-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Relationship between Proteinuria and Coronary Risk: A Systematic Review and Meta-Analysis
Background:
Markers of kidney dysfunction such as proteinuria or albuminuria have been reported to be associated with coronary heart disease, but the consistency and strength of any such relationship has not been clearly defined. This lack of clarity has led to great uncertainty as to how proteinuria should be treated in the assessment and management of cardiovascular risk. We therefore undertook a systematic review of published cohort studies aiming to provide a reliable estimate of the strength of association between proteinuria and coronary heart disease.Methods and Findings:
A meta-analysis of cohort studies was conducted to obtain a summary estimate of the association between measures of proteinuria and coronary risk. MEDLINE and EMBASE were searched for studies reporting an age - or multivariate-adjusted estimate and standard error of the association between proteinuria and coronary heart disease. Studies were excluded if the majority of the study population had known glomerular disease or were the recipients of renal transplants. Two independent researchers extracted the estimates of association between proteinuria (total urinary protein >300 mg/d), microalbuminuria (urinary albumin 30–300 mg/d), macroalbuminuria (urinary albumin >300 mg/d), and risk of coronary disease from individual studies. These estimates were combined using a random-effects model. Sensitivity analyses were conducted to examine possible sources of heterogeneity in effect size. A total of 26 cohort studies were identified involving 169,949 individuals and 7,117 coronary events (27% fatal). The presence of proteinuria was associated with an approximate 50% increase in coronary risk (risk ratio 1.47, 95% confidence interval [CI] 1.23–1.74) after adjustment for known risk factors. For albuminuria, there was evidence of a dose–response relationship: individuals with microalbuminuria were at 50% greater risk of coronary heart disease (risk ratio 1.47, 95% CI 1.30–1.66) than those without; in those with macroalbuminuria the risk was more than doubled (risk ratio 2.17, 1.87–2.52). Sensitivity analysis indicated no important differences in prespecified subgroups.Conclusion:
These data confirm a strong and continuous association between proteinuria and subsequent risk of coronary heart disease, and suggest that proteinuria should be incorporated into the assessment of an individual's cardiovascular risk.
Published in the journal: The Relationship between Proteinuria and Coronary Risk: A Systematic Review and Meta-Analysis. PLoS Med 5(10): e207. doi:10.1371/journal.pmed.0050207
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.0050207Summary
Background:
Markers of kidney dysfunction such as proteinuria or albuminuria have been reported to be associated with coronary heart disease, but the consistency and strength of any such relationship has not been clearly defined. This lack of clarity has led to great uncertainty as to how proteinuria should be treated in the assessment and management of cardiovascular risk. We therefore undertook a systematic review of published cohort studies aiming to provide a reliable estimate of the strength of association between proteinuria and coronary heart disease.Methods and Findings:
A meta-analysis of cohort studies was conducted to obtain a summary estimate of the association between measures of proteinuria and coronary risk. MEDLINE and EMBASE were searched for studies reporting an age - or multivariate-adjusted estimate and standard error of the association between proteinuria and coronary heart disease. Studies were excluded if the majority of the study population had known glomerular disease or were the recipients of renal transplants. Two independent researchers extracted the estimates of association between proteinuria (total urinary protein >300 mg/d), microalbuminuria (urinary albumin 30–300 mg/d), macroalbuminuria (urinary albumin >300 mg/d), and risk of coronary disease from individual studies. These estimates were combined using a random-effects model. Sensitivity analyses were conducted to examine possible sources of heterogeneity in effect size. A total of 26 cohort studies were identified involving 169,949 individuals and 7,117 coronary events (27% fatal). The presence of proteinuria was associated with an approximate 50% increase in coronary risk (risk ratio 1.47, 95% confidence interval [CI] 1.23–1.74) after adjustment for known risk factors. For albuminuria, there was evidence of a dose–response relationship: individuals with microalbuminuria were at 50% greater risk of coronary heart disease (risk ratio 1.47, 95% CI 1.30–1.66) than those without; in those with macroalbuminuria the risk was more than doubled (risk ratio 2.17, 1.87–2.52). Sensitivity analysis indicated no important differences in prespecified subgroups.Conclusion:
These data confirm a strong and continuous association between proteinuria and subsequent risk of coronary heart disease, and suggest that proteinuria should be incorporated into the assessment of an individual's cardiovascular risk.Introduction
Over recent decades, substantial progress has been made in understanding the role that “classical risk factors”—namely blood pressure, smoking, cholesterol, diabetes, and obesity—play in the aetiology of coronary heart disease (CHD) [1–6]. Subsequent studies and meta-analyses have identified other factors that may provide additional important predictive information regarding the risk of CHD, including inflammatory markers [7], haemostatic factors [8,9], left ventricular hypertrophy, and markers of kidney dysfunction [10].
Kidney disease is highly prevalent worldwide [11–13], affecting approximately one in six adults in Western countries. An association between severe kidney failure and accelerated cardiovascular disease (CVD) has long been recognized [14]. Markers of early kidney disease, such as the presence of albumin or protein in the urine, have been reported to be associated with increased risk of CHD in the general population [15]. However, inconsistencies in both the direction and magnitude of the reported relationship have led to uncertainty about the practical benefit of measuring these indices in quantifying an individual's future coronary risk. Variations in the definition, measurement, and assessment of proteinuria have added to the confusion, with some studies reporting on the relationship between urinary albumin excretion (micro - or macroalbuminuria) and coronary risk, while others have used total urinary protein excretion, of which albumin is a component.
Previous reports have suggested a positive association between proteinuria and CHD risk [10], but the strength, consistency, and independence of the relationship have not been defined. Hence, the aim of the current study was to provide reliable estimates of the strength and nature of the association between proteinuria (urinary protein excretion of >300 mg/d), microalbuminuria (30–300 mg/d of urinary albumin excretion), and macroalbuminuria (>300 mg/d) with subsequent risk of CHD in the general population and in predefined subgroups (diabetes, gender, and ethnicity).
Methods
Data Sources and Searches
We performed a systematic review of the available literature according to the MOOSE guidelines [16] for the conduct of meta-analyses of observational studies. Relevant studies published between 1966 and November 2006 were identified from CINAHL (http://www.ebscohost.com/cinahl/), EMBASE (http://www.embase.com/), and MEDLINE (http://ovidsp.tx.ovid.com/spb/ovidweb.cgi and http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed) using a combined text word and MeSH heading search strategy that included all spellings of proteinuria, albuminuria, microalbuminuria, or macroalbuminuria combined with coronary heart disease, ischemic heart disease, myocardial infarction, and angina pectoris, and limited to cohort studies. References from identified studies were manually scanned to identify any other relevant studies.
Study Selection and Data Extraction
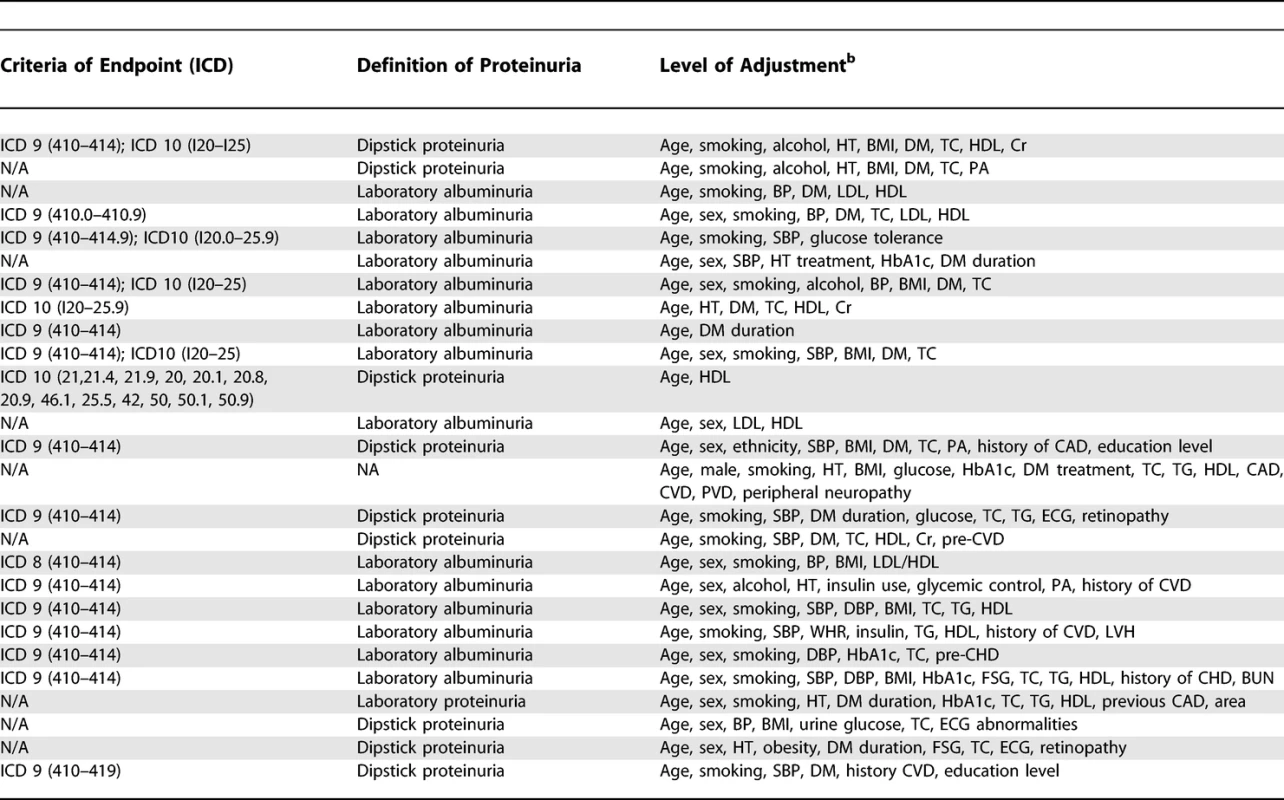
Cohort studies were included if they reported quantitative estimates of the age and sex-adjusted relative risk (RR) for fatal or nonfatal CHD associated with any level of albuminuria or proteinuria (Table 1), together with an estimate of variance (standard error or 95% confidence intervals [CIs]). Studies were excluded if the study population chiefly comprised pathological subgroups (including hospital-based populations or those that the majority of the study population comprised individuals with a history of myocardial infarction, chronic kidney disease, or renal transplantation), if they reported the estimate of effect with no means by which to derive the standard error, or if the estimate was not adjusted for age. Reports from clinical trials were excluded to avoid potential modification of the nature and magnitude of the relationship between proteinuria and CHD by the intervention studied (e.g., blood pressure lowering). Studies reported in languages other than English were included.
Tab. 1. 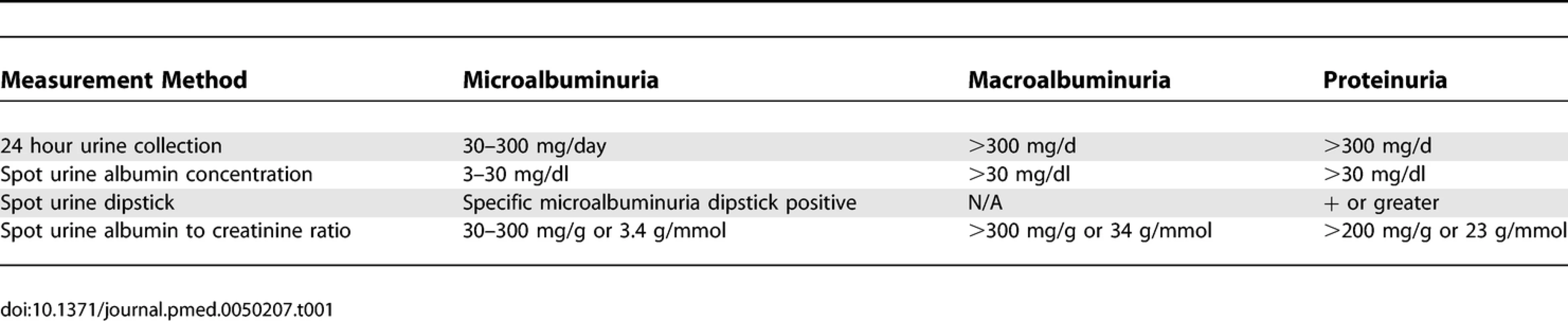
Definitions of Albuminuria and Proteinuria CHD mortality was defined as death attributed to ICD9 (International Classification of Diseases and Related Health Problems, Ninth Revision) codes 410 to 414.9 and ICD10 codes I20.0 to I25.9. The prespecified definitions of proteinuria are shown in Table 1. The literature search and data extraction were conducted by two of the authors (CV and TN). Where there was disagreement over the eligibility of a study, three additional authors reviewed the paper until a consensus was reached (RH, VP, FB).
Data Synthesis and Analyses
Summary estimates of RR were obtained using a random effects model. Separate summary estimates were obtained for the RR associated with microalbuminuria, macroalbuminuria, any albuminuria (micro or macro), and proteinuria. In addition, some studies reported sex-specific risk ratios for the strength of the association, and these were pooled if a single overall estimate was not provided.
The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic [17]. The 95% CI of the I2 statistic was calculated using the method described by Higgins et al. [18]. We investigated possible sources of heterogeneity by comparing summary results obtained when studies were grouped according to specific characteristics. Tests for heterogeneity were performed using meta-regression analysis. Publication bias was assessed using the Egger test and represented graphically using funnel plots plotting the natural log of the RR versus its standard error. The trim and fill analysis for publication bias was performed using Duval and Tweedie's methods [19]. A p-value below 0.05 was considered statistically significant in all analyses. All analyses were performed using STATA (Release 9.2; Stata Corporation, http://www.stata.com/).
Results
Literature Search and Characteristics of Studies
The electronic search yielded a total of 3,653 articles, of which 297 reports were reviewed in full text (see Figure 1). Of these, 26 studies were eligible for inclusion [20–45], including information on 169,949 individuals and 7,117 CHD events (27% fatal). Study size ranged from 146 to 90,363 participants and the average duration of participant follow-up was 4–27 y (Table 2).
Fig. 1. Identification Process for Eligible Studies 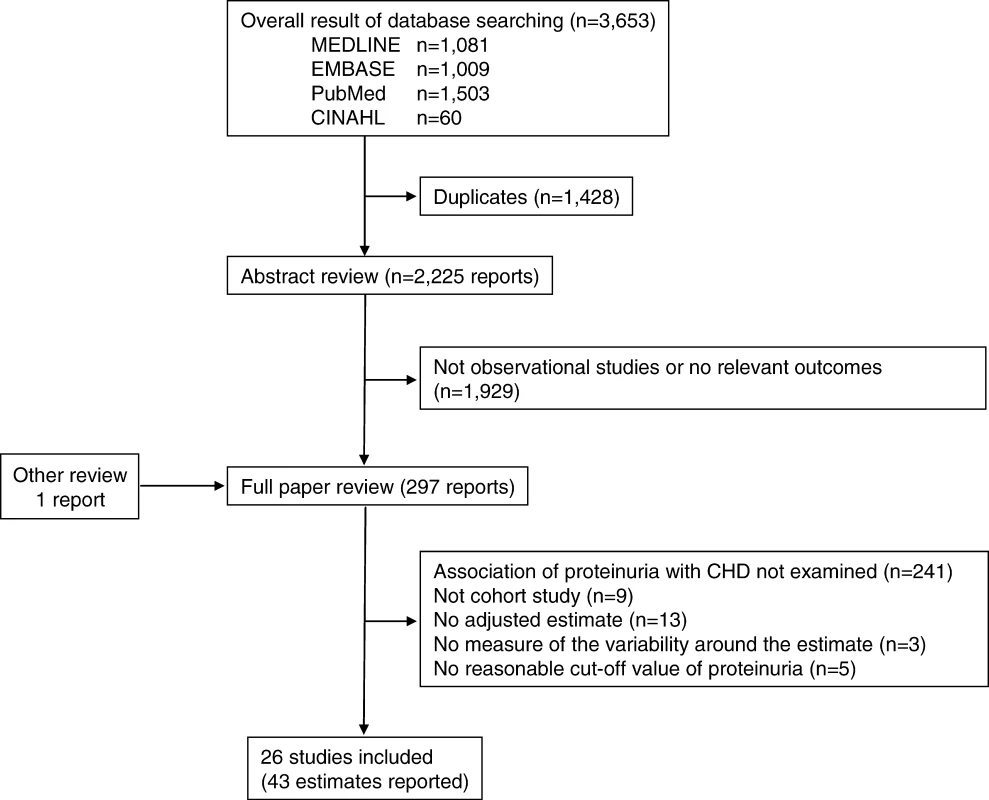
Tab. 2. 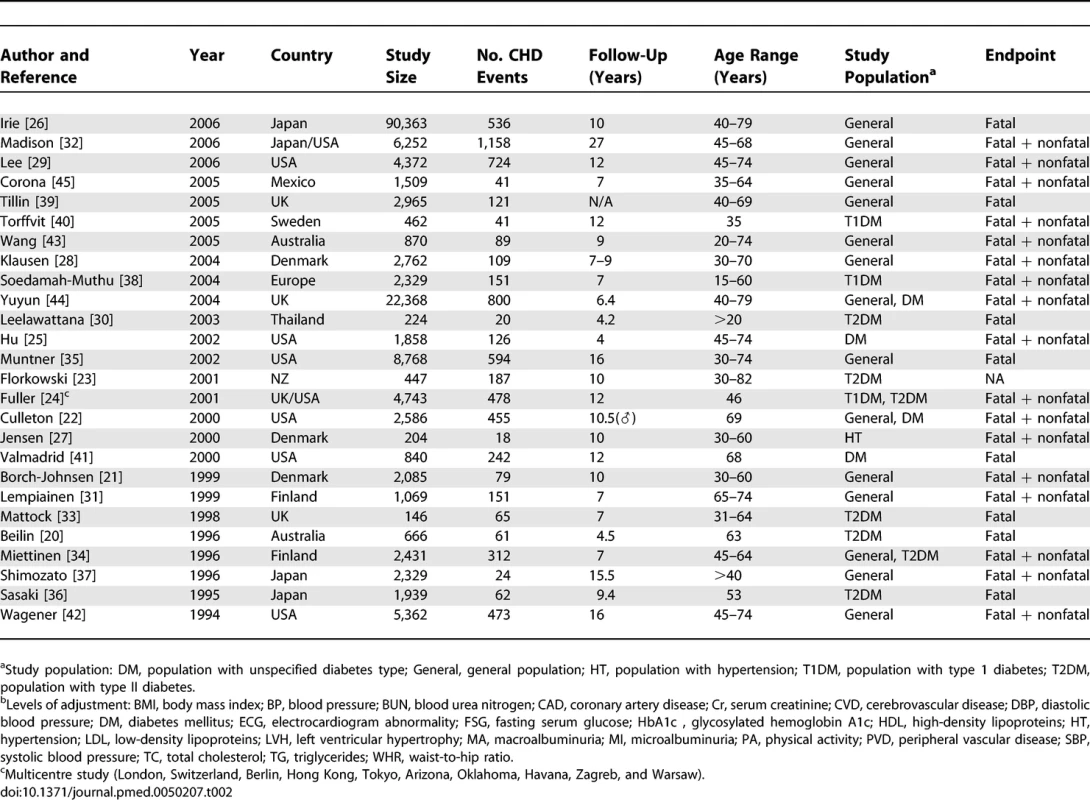
Characteristics of Studies Reporting on the Association between Proteinuria and Subsequent Risk of Coronary Heart Disease In total, 26 studies reported the RR for CHD associated with a measure of proteinuria, and some reported the relationship for more than one threshold of proteinuria: ten studies reported on the association between total proteinuria and CHD; seven studies reported on the association between microalbuminuria and CHD; nine studies reported on the association between macroalbuminuria and CHD; and six studies reported on the association between any level of albuminuria and CHD. Levels of proteinuria were measured using either urinary dipstick tests for protein or the measurement of the UPCR (urinary protein–creatinine ratio) on spot urine specimens. The former was used in the majority of studies (n = 9). Levels of albuminuria were estimated by measurement of the UACR (urinary albumin–creatinine ratio) on spot urine specimens. Twenty-three studies reported hazard ratios, and three reported odds ratios. Twenty-five of the studies provided an estimate that had been adjusted for major CHD risk factors including blood pressure, smoking, diabetes, and cholesterol (Table 2).
Association between Proteinuria and Subsequent Risk of CHD
A total of 16 estimates from ten studies including 124,997 patients reported on the relationship between total proteinuria and the risk of subsequent CHD (Figure 2). The mean-weighted estimate from these studies indicated that individuals with proteinuria have an approximately 50% greater risk of CHD compared with those without the condition: RR 1.47 (95% CI 1.23–1.74). There was considerable heterogeneity across these studies (p < 0.006) but no significant evidence of publication bias (p = 0.11).
Fig. 2. Summary Risk Ratio (95% Confidence Intervals) for the Association of Proteinuria with the Risk of Coronary Heart Disease in Population-Based Cohort Studies 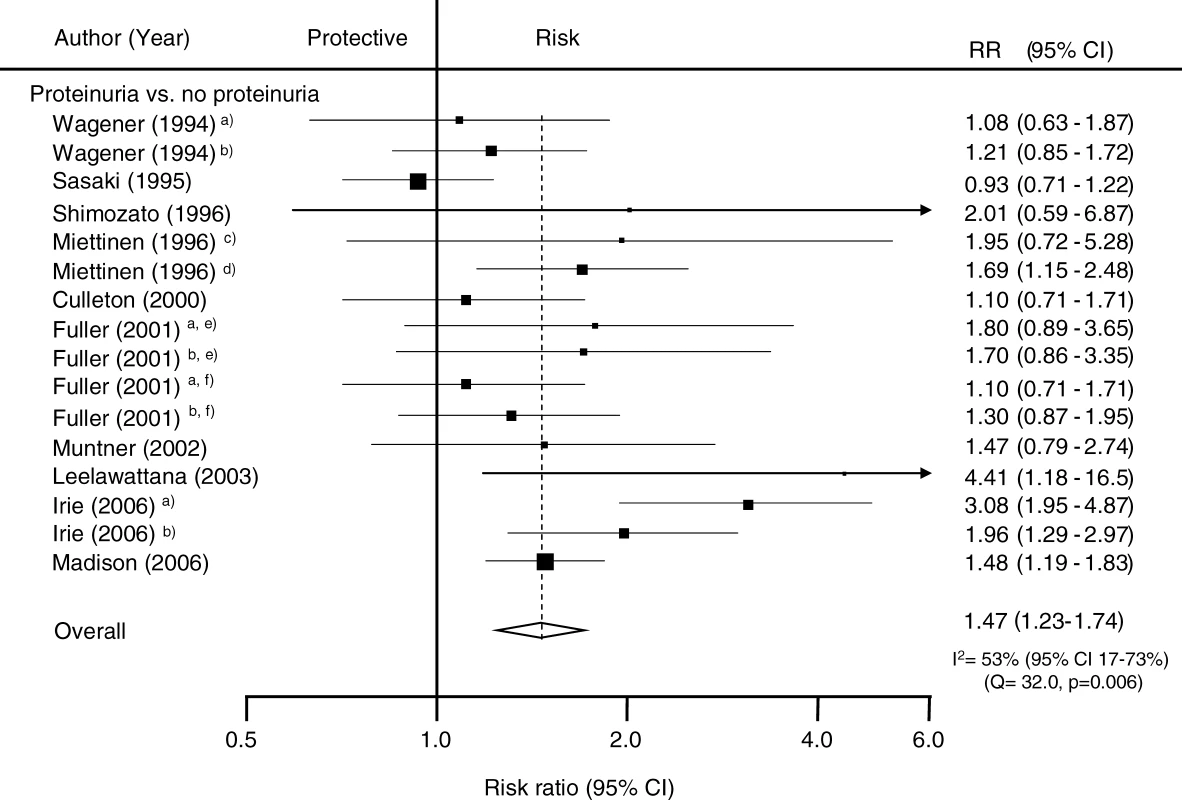
The black squares are inversely proportional to the variance of the study and the horizontal lines represent the 95% CIs. Footnotes: Four studies reported estimates separated according to subgroups including (a) female, (b) male, (c) people without diabetes, (d) people with diabetes, (e) people with type 1 diabetes, and (f) people with type 2 diabetes. References: Culleton 2000 [22]; Fuller 2001 [24]; Irie 2006 [26]; Leelawattana 2003 [30]; Madison 2006 [32]; Miettinen 1996 [34]; Muntner 2002 [35]; Sasaki 1995 [36]; Shimozato 1996 [37]; Wagener 1994 [42]. Potential sources of heterogeneity in the strength of the association between proteinuria and CHD were examined by conducting sensitivity analyses (Figure 3). There was no evidence that the strength of the association differed according to diabetes status, ethnicity (Asian versus non-Asian, where most Asian participants were from Japan), fatal versus nonfatal CHD outcomes, laboratory versus dipstick measurement of proteinuria, duration of study follow-up, or study size (all p-values for heterogeneity > 0.1).
Fig. 3. Examination of Potential Sources of Heterogeneity between Studies of Proteinuria and CHD According to Study or Participant Characteristics 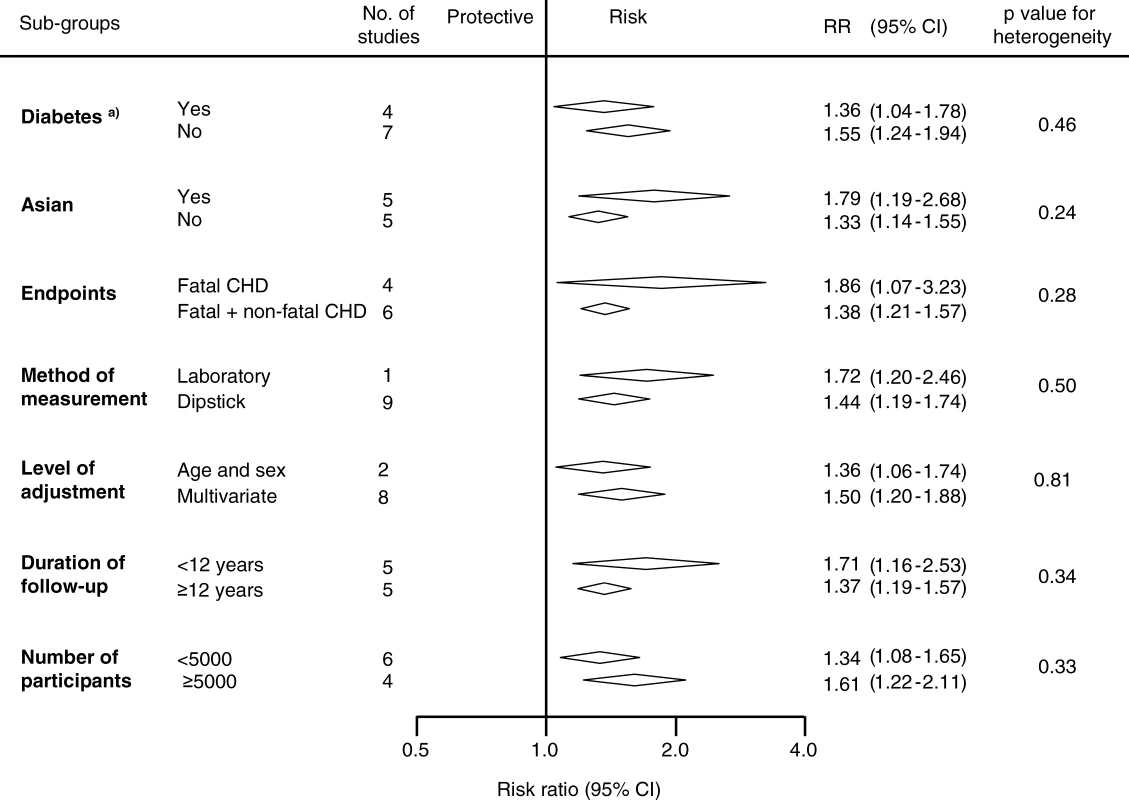
Conventions as in Figure 2. Association between Albuminuria and Subsequent Risk of CHD
In the seven studies (n = 31,591 participants) for which information was available, individuals with microalbuminuria had a 50% greater risk of subsequent CHD compared with individuals without, and there was no evidence of heterogeneity among included studies: RR 1.47 (95% CI 1.30–1.66); p for heterogeneity = 0.48 (Figure 4). Evidence of significant publication bias was identified using the Egger test (p = 0.01), and after correction for its presence the estimate of the association was marginally reduced to 1.42 (95% CI 1.23–1.64). A total of nine studies (n = 34,834 participants) compared the risk of CHD among individuals with and without macroalbuminuria. Individuals in whom macroalbuminuria was detected had double the risk of CHD compared with individuals without, with no evidence of publication bias: RR 2.17, 95% CI 1.87–2.52 (Figure 4). Even after accounting for the fact that we are comparing different levels of albuminuria with the same control group by using the Bonferroni correction, the pooled estimates of microalbuminuria and macroalbuminuria remained highly significant (both p < 0.0001 after Bonferroni correction).
Fig. 4. Summary Risk Ratio (95% Confidence Intervals) for the Association of Albuminuria with the Risk of Coronary Heart Disease in Population-Based Cohort Studies 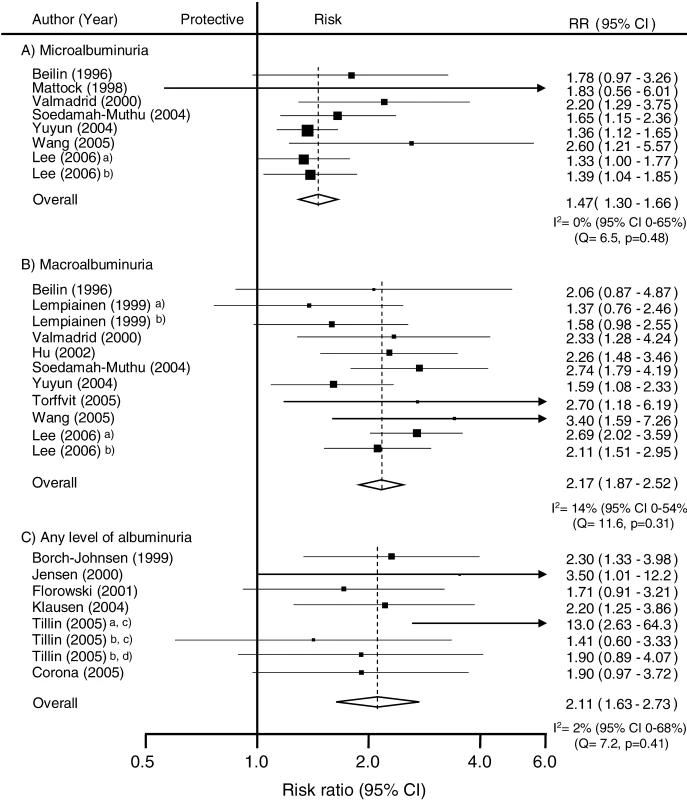
(A) Studies reporting risk estimate of microalbuminuria compared to normoalbuminuria. In those six studies (n = 9,972 participants) that reported on the association between any level of albuminuria the RR for CHD among individuals with albuminuria was twice that of normoalbuminuric individuals with no evidence of publication bias: RR 2.11, 95% CI 1.63–2.73; p for heterogeneity = 0.41 (Figure 4).
To examine whether there was a dose–response association between albuminuria and CHD the following analysis was restricted to those six studies (n = 31,445 participants) that had reported separately on the association between microalbuminuria and macroalbuminuria and subsequent CHD (Figure 5). Among these studies, compared with normoalbuminuric individuals, those with microalbuminuria had a 50% greater risk of CHD (RR 1.48, 95% CI 1.30–1.68) and in those with macroalbuminuria, the risk of CHD was more than doubled (RR 2.55, 95% CI 2.09–3.11; p for heterogeneity < 0.0001).
Fig. 5. Subgroup Analysis of Comparisons within Studies Reporting Associations of Proteinuria and Albuminuria with Coronary Heart Disease Risk 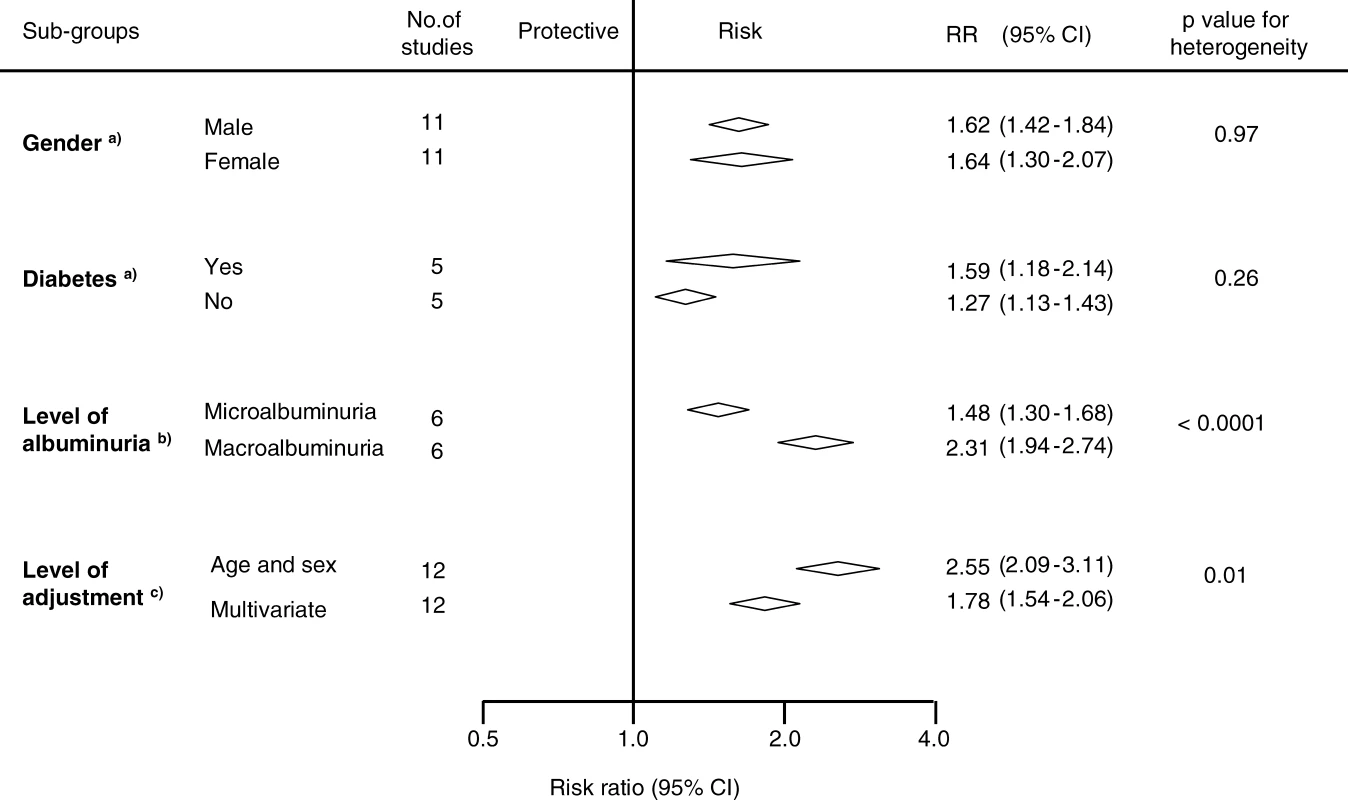
Conventions as in Figure 2. Impact of Adjustment for Major Cardiovascular Risk Factors on the Strength of the Association
A total of 12 studies (n = 138,003 participants) reported estimates of the strength of the association between either proteinuria or albuminuria that were adjusted first, by age and then by other known cardiovascular risk factors (i.e., blood pressure, smoking, lipids and diabetes). The overall age-adjusted summary estimate was 2.55 (95% CI 2.09–3.11), which was reduced by approximately 50% to 1.78 (95% CI 1.54–2.06; p for heterogeneity = 0.01, Figure 5).
Discussion
This overview of the epidemiological evidence suggests that proteinuria is independently associated with increased risk of subsequent CHD. The results from this meta-analysis of 26 cohort studies, including information on over 7,000 CHD events among almost 170,000 individuals, suggest that people with proteinuria have a risk of CHD that is at least 50% greater than those without. Moreover, there was some evidence to indicate a dose–response relationship such that the strength of the association was substantially higher among individuals with macroalbuminuria compared with those with microalbuminuria. Furthermore, the relationship was consistent across diverse population subgroups including individuals with and without diabetes. The included studies were largely population-based suggesting that these findings are broadly generalisable and less likely to have been affected by interventions (e.g., blood pressure lowering) than data from clinical trials.
The magnitude of the risk is similar to that associated with many classical risk factors, and stronger than many more recently discovered risk factors [7,9] suggesting that the addition of proteinuria may improve the predictive ability of commonly used cardiovascular risk prediction formulae. This is being increasingly recognized, with the most recent cardiovascular disease prevention guidelines from the American Heart Association [46] suggesting that individuals with proteinuria should be considered to be at similar risk to people with established CHD. Our data suggest that proteinuria is likely to improve the ability to predict coronary risk. Further studies will be required to assess whether the performance of commonly used risk prediction tools can be improved by the inclusion of proteinuria in cardiovascular risk prediction formulae.
Although these data suggest that albuminuria may be a stronger predictor of coronary risk than urinary total protein excretion, this may be an artefact of the data, since the majority of studies that measured urinary protein used dipstick tests, which have a lower sensitivity and specificity than do laboratory estimates. Unfortunately, as only one of the included studies used laboratory estimates to measure proteinuria, we had limited statistical power in the sensitivity analysis to be able to detect any real difference between the two methods. The included studies did not use a specific methodology for the collection of the urine specimens, adding a further potential source of variability [47].
The relationship between proteinuria and CHD was similar in individuals with and without diabetes, and in the other subgroups studied. We were unable to assess whether proteinuria conferred an increased risk of coronary disease separately to other manifestations of chronic kidney disease, although others have recently reported that these risks are additive [48]. Although the impact of therapies that reduce proteinuria (e.g., blood pressure–lowering agents) have been the subject of reasonably large trials in people with diabetes and proteinuria [49,50], fewer data are available regarding the effects of these therapies in individuals without diabetes and with proteinuria. The Heart Outcomes Protection Evaluation (HOPE [51]) suggested that a somewhat greater magnitude of cardiovascular protection may be associated with the use of the angiotensin-converting enzyme ramipril in participants with albuminuria at baseline (relative risk reduction of 26% versus 15%), although the statistical significance of this difference was not reported. Data from other trials suggest that the reduction in urinary protein excretion achieved in an individual predicts the reduction in the risk of subsequent cardiovascular events [52,53]. The confirmation of a differential cardiovascular protective efficacy for blood pressure–lowering agents according to baseline proteinuria in future studies would further increase the value of adding proteinuria to cardiovascular risk prediction tools that guide decisions regarding the use of preventative therapies.
Our systematic review could not assess whether proteinuria plays a causative role in the development of CHD, as this would require evidence from randomised controlled trials of therapies that acted mainly by reducing proteinuria. It has been suggested that proteinuria may simply be a marker of early vascular disease, as propounded in the “Steno hypothesis.” This hypothesis contends that the presence of proteinuria reflects widespread early vascular disease and abnormal endothelial function (including that in the glomerular vasculature), and as such may be a marker of the severity and duration of other risk factors (e.g., elevated blood pressure) rather than playing a pathogenic role per se [54].
An important limitation of the current analyses is its reliance upon published summary data (rather than individual participant data), which impairs the examination of the impact of adjustment for known CHD risk factors. In those studies that provided both adjusted and unadjusted estimates, the association between proteinuria and CHD was significantly attenuated by about 50% after adjustment. Such a significant attenuation in effect size suggests that residual confounding is likely to remain and, further, that the summary result presented here may be an overestimate of the true magnitude of the association between proteinuria and CHD. Some evidence of publication bias was also identified, although after correcting for it, the associations remained strong and significant. Conversely, the strength of the relationship may have been underestimated due to the impact of regression dilution bias [55], in which a single measurement of a risk factor may underestimate the strength of its relationship to a disease. The effect of regression dilution bias is supported by the findings of the study by Madison et al. [32], in which proteinuria present on two separate measurements was associated with a much higher risk of subsequent coronary heart disease than transient proteinuria (RR 3.72, 95% CI 2.62–5.27 versus 1.48, 1.19–1.83). Despite these limitations, the results of this systematic review represent the most precise and accurate estimate of the strength of the relationship between proteinuria and CHD currently available.
The findings of this study therefore strongly support a role for the evaluation of proteinuria in the prediction of CHD risk. They suggest that the use of strategies to reduce proteinuria, and better targeting of other cardioprotective therapies, may help to reduce the overall burden of CHD. Studies to assess the impact of these strategies are warranted.
Zdroje
1. YusufSHawkenSOunpuuSDansTAvezumA
2004
Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study.
Lancet
364
937
952
2. ZhangXPatelAHoribeHWuZBarziF
2003
Cholesterol, coronary heart disease, and stroke in the Asia Pacific region.
Int J Epidemiol
32
563
572
3. WoodwardMZhangXBarziFPanWUeshimaH
2003
The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region.
Diabetes Care
26
360
366
4. WoodwardMLamTHBarziFPatelAGuD
2005
Smoking, quitting, and the risk of cardiovascular disease among women and men in the Asia-Pacific region.
Int J Epidemiol
34
1036
1045
5. Ni MhurchuCRodgersAPanWHGuDFWoodwardM
2004
Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants.
Int J Epidemiol
33
751
758
6. KengneAPPatelABarziFJamrozikKLamTH
2007
Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region.
J Hypertens
25
1205
1213
7. DaneshJWheelerJGHirschfieldGMEdaSEiriksdottirG
2004
C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease.
N Engl J Med
350
1387
1397
8. DaneshJWhincupPWalkerMLennonLThomsonA
2001
Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis.
Circulation
103
2323
2327
9. DaneshJLewingtonSThompsonSGLoweGDCollinsR
2005
Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis.
JAMA
294
1799
1809
10. SarnakMJLeveyASSchoolwerthACCoreshJCulletonB
2003
Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention.
Circulation
108
2154
2169
11. CoreshJAstorBCGreeneTEknoyanGLeveyAS
2003
Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey.
Am J Kidney Dis
41
1
12
12. ChadbanSJBrigantiEMKerrPGDunstanDWWelbornTA
2003
Prevalence of kidney damage in Australian adults: The AusDiab kidney study.
J Am Soc Nephrol
14
S131
138
13. WhiteSLCassAAtkinsRCChadbanSJ
2005
Chronic kidney disease in the general population.
Adv Chronic Kidney Dis
12
5
13
14. LindnerACharraBSherrardDScribnerB
1974
Accelerated atherosclerosis in prolonged maintenance haemodialysis.
New Engl J Med
290
69
701
15. de ZeeuwDParvingHHHenningRH
2006
Microalbuminuria as an early marker for cardiovascular disease.
J Am Soc Nephrol
17
2100
2105
16. StroupDFBerlinJAMortonSCOlkinIWilliamsonGD
2000
Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group.
JAMA
283
2008
2012
17. HigginsJPThompsonSGDeeksJJAltmanDG
2003
Measuring inconsistency in meta-analyses.
BMJ
327
557
560
18. HigginsJPThompsonSG
2002
Quantifying heterogeneity in a meta-analysis.
Stat Med
21
1539
1558
19. DuvalSTweedieR
2000
Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis.
Biometrics
56
455
463
20. BeilinJStantonKGMcCannVJKnuimanMWDivitiniML
1996
Microalbuminuria in type 2 diabetes: An independent predictor of cardiovascular mortality.
Aust N Z J Med
26
519
525
21. Borch-JohnsenKFeldt-RasmussenBStrandgaardSSchrollMJensenJS
1999
Urinary albumin excretion: An independent predictor of ischemic heart disease.
Arterioscler Thromb Vasc Biol
19
1992
1997
22. CulletonBFLarsonMGParfreyPSKannelWBLevyD
2000
Proteinuria as a risk factor for cardiovascular disease and mortality in older people: A prospective study.
Am J Med
109
1
8
23. FlorkowskiCMScottRSCoopePAMoirCL
2001
Predictors of mortality from type 2 diabetes mellitus in Canterbury, New Zealand; a ten-year cohort study.
Diabetes Res Clin Pract
53
113
120
24. FullerJHStevensLKWangSLLeeETKeenH
2001
Risk factors for cardiovascular mortality and morbidity: The WHO multinational study of vascular disease in diabetes.
Diabetologia
44
S54
S64
25. HuDJablonskiKASparlingYHRobbinsDCLeeET
2002
Accuracy of lipoprotein lipids and apoproteins in predicting coronary heart disease in diabetic American Indians: the Strong Heart Study.
Ann Epidemiol
12
79
85
26. IrieFIsoHSairenchiTFukasawaNYamagishiK
2006
The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population.
Kidney Int
69
1264
1271
27. JensenJSFeldt-RasmussenBStrandgaardSSchrollMBorch-JohnsenK
2000
Arterial hypertension, microalbuminuria, and risk of ischemic heart disease.
Hypertension
35
898
903
28. KlausenKBorch-JohnsenKFeldt-RasmussenBJensenGClausenP
2004
Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes.
Circulation
110
32
35
29. LeeYTFujitaTShimamotoKWuZZhuJ
2006
Other health matters to be considered for the Asian population: Hypertensive patients at high risk.
Int J Clin Pract
60
17
19
30. LeelawattanaRRattarasarnCLimASoonthornpunSSetasubanW
2003
Causes of death, incidence and risk factors of cardiovascular diseases in Thai type 2 diabetic patients: A 5-year follow-up study.
Diabetes Res Clin Pract
60
183
189
31. LempiainenPMykkanenLPyoralaKLaaksoMKuusistoJ
1999
Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men.
Circulation
100
123
128
32. MadisonJRSpiesCSchatzIJMasakiKChenR
2006
Proteinuria and risk for stroke and coronary heart disease during 27 years of follow-up: The Honolulu Heart Program.
Arch Intern Med
166
884
889
33. MattockMBBarnesDJVibertiGKeenHBurtD
1998
Microalbuminuria and coronary heart disease in NIDDM: an incidence study.
Diabetes
47
1786
1792
34. MiettinenHHaffnerSMLehtoSRonnemaaTPyoralaK
1996
Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects.
Stroke
27
2033
2039
35. MuntnerPHeJHammLLoriaCWheltonPK
2002
Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States.
J Am Soc Nephrol
13
745
753
36. SasakiAHoriuchiNHasegawaKUeharaM
1995
Mortality from coronary heart disease and cerebrovascular disease and associated risk factors in diabetic patients in Osaka District, Japan.
Diabetes Res Clin Pract
27
77
83
37. ShimozatoMNakayamaTYokoyamaTYoshi-ikeNYamaguchiM
1996
A 15.5-year cohort study on risk factors for possible myocardial infarction and sudden death within 24 hours in a rural Japanese community.
J Epidemiol
6
15
22
38. Soedamah-MuthuSSChaturvediNToellerMFerrissBReboldiP
2004
Risk factors for coronary heart disease in type 1 diabetic patients in Europe: The EURODIAB Prospective Complications Study.
Diabetes Care
27
530
537
39. TillinTForouhiNMcKeiguePChaturvediN
2005
Microalbuminuria and coronary heart disease risk in an ethnically diverse UK population: a prospective cohort study.
Journal of the American Society of Nephrology: JASN
16
3702
3710
40. TorffvitOLovestam-AdrianMAgardhEAgardhCD
2005
Nephropathy, but not retinopathy, is associated with the development of heart disease in Type 1 diabetes: A 12-year observation study of 462 patients.
Diabet Med
22
723
729
41. ValmadridCTKleinRMossSEKleinBEK
2000
The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus.
Archives Intern Med
160
1093
1100
42. WagenerDKHarrisTMadansJH
1994
Proteinuria as a biomarker: Risk of subsequent morbidity and mortality.
Environ Res
66
160
172
43. WangZHoyWE
2005
Albuminuria and incident coronary heart disease in Australian Aboriginal people.
Kidney Int
68
1289
1293
44. YuyunMFKhawKTLubenRWelchABinghamS
2004
A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: The EPIC-Norfolk Study.
Am J Epidemiol
159
284
293
45. CoronaAJMartinezDRAvilaMHHaffnerSWilliamsK
2005
Microalbuminuria as a predictor of myocardial infarction in a Mexican population: the Mexico City Diabetes Study.
Kidney Int Suppl
S34
39
46. MoscaLBankaCLBenjaminEJBerraKBushnellC
2007
Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update.
Circulation
115
1481
1501
47. GansevoortRTBrinkmanJBakkerSJDe JongPEde ZeeuwD
2006
Evaluation of measures of urinary albumin excretion.
Am J Epidemiol
164
725
727
48. HallanSAstorBRomundstadSAasarodKKvenildK
2007
Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study.
Arch Intern Med
167
2490
2496
49. LewisEJHunsickerLGClarkeWRBerlTPohlMA
2001
Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes.
N Engl J Med
345
851
860
50. BrennerBMCooperMEde ZeeuwDKeaneWFMitchWE
2001
Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.
N Engl J Med
345
861
869
51. YusufSSleightPPogueJBoschJDaviesR
2000
Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators.
N Engl J Med
342
145
153
52. de ZeeuwDRemuzziGParvingHHKeaneWFZhangZ
2004
Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL.
Kidney Int
65
2309
2320
53. IbsenHOlsenMHWachtellKBorch-JohnsenKLindholmLH
2005
Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study.
Hypertension
45
198
202
54. DeckertTFeldt-RasmussenBBorch-JohnsenKJensenTKofoed-EnevoldsenA
1989
Albuminuria reflects widespread vascular damage. The Steno hypothesis.
Diabetologia
32
219
226
55. MacMahonSPetoRCutlerJCollinsRSorlieP
1990
Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias.
Lancet
335
765
774
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2008 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Why Current Publication Practices May Distort Science
- Faecal and Urinary Incontinence after Multimodality Treatment of Rectal Cancer
- The Relationship between Proteinuria and Coronary Risk: A Systematic Review and Meta-Analysis
- Off-Label Promotion, On-Target Sales
- Why Treatment Fails in Type 2 Diabetes
- Health Benefits of Universal Influenza Vaccination Strategy
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Relationship between Proteinuria and Coronary Risk: A Systematic Review and Meta-Analysis
- Health Benefits of Universal Influenza Vaccination Strategy
- Off-Label Promotion, On-Target Sales
- Why Treatment Fails in Type 2 Diabetes
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy