-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
De Novo Origins of Human Genes
article has not abstract
Published in the journal: De Novo Origins of Human Genes. PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002381
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002381Summary
article has not abstract
Where do new genes come from? For a long time the answer to that question has simply been “from other genes”. The most prolific source of new loci in eukaryotic genomes is gene duplication in all its guises: exon shuffling, tandem duplication, retrocopying, segmental duplication, and genome duplication. However, in recent years there has been a growing appreciation of the oft-dismissed possibility of evolution of new genes from scratch (i.e., de novo) as a rare but consistent feature of eukaryotic genomes [1], [2].
Pioneering work identified several de novo genes in Drosophila [3]–[5], and since then, additional Drosophila cases have been identified [6], as well as cases in yeast [7], [8], Plasmodium [9], rice [10], mouse [11], primates [12], and human [13], [14]. It would appear that whenever anyone makes the effort to search, candidate novel genes are found.
In this issue of PLoS Genetics, Wu et al. [15] report 60 putative de novo human-specific genes. This is a lot higher than a previous, admittedly conservative, estimate of 18 such genes [13], [16]. The genes identified share broad characteristics with other reported de novo genes [13]: they are short, and all but one consist of a single exon. In other words, the genes are simple, and their evolution de novo seems plausible. The potential evolution of complex features such as intron splicing and protein domains within de novo genes remains somewhat puzzling. However, features such as proto-splice sites may pre-date novel genes [9], [17], and the appearance of protein domains by convergent evolution may be more likely than previously thought [2].
The operational definition of a de novo gene used by Wu et al. [15] means that there may be an ORF (and thus potentially a protein-coding gene) in the chimpanzee genome that is up to 80% of the length of the human gene (for about a third of the genes the chimpanzee ORF is at least 50% of the length of the human gene). This is a more lenient criterion than employed by other studies, and this may partly explain the comparatively high number of de novo genes identified. Some of these cases may be human-specific extensions of pre-existing genes, rather than entirely de novo genes—an interesting, but distinct, phenomenon.
Limitations in Defining and Identifying De Novo Genes
A major consideration in these studies is the reliable definition and identification of de novo genes. If a sequence similarity search fails to return a plausible homolog, then it may be that you are dealing with a novel gene. However, it is necessary to exclude the alternative hypothesis of recent loss in sister lineages as well as the possibility that this is a rapidly evolving gene with highly divergent, but extant, homologs.
Wu et al. [15] have employed a strategy similar to that of Knowles and McLysaght [13] to search within the human genome for candidate novel loci. The search protocol requires positive evidence of the absence of the gene from other primate lineages in order to show that it is not a gene that has diverged beyond recognition from its homologs (orthologous DNA is identifiable), nor is it a gene that has been recently lost in sister lineages (the ancestral sequence is inferred to carry a disablement) (Figure 1).
Fig. 1. Evidence in the detection of novel genes. 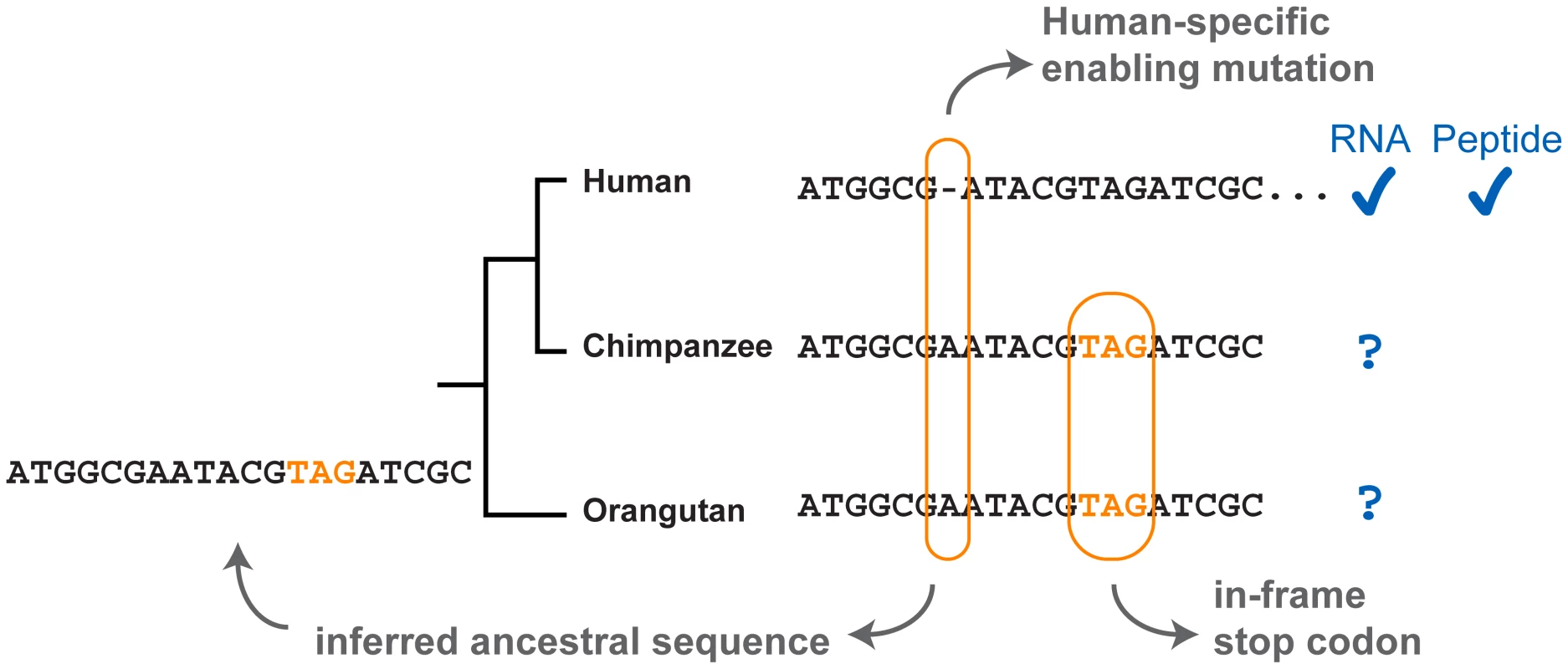
A hypothetical example where a novel human ORF is created by a human-specific deletion. The 1 bp deletion shifts a downstream stop codon out of frame. Because the deletion is not shared by other primates, the ancestral sequence is inferred to carry the in-frame stop. The authenticity of the novel human gene can be confirmed with transcription and translation evidence. A serious limitation in this approach is that it relies on existing gene lists that have been annotated using criteria that usually include the presence of a homolog in other genomes. Novel genes fail to meet this criterion by definition, thus they are usually not reliably annotated. Wu et al.'s study [15] highlights the volatility of the annotation of putatively novel genes—over half of the candidate de novo genes they identified are not included in the more recent Ensembl gene lists they used (version 56), and by version 60 only six of these genes were still listed.
It would be preferable to have a method of identifying novel genes that used more direct evidence of gene expression. Sequenced peptides and ESTs can be used to confirm that a putative gene is operational, but these data are not currently suitable for identifying protein-coding genes from first principles: the peptide databases usually only list peptides belonging to already-annotated genes [18]; and the high rate of promiscuous transcription of the genome, particularly in testis, where several of Wu et al.'s genes [15] were expressed at their highest, means that transcription alone is not sufficient to recognize a gene [1], [19].
However, care must also be taken to ensure that the ancestral sequence can reliably be inferred to be non-coding. Wu et al. [15] restricted their search to chimpanzee and orangutan genomes, but in at least one case (ENSG00000221972) gorilla and gibbon share the “human-specific” mutation, making this case equivocal. Ideally, the putative non-coding sequences should be investigated for evidence of transcription and translation to support the inference of absence of coding capacity.
Future Challenges
Though Wu et al. [15] have contributed to our growing knowledge of de novo gene evolution, we still lack a definitive list of de novo–originated genes in the human genome—mainly due to issues concerning genome annotation and the stringent criteria required to reliably identify cases. A comprehensive list of de novo genes in human as well as in other primates would open up the opportunity to examine the survivorship of these genes and investigate their specific contribution to phenotype.
The observation by Wu et al. [15] that some of the candidate de novo genes are expressed at their highest in brain tissues and testis is interesting, but by no means proves they are functional. A major challenge remains to demonstrate functionality of the de novo genes. This is particularly difficult for human-specific genes, where there is perhaps the greatest interest, but there are also the greatest limitations in terms of possible experiments.
What Does This Tell Us about Human–Chimpanzee Divergence?
Though it remains to be seen if any of the genes is functional, a clear picture is developing of de novo evolution as a process that can create genetic novelty, upon which there is at least the opportunity for natural selection to act. It has been argued that the capacity for innovation generated by novel genes is particularly important for the evolution of lineage-specific traits [20].
It is now common knowledge that human and chimpanzee DNA differ by only 1% (more accurately, they differ in 1% of alignable regions of genome, with a further 3% divergence due to lineage-specific indels [21]). This fact lies in stark contrast to the large phenotypic differences between the two species [22]. The study by Wu et al. [15], along with the previous reports of de novo genes in human, shows that even within highly similar regions of DNA, we can pinpoint small changes at the nucleotide level—base substitutions and indels—that have the potential to generate large phenotypic effects.
Zdroje
1. KaessmannH 2010 Origins, evolution, and phenotypic impact of new genes. Genome Res 20 1313 1326
2. TautzDDomazet-LosoT 2011 The evolutionary origin of orphan genes. Nat Rev Genet 12 692 702
3. BegunDJLindforsHAKernADJonesCD 2007 Evidence for de novo evolution of testis-expressed genes in the Drosophila yakuba/Drosophila erecta clade. Genetics 176 1131 1137
4. BegunDJLindforsHAThompsonMEHollowayAK 2006 Recently evolved genes identified from Drosophila yakuba and D. erecta accessory gland expressed sequence tags. Genetics 172 1675 1681
5. LevineMTJonesCDKernADLindforsHABegunDJ 2006 Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc Natl Acad Sci U S A 103 9935 9939
6. ZhouQZhangGZhangYXuSZhaoR 2008 On the origin of new genes in Drosophila. Genome Res 18 1446 1455
7. CaiJZhaoRJiangHWangW 2008 De novo origination of a new protein-coding gene in Saccharomyces cerevisiae. Genetics 179 487 496
8. LiDDongYJiangYJiangHCaiJ 2010 A de novo originated gene depresses budding yeast mating pathway and is repressed by the protein encoded by its antisense strand. Cell Res 20 408 420
9. YangZHuangJ 2011 De novo origin of new genes with introns in Plasmodium vivax. FEBS Lett 585 641 644
10. XiaoWLiuHLiYLiXXuC 2009 A rice gene of de novo origin negatively regulates pathogen-induced defense response. PLoS ONE 4 e4603 doi:10.1371/journal.pone.0004603
11. HeinenTJAJStaubachFHämingDTautzD 2009 Emergence of a new gene from an intergenic region. Curr Biol 19 1527 1531
12. Toll-RieraMBoschNBelloraNCasteloRArmengolL 2009 Origin of primate orphan genes: a comparative genomics approach. Mol Biol Evol 26 603 612
13. KnowlesDGMcLysaghtA 2009 Recent de novo origin of human protein-coding genes. Genome Res 19 1752 1759
14. LiC-YZhangYWangZZhangYCaoC 2010 A human-specific de novo protein-coding gene associated with human brain functions. PLoS Comput Biol 6 e1000734 doi:10.1371/journal.pcbi.1000734
15. WuD-DIrwinDMZhangY-P 2011 De novo origin of human protein coding genes. PLoS Genet 7 e1002379 doi:10.1371/journal.pgen.1002379
16. SiepelA 2009 Darwinian alchemy: Human genes from noncoding DNA. Genome Research 19 1693 1695
17. YandellMBaileyAMMisraSShuSWielC 2005 A computational and experimental approach to validating annotations and gene predictions in the Drosophila melanogaster genome. Proc Natl Acad Sci U S A 102 1566 1571
18. DuncanMWAebersoldRCaprioliRM 2010 The pros and cons of peptide-centric proteomics. Nat Biotechnol 28 659 664
19. BirneyEStamatoyannopoulosJADuttaAGuigoRGingerasTR 2007 Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799 816
20. KhalturinKHemmrichGFrauneSAugustinRBoschTCG 2009 More than just orphans: are taxonomically-restricted genes important in evolution? Trends Genet 25 404 413
21. Chimpanzee Sequencing and Analysis Consortium 2005 Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437 69 87
22. VarkiAAltheideTK 2005 Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Research 15 1746 1758
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 11- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



