials to provide survival benefits beyond those achievable with CT. EGFR TKI therapy such as erlotinib has produced an OS benefit in the salvage setting. However, the use of EGFR TK inhibitors based on current data seems to be oriented to the subset of patients with NSCLC.
Antiangiogenic targeted therapies such as bevacizumab have demonstrated a survival benefit in the first line therapy and are being actively studied with multiple CT combinations and other new targeted therapies as well.
Lung cancer is a heterogeneous disease with multiple mutations. It is unlikely that only one signalling pathway is driving the oncogenic behaviour of tumours. Therefore, multikinase inhibitors represent a group of new targeted therapies that can play a major role in the treatment of NSCLC in the near future.
The author declares he has no potential conflicts of interest concerning drugs, pruducts, or services used in the study.
Autor deklaruje, že v souvislosti s předmětem studie nemá žádné komerční zájmy.The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Luis Mendoza, MD, PhD
INC Research, City Empiria
Na Strzi 65/1702
140 64 Prague 4
e-mail: Lmendoza@incresearch.com
Zdroje
1. Rapp E, Pater JL, Willan A et al. Chemotheraphy can prolong survival in patients with advanced non smalll-cell lung cancer – report of a Canadian multicenter randomized trial. J Clin Oncol 1988; 6 : 633–641.
2. Breathnach OS, Freidlin B, Conley B et al. Twenty-two years of phase II trials for patients with advanced non small cell lung cancer: sobering results. J Clin Oncol 2001; 19 : 1734–1742.
3. Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001; 37 (Suppl 4): 9–15.
4. Ohsaki Y, TANNO S, Fujita Y et al. Epidermal growth factor receptor expression correlates with poor prognosis in non small cell lung cancer patients with p53 overexpression. Oncol Rep 2000; 7 : 603–607.
5. Hirsch FR, Varella-Garcia M, Bunn PA Jr et al. Epidermal growth factor receptor in non small cell lung carcinomas: correlation between gene copy number and protein expression and impact in prognosis. J Clin Oncol 2003; 21(20): 3798–3807.
6. Onn A, Choe DH, Herbst RS et al. Tumor cavitation in stage I non small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology 2005; 237(1): 342–347.
7. Dacic S, Flanagan M, Cieply K et al. Significancer of EGFR protein expression and gene amplification in non small cell lung carcinoma. Am J Clin Pathol 2006; 125(6): 860–865.
8. Wislez M, Antoine M, Poulot V et al. IFCT0401-bio trial: predictive biological markers for disease control of patients with non resectable, adenocarcinoma with bronchioloalveolar features treated with gefitinib. Proc Am Soc Clin Oncol 2007; 25 : 18. Abstract 7653.
9. Nakamura H, Kawasaki N, Taguchi M et al. Survival impact ofeppidermal growth factor receptor overexpression in patients with non small cell lung cancer: a meta analysis. Thorax 2006; 61(2): 140–145.
10. Dassonville O, Bozec A, Fischell JL et al. EGFR targeting therapies: monoclonal antibodies versus tyrosine kinase inhibitors: similarities and differences. Crit Rev Oncol Hematol 2007; 62(1): 53–61.
11. Fukuoka M, Yano S, Giaccone G et al. Multi institutional randomized phase II trial of gefitinib for previously treated patients with advanced no-small cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 2003; 21 : 2237–2246.
12. Kris MG, Natale RB, Hebst RS et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor TK, in symptomatic patients with non small cell lung cancer: a randomized trial. JAMA 2003; 290 : 2149–2158.
13. Giaccone G, Herbst RS, Manegold C et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non small cell lung cancer: a phase II trial – INTACT 1. J Clin Oncol 2004; 22 : 777–784.
14. Herbst RS, Giaccone G, Schiller JH et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non small cell lung cancer: a phase III trial – INTACT 2. J Clin Oncol 2004; 22 : 785–794.
15. Thatcher N, Chang A, Parikh P et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non small cell lung cancer: results from a randomized, placebo-controlled, multicenter study (Iressa Survical Evaluation in Lung Cancer). Lancet 2005; 366 : 1527–1537.
16. AstraZeneca. Gefitinib (IressaTM) marketing authorisation application withdrawn in EU. January 4, 2005. Available from: http://www.astrazeneca.com/pressrelease/4442.aspx.
17. National Cancer Institute. Clinical Trial of Gefitinib for Advanced Lung Cancer Closes Early. April 18, 2005. Available from: http://www.nci.nih.gov/newscenter/pressreleases/gefitinibNSCLC.
18. Cufer T, Vrdoljak E, Gaafar R et al. Phase II, open-label, randomized study (SIGN) of single agent gefitinib (IRESSA) or docetaxel as second line therapy in patients with advanced (stage IIIb or IV) non small cell lung cancer. Anticancer Drugs 2006; 17(4): 401–409.
19. Perez-Soler R, Chachoua A, Hammond LA et al. Determinats of tumor response and survival with erlotinib in patients with non small cell lung cancer. J Clin Oncol 2004; 22(16): 3238–3247.
20. Giaccone G, Gallegos Ruiz M, Le Chevalier T et al. Erlotinib for frontline treatment of advanced non small cell lung cancer: a phase II study. Clin Cancer Res 2006; 12 (20 Pt 1): 6049–6055.
21. Jackman DM, Yeap BY, Lindeman NI et al. Phase II clinical trial of chemotheraphy-naïve patients > or = 70 years of age treated with erlotinib for advanced non small cell lung cancer. J Clin Oncol 2007; 25(7): 760–766.
22. Gatzemeier U, Pluzanska A, Szczesna A et al. Results of a phase III trial of erlotinib (OSI–774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non small cell lung cancer (NSCLC). J Clin Oncol 2007; 25(12): 1545–1552.
23. Herbst RS, Prager D, Hermann R et al. TRIBUTE: a phae III trial of erlotinib hydrocloride (OSI–774) combined with carboplatin and paclitaxel CT in advanced non small cell lung cancer. J Clin Oncol 2005; 23(25): 5892–5899.
24. Shepherd FA, Rodrigues Pereira JR, Ciuleanu T et al. Erlotinib in previously treated non small cell-lung cancer. N Engl J Med 2005; 353 : 123–132.
25. Janne PA, Gurubhagavatula S, Yeap BY et al. Outcomes of patients with advanced non small cell lung cancer treated with gefitinib (D1839, “Iressa”) on an expanded access study. Lung Cancer 2004; 44(2): 221–230.
26. Lee DH, Han JY, Yu SY et al. The role of gefitinib treatment for Korean never-smokers with advanced or metastatic adenocarcinoma of the lung: a prospective study. J Thorac Oncol 2006; 1(9): 965–971.
27. Tsao MS, Sakurada A, Cutz JC et al. Erlotinib in lung cancer: molecular and clinical predictors of outcome. N Engl J Med 2005; 353(2): 133–144.
28. Lu JF, Eppler SM, Wolf J et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure safety relationship in patients with non small cell lung cancer. Clin Pharmacol Ther 2006; 80(2): 136–145.
29. Hirsch FR, Varella-Garcia M, Bunn PA Jr et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controled study in advanced non small cell lung cancer. Clin Oncol 2006; 24(1): 5034–5042.
30. Laack E, Schneider C, Gutjahr T et al. Association between different potential predictive markers from TRUST, a trial of erlotinib in non small cell lung cancer. J Proc Am Soc Clin Oncol 2007; 25 : 18. Abstract 7651.
31. Bell DW, Lynch TJ, Haserlat SM et al. Epidermal growth factor receptor mutations and gene amplification in non small cell lung cancer molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 2005; 23(11): 8081–8092.
32. Yang SH, Mechanic LE, Yang P et al. Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non small cell lung cancer. Clin Cancer Res 2005; 11(6): 2106–2110.
33. Murray S, Timotheadou E, Linardou H et al. Mutations of the epidermal growth factor receptor tyrosine kinase domain and associations with clinicopathological features in non small cell lung cancer patients. Lung Cancer 2006; 52(2): 225–233.
34. Niho S, Kubota K, Goto K et al. First line single agent treatment with gefitinib in patients with advanced non small cell lung canecr: a phase II study. J Clin Oncol 2006; 24(1): 1599–1603.
35. Ohtsuka K, Ohnishi H, Furuyashiki G et al. Clinico-pathological and biological significancer of tyrosine kinase domain gene mutations and overexpression of epidermal growth factor receptor for lung adenocarcinoma. J Thorac Oncol 2006; 1(8): 787–795.
36. Wu YL, Zhong WZ, Li LY et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non small cell lung cancer: a meta analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007; 2(5): 430–439.
37. Kashii T, Okamoto I, Urata Y et al. EGFR mutation based phase II multicenter trial of gefitinib in advanced non small cell lung cancer patients: results of West Japan Thoracic Oncology Group Trial (WJTOG0403). 31st ESMO Congress 2006. Abstract 717.
38. Porta R, Terrasa J, Rolfo C et al. Deciding on second - or third line erlotinib in stage IV non small cell lung cancer patients based on the presence of mutations in the TK domain of the epidermal growth factor receptor. 31st ESMO Congress 2006. Abstract 719.
39. Massuti B, Reguart N, Vivanco GL et al. First line erlotinib in stage IV non small cell lung cancer patients with mutations in the TK domain of the epidermal growth factor receptor. 31st ESMO Congress 2006. Abstract 720.
40. Cappuzzo F, Hirsh FR, Rossi E et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non small cell lung cancer. J Natl Cancer Inst 2005; 97 : 643–655.
41. Lynch TJ, Lilenbaum R, Bonomi P et al. A phase II trial of cetuximab as therapy for recurrent non small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2004; 23 : 634. Abstract 7084.
42. Rosell R, Robinet G, Szczesna A et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first line therapy in EGFR-expressing advanced non small cell lung cancer. Ann Oncol 2008; 19(2): 362–369.
43. Pirker R, Szczesna A, Von Pawel J et al. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first line treatment of patients with advanced non small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2008; 26. Abstract 3.
44. Crawford J, Sandler AB, Hammond LA et al. ABX-EGF in combination with paclitaxel and carboplatin for advanced non small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2004; 23 : 634. Abstract 7083.
45. Cappuzzo F, Varella-Garcia M, Shigematsu H et al. Increased HER2 gene copy number associated with response to gefitinib therapy in epidermal growth factor receptor-positive non small cell lung cancer patients. J Clin Oncol 2005; 23(22): 5007–5018.
46. Swanton C, Futreal A, Eisen T. Her2-targeted therapies in non small cell lung cancer. Clin Cancer Res 2006; 12 (14 Pt 2): 4377–4383.
47. Langer CJ, Stephenson P, Thor A et al. Trastuzumab in the treatment of advanced non small cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J Clin Oncol 2004; 22(7): 1180–1187.
48. Herbst RS, Davies AM, Natale RB et al. Efficacy and safety of single agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin Cancer Res 2007; 13(20): 6175–6181.
49. Herbst RS, Hidalgo M, Pierson AS et al. Angiogenesis inhibitors in clinical development for lung cancer. Semin Oncol 2002; 29 (Suppl 4): 66–77.
50. Johnson DH, Fehrenbacher L, Novotny WF et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non small cell lung cancer. J Clin Oncol 2004; 22 : 2184–2191.
51. Sandler AB, Gray R, Perry M et al. Paclitaxel-Carboplatin Alone or with bevacizumab for non small cell lung cancer. N Engl J Med 2006; 355 : 2542–2550.
52. Manegold C, Von Pawel J, Zatloukal P et al. Randomised, double-blind multicentre phase III study of bevacizumab in combination with cisplatin and gemcitabine in chemotherapy-naïve patients with advanced or recurrent non squamous non small cell lung cancer (NSCLC): BO17704. Proc Am Soc Clin Oncol 2007; 25 : 18. Abstract LBA 7514.
53. Laurie SA, Gauthier I, Arnold A et al. Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non small cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol 2008; 26(11): 1871–1878.
54. Wedge SR, Ogilvie DJ, Dukes M et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 2002; 62 : 4645–4655.
55. Ciardiello F, Bianco R, Caputo R et al. Antitumor activity of ZD6474, a VEGF receptor TK inhibitor, in human cancer cells with acquired resistance o antiepidermal growth factor receptor therapy. Clin Cancer Res 2004; 10 : 784–793.
56. Taguchi F, Koh Y, Koizumi F et al. Anticancer effects of ZD6474, a VEGF receptor TK inhibitor, in gefitinib (Iressa)-sensitive and resistant xenograft models. Cancer Sci 2004; 95 : 984–989.
57. Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic nd therapeutic implications. J Clin Oncol 2005; 23(14): 3243–3256.
58. Johnson BE, Ma P, West H et al. Preliminary phase II safety evaluation of ZD6474, in combination with carboplatin and paclitaxel, as 1st line treatment in patients with NSCLC. Proc Am Soc Clin Oncol 2005; 23 : 16. Abstract 7102.
59. Heymach JV, Johnson BE, Prager D et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small cell lung cancer. J Clin Oncol 2007; 25(27): 4270–4277.
60. Natale RB, Bodkin D, Govindan R et al. ZD6474 versus gefitinib in patients with advanced NSCLC: Final results from a two part, double-blind, randomized phase II trial. Proc Am Soc Clin Oncol 2006; 24 : 18. Abstract 7000.
61. Wilhelm SM, Carter C, Tang LY et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and target the RAF/MEK/ERK pathway and receptor TKs involved in tumor progression and angiogenesis. Cancer Res 2005; 64 : 7099–7109.
62. Adams J, Huang P, Patrick D. A strategy for the design of multiple inhibitors for kinase-medited signalling in agiogenesis. Curr Opin Chem Biol 2002; 6 : 486–492.
63. Carter CA, Chen C, Brink C et al. Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non small cell lung carcinoma. Cancer Chemother Pharmacol 2007; 59(2): 183–195.
64. Liu B, Barett T, Choyke P et al. A phase II study BAY 43–9006 (Sorafenib) in patients with relapsed non small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol 2006; 24 : 18. Abstract 17119.
65. Gatzemeier U, Blumenschein G, Fosella F et al. Phase II trial of single agent sorafenib in patients with advanced non small cell lung carcinoma. Proc Am Soc Clin Oncol 2006; 24 : 18. Abstract 7002.
66. O’Farrell AM, Abrams TJ, Yuen HA et al. SU11248 is a novel FLT3 TK inhibitor with potent activity in vitro and in vivo. Blood 2003; 101 : 3597–3605.
67. Socinski MA, Novello S, Brahmer JR et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non small cell lung cancer. J Clin Oncol 2008; 26(4): 650–656.
68. Brahmer JR, Govindan S, Novello R et al. Efficacy and safety of continuous daily sunitinib dosing in previously treated advanced non small cell lung cancer (NSCLC): Results from a phase II study. Proc Am Soc Clin Oncol 2007; 25 : 18. Abstract 7542.
Štítky
Detská onkológia Chirurgia všeobecná Onkológia
Článok vyšiel v časopiseKlinická onkologie
Najčítanejšie tento týždeň
2009 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Nejasný stín na plicích – kazuistika
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Targeted Therapies in the Treatment of Advanced Non Small Cell Lung Cancer: Update
- Primary Pulmonary Sarcomas
- IKARUS Project – Incidence of Bone Events in Breast Cancer: Retrospective Analysis of Patients in Oncological Centres in the Czech Republic and Slovakia
- Experience in Data Management of the Clinical Retrospective Project in Czech and Slovak Oncology Centres (IKARUS Project)
- Radical Surgery and Intensive Chemotherapy Are Necessaryfor Successful Treatment of Osteosarcoma
- Treatment with Sunitinib and Hypothyroidism – a Case Report and Overview of Literature
- Iris Metastasis as the First Sign of Small Cell Lung Carcinoma with Metastatic Involvement of the Mediastinum
- Palliative and Hospic Care in the Czech Republic and in Europe
- Modernizace a obnova přístrojového vybavení Centra komplexní onkologické péče Nemocnice České Budějovice
- Zápis ze schůze výboru České onkologické společnosti dne 16. 6. 2009 v Hradci Králové
- Strouhal E., Němečková A. Trpěli i dávní lidé nádory? Historie a paleopatologie nádorů, zvláště zhoubných.Praha: Karolinum 2008. 193 str. ISBN 978-80-246-1481-6.
- Klinická onkologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Primary Pulmonary Sarcomas
- Palliative and Hospic Care in the Czech Republic and in Europe
- Radical Surgery and Intensive Chemotherapy Are Necessaryfor Successful Treatment of Osteosarcoma
- Treatment with Sunitinib and Hypothyroidism – a Case Report and Overview of Literature
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



