-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Converging on the Origins of Axonal Ion Channel Clustering
article has not abstract
Published in the journal: Converging on the Origins of Axonal Ion Channel Clustering. PLoS Genet 5(1): e32767. doi:10.1371/journal.pgen.1000340
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000340Summary
article has not abstract
The high-density clustering of voltage-gated Na+ channels and KCNQ2/3 K+ channels at the axon initial segment (AIS; see Figure 1A) and nodes of Ranvier (Figure 1B) is essential for the final integration of synaptic inputs and the initiation and rapid propagation of action potentials (APs) in neurons [1]–[3]. The AIS also marks the transition between the axonal and somatodendritic domains of the neuron, and its molecular integrity is required to maintain neuronal polarity [4],[5]. Thus, the AIS acts as both a key functional and structural bridge between neuronal input and output. Previously, the cytoskeletal and scaffolding protein ankyrinG was shown to be essential for the clustering of Na+ channels at the AIS and at the peripheral nervous system nodes of Ranvier [6]–[8]. Efforts to identify the molecular basis of Na+ channel clustering at the AIS showed that all mammalian Na+ channels have a cytoplasmic anchor motif that mediates their interaction (and AIS clustering) with ankyrinG [9],[10]. Subsequently, a comparison between Na+ channels and KCNQ2/3 K+ channels revealed the surprising fact that these two types of channels have the ankyrinG interaction and anchor motif in common [11]. While these anchor motifs are highly conserved among vertebrates, they are not found among invertebrates. This observation led to the fascinating question of how and why ion channels from two different gene families evolved a common amino acid sequence that mediates their clustering and localization at the AIS and nodes of Ranvier.
Fig. 1. Ion channels are clustered in mammalian axons at the AIS and nodes of Ranvier. 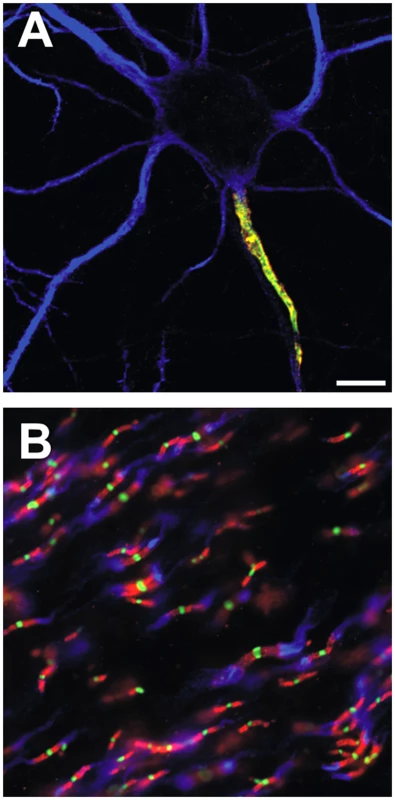
(A) A hippocampal neuron in culture is labeled for Na+ channels (red), the cytoskeletal and ankyrinG interacting protein βIV spectrin (green), and MAP2 (blue), to indicate the somatodendritic domain. (B) Nodes of Ranvier are triple-labeled for Nav channels (green), caspr to label paranodal junctions (red), and Kv1 K+ channels to label juxtaparanodal regions of myelinated axons. In a remarkable follow-up paper published in a recent issue of PLoS Genetics, Hill et al. [12] have now addressed this question by identifying key steps in the evolution of ion channel clustering on axons. They performed a phylogenetic analysis of invertebrate and vertebrate Na+ channels to define where along the evolutionary tree the AIS targeting motifs arose. They found that the first Na+ channels with anchor motifs arose in basal chordates, such as amphioxus, and that all orthologous Na+ channel genes in jawed vertebrates contain this anchor motif. The anchor motif is encoded by a single exon that is not found among invertebrates. Hill et al. [12] then used Na+ channel antibodies directed against a highly conserved sequence in the inactivation gate to discern the distribution of Na+ channels in dye-filled motor neurons from lamprey, a primitive, jawless vertebrate lacking myelin. They observed a narrow region of the axon, adjacent to the neuronal cell body, that was highly enriched in Na+ channels and that resembled the corresponding AIS found in mammals (Figure 1A). Consistent with the idea that these channels underlie the AP in lamprey neurons, previous studies showed that lamprey motor neuron APs are initiated in the proximal axon [13]. Thus, Hill et al. [12] demonstrate that a morphologically, molecularly, and functionally distinct AIS arose in basal chordates before the evolution of myelin and nodes of Ranvier.
Although KCNQ2/3 K+ channel homologs could be identified in lamprey, in contrast to Na+ channels, they lacked an AIS anchor motif. Instead, Hill et al. [12] found that the KCNQ2/3 K+ channel AIS anchor motif arose after lampreys diverged from other vertebrates, in a common ancestor of shark and humans. This sequence of evolutionary events is approximately coincident with that for the evolution of myelin, suggesting that the unique properties of vertebrate myelinated axons (i.e., saltatory conduction) drove the molecular evolution of the KCNQ2/3 K+ channels so that they, too, localized at nodes and the AIS.
The results of Hill et al. [12] are both significant and profound. Their results provide a first glimpse into the evolutionary origins of ion channel clustering along axons. The phylogenetic analysis of the anchor motifs in Na+ and KCNQ2/3 K+ channels suggests that nodes of Ranvier evolved from the previously developed AIS. This view is consistent with the AIS being specified intrinsically and assembled by the neuron, but node formation is initiated by, and requires, extrinsic factors derived from myelinating glia, some of which have recently been described [14]. Thus, channel clustering at the AIS may have been a key evolutionary event, facilitating the subsequent development of myelin, saltatory conduction, and the complex vertebrate nervous system.
The analysis of Na+ and KCNQ2/3 K+ channel clustering mechanisms also provides a remarkable instance of convergent evolution. While many examples exist for functional and anatomical convergence as a consequence of different organisms occupying similar ecological niches, the work of Hill et al. [12] demonstrates that the AIS anchor motifs are analogous structures that arose through molecular convergence, implying that Na+ and KCNQ2/3 K+ channels occupy a similar molecular and functional niche. The restricted localization of these channels to the nodes and the AIS strongly supports this view. However, because ankyrinG is a large scaffolding protein (two splice variants of 270 kD and 480 kD have been reported at the AIS and nodes [15]) that participates in numerous protein–protein interactions, the following question arises: Why should Na+ channels and KCNQ2/3 K+ channels evolve nearly identical anchor motifs? One possible explanation is that structural constraints in ankyrinG limit the number of available binding sites for interacting proteins, resulting in Na+ and KCNQ2/3 K+ channels competing for the same binding site. In this scenario, neurons could use this competition to modulate and change the biophysical properties of their spike-generating machinery at the AIS in response to different signals. Intriguingly, recent evidence also indicates that sequences in and adjacent to the Na+ channel AIS anchor motif can be phosphorylated by the kinase CK2, thereby increasing a channel's affinity for ankyrinG by ∼1,000-fold [16]. It is interesting to speculate that given the central role of the AIS in AP initiation, additional levels of control may have evolved to permit dynamic and plastic regulation of AIS firing properties.
Besides initiating the AP, the AIS also functions to maintain neuronal polarity. Loss of ankyrinG completely disrupts neuronal polarity, leading to axons with the molecular characteristics of dendrites [5]. In the future, it will be interesting to determine key points in evolution that resulted in clearly defined neuronal polarity. One critical question will be whether clustering of Na+ channels was driven by neuronal polarity, or vice versa. These questions are appealing and compelling not only because they provide clues into the origins of ion channel clustering, but because they provide a window into the very origins of our own success.
Zdroje
1. KoleMH
IlschnerSU
KampaBM
WilliamsSR
RubenPC
2008 Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11 178 186
2. SchwarzJR
GlassmeierG
CooperEC
KaoTC
NoderaH
2006 KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol 573 17 34
3. ShahMM
MiglioreM
ValenciaI
CooperEC
BrownDA
2008 Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 105 7869 7874
4. WincklerB
ForscherP
MellmanI
1999 A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397 698 701
5. HedstromKL
OgawaY
RasbandMN
2008 AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183 635 640
6. HedstromKL
XuX
OgawaY
FrischknechtR
SeidenbecherCI
2007 Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol 178 875 886
7. ZhouD
LambertS
MalenPL
CarpenterS
BolandLM
1998 AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143 1295 1304
8. DzhashiashviliY
ZhangY
GalinskaJ
LamI
GrumetM
2007 Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol 177 857 870
9. GarridoJJ
GiraudP
CarlierE
FernandesF
MoussifA
2003 A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300 2091 2094
10. LemailletG
WalkerB
LambertS
2003 Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem 278 27333 27339
11. PanZ
KaoT
HorvathZ
LemosJ
SulJ-Y
2006 A common ankyrin-G-based mechanism retains KCNQ and Nav channels at electrically active domains of the axon. J Neurosci 26 2599 2613
12. HillAS
NishinoA
NakajoK
ZhangG
FinemanJR
2008 Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet 4(12) e1000317 doi:10.1371/journal.pgen.1000317
13. TeravainenH
RvainenCM
1971 Fast and slow motorneurons to body muscle of the sea lamprey. J Neurophysiol 34 990 998
14. SusukiK
RasbandMN
2008 Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol 20 616 623
15. KordeliE
LambertS
BennettV
1995 AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem 270 2352 2359
16. BréchetA
FacheMP
BrachetA
FerracciG
BaudeA
2008 Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interaction with ankyrin G. J Cell Biol E-pub ahead of print. doi:10.1083/jcb.200805169
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2009 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Order and Disorder during Divergence
- Converging on the Origins of Axonal Ion Channel Clustering
- Mouse Genome-Wide Association Mapping Needs Linkage Analysis to Avoid False-Positive Loci
- Why Is the Correlation between Gene Importance and Gene Evolutionary Rate So Weak?
- A Microhomology-Mediated Break-Induced Replication Model for the Origin of Human Copy Number Variation
- A Novel Role for in the Parallel Evolution of Depigmentation in Independent Populations of the Cavefish
- Copy Number Variation and the Co-Evolution of Primate and Viral Genomes
- Multiple Chromosomal Rearrangements Structured the Ancestral Vertebrate -Bearing Protochromosomes
- A -Acting Diversification Activator Both Necessary and Sufficient for AID-Mediated Hypermutation
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A -Acting Diversification Activator Both Necessary and Sufficient for AID-Mediated Hypermutation
- Order and Disorder during Divergence
- Mouse Genome-Wide Association Mapping Needs Linkage Analysis to Avoid False-Positive Loci
- Why Is the Correlation between Gene Importance and Gene Evolutionary Rate So Weak?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



