-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials
article has not abstract
Published in the journal: CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med 7(3): e32767. doi:10.1371/journal.pmed.1000251
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.1000251Summary
article has not abstract
Introduction
Randomised controlled trials, when appropriately designed, conducted, and reported, represent the gold standard in evaluating healthcare interventions. However, randomised trials can yield biased results if they lack methodological rigour [1]. To assess a trial accurately, readers of a published report need complete, clear, and transparent information on its methodology and findings. Unfortunately, attempted assessments frequently fail because authors of many trial reports neglect to provide lucid and complete descriptions of that critical information [2],[3],[4].
That lack of adequate reporting fuelled the development of the original CONSORT (Consolidated Standards of Reporting Trials) statement in 1996 [5] and its revision five years later [6],[7],[8]. While those statements improved the reporting quality for some randomised controlled trials [9],[10], many trial reports still remain inadequate [2]. Furthermore, new methodological evidence and additional experience has accumulated since the last revision in 2001. Consequently, we organised a CONSORT Group meeting to update the 2001 statement [6],[7],[8]. We introduce here the result of that process, CONSORT 2010.
Intent of CONSORT 2010
The CONSORT 2010 Statement is this paper including the 25 item checklist in the table (Table 1) and the flow diagram (Figure 1). It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Other trial designs, such as cluster randomised trials and non-inferiority trials, require varying amounts of additional information. CONSORT extensions for these designs, [11],[12] and other CONSORT products, can be found through the CONSORT website (http://www.consort-statement.org). Along with the CONSORT statement, we have updated the explanation and elaboration article, [13] which explains the inclusion of each checklist item, provides methodological background, and gives published examples of transparent reporting.
Fig. 1. Flow diagram of the progress through the phases of a parallel randomised trial of two groups (that is, enrolment, intervention allocation, follow-up, and data analysis). 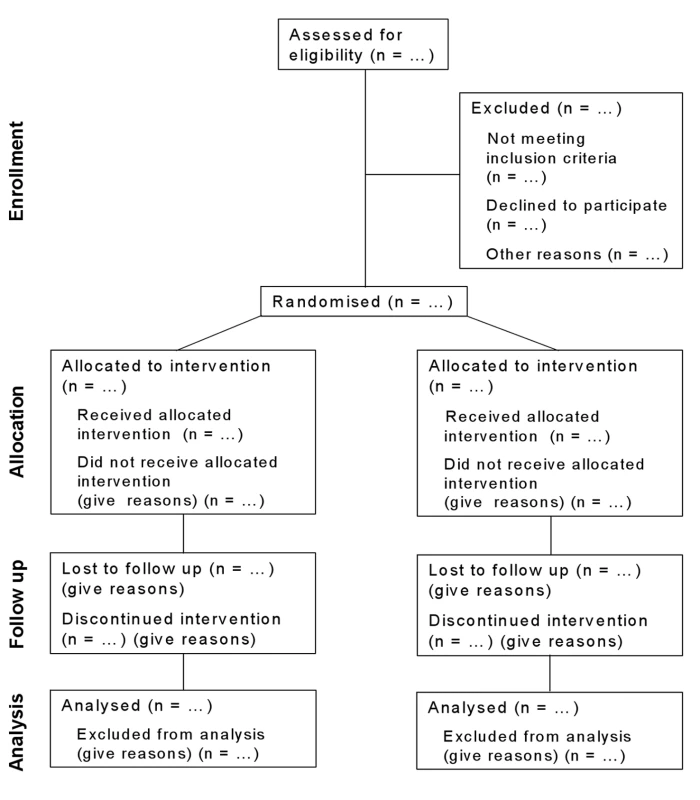
(For a downloadable version of this diagram see Figure S1 or the CONSORT website.) Tab. 1. CONSORT 2010 checklist of information to include when reporting a randomised trial (for a downloadable version of this checklist see Text S1 or the CONSORT website).* 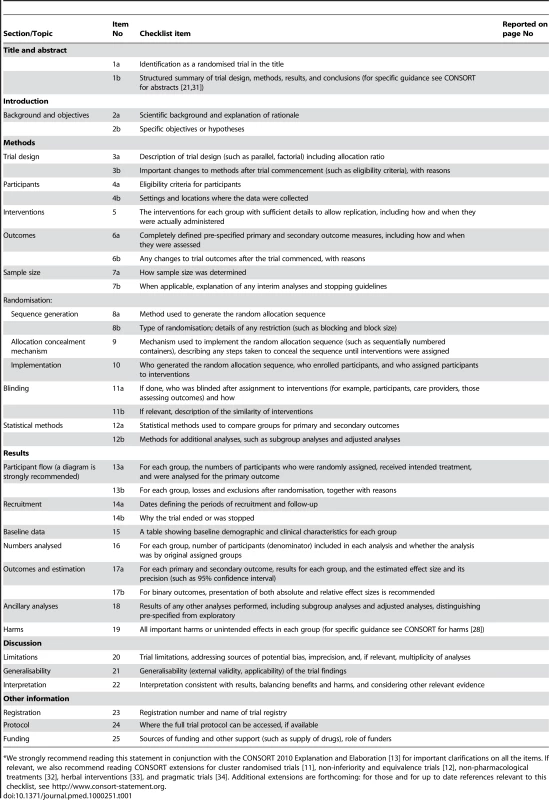
*We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration [13] for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials [11], non-inferiority and equivalence trials [12], non-pharmacological treatments [32], herbal interventions [33], and pragmatic trials [34]. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see http://www.consort-statement.org. Diligent adherence by authors to the checklist items facilitates clarity, completeness, and transparency of reporting. Explicit descriptions, not ambiguity or omission, best serve the interests of all readers. Note that the CONSORT 2010 Statement does not include recommendations for designing, conducting, and analysing trials. It solely addresses the reporting of what was done and what was found.
Nevertheless, CONSORT does indirectly affect design and conduct. Transparent reporting reveals deficiencies in research if they exist. Thus, investigators who conduct inadequate trials, but who must transparently report, should not be able to pass through the publication process without revelation of their trial's inadequacies. That emerging reality should provide impetus to improved trial design and conduct in the future, a secondary indirect goal of our work. Moreover, CONSORT can help researchers in designing their trial.
Background to CONSORT
Efforts to improve the reporting of randomised controlled trials accelerated in the mid-1990s, spurred partly by methodological research. Researchers had shown for many years that authors reported such trials poorly, and empirical evidence began to accumulate that some poorly conducted or poorly reported aspects of trials were associated with bias [14] Two initiatives aimed at developing reporting guidelines culminated in one of us (DM) and Drummond Rennie organising the first CONSORT statement in 1996. [5] Further methodological research on similar topics reinforced earlier findings [15] and fed into the revision of 2001. [6],[7],[8] Subsequently, the expanding body of methodological research informed the refinement of CONSORT 2010. More than 700 studies comprise the CONSORT database (located on the CONSORT website), which provides the empirical evidence to underpin the CONSORT initiative.
Indeed, CONSORT Group members continually monitor the literature. Information gleaned from these efforts provides an evidence base on which to update the CONSORT statement. We add, drop, or modify items based on that evidence and the recommendations of the CONSORT Group, an international and eclectic group of clinical trialists, statisticians, epidemiologists, and biomedical editors. The CONSORT Executive (KFS, DGA, DM) strives for a balance of established and emerging researchers. The membership of the group is dynamic. As our work expands in response to emerging projects and needed expertise, we invite new members to contribute. As such, CONSORT continually assimilates new ideas and perspectives. That process informs the continually evolving CONSORT statement.
Over time, CONSORT has garnered much support. More than 400 journals, published around the world and in many languages, have explicitly supported the CONSORT statement. Many other healthcare journals support it without our knowledge. Moreover, thousands more have implicitly supported it with the endorsement of the CONSORT statement by the International Committee of Medical Journal Editors (http://www.icmje.org). Other prominent editorial groups, the Council of Science Editors and the World Association of Medical Editors, officially support CONSORT. That support seems warranted: when used by authors and journals, CONSORT seems to improve reporting. [9]
Development of CONSORT 2010
Thirty one members of the CONSORT 2010 Group met in Montebello, Canada, in January 2007 to update the 2001 CONSORT statement. In addition to the accumulating evidence relating to existing checklist items, several new issues had come to prominence since 2001. Some participants were given primary responsibility for aggregating and synthesising the relevant evidence on a particular checklist item of interest. Based on that evidence, the group deliberated the value of each item. As in prior CONSORT versions, we kept only those items deemed absolutely fundamental to reporting a randomised controlled trial. Moreover, an item may be fundamental to a trial but not included, such as approval by an institutional ethical review board, because funding bodies strictly enforce ethical review and medical journals usually address reporting ethical review in their instructions for authors. Other items may seem desirable, such as reporting on whether on-site monitoring was done, but a lack of empirical evidence or any consensus on their value cautions against inclusion at this point. The CONSORT 2010 Statement thus addresses the minimum criteria, although that should not deter authors from including other information if they consider it important.
After the meeting, the CONSORT Executive convened teleconferences and meetings to revise the checklist. After seven major iterations, a revised checklist was distributed to the larger group for feedback. With that feedback, the executive met twice in person to consider all the comments and to produce a penultimate version. That served as the basis for writing the first draft of this paper, which was then distributed to the group for feedback. After consideration of their comments, the executive finalised the statement.
The CONSORT Executive then drafted an updated explanation and elaboration manuscript, with assistance from other members of the larger group. The substance of the 2007 CONSORT meeting provided the material for the update. The updated explanation and elaboration manuscript was distributed to the entire group for additions, deletions, and changes. That final iterative process converged to the CONSORT 2010 Explanation and Elaboration. [13]
Changes in CONSORT 2010
The revision process resulted in evolutionary, not revolutionary, changes to the checklist (Table 1), and the flow diagram was not modified except for one word (Figure 1). Moreover, because other reporting guidelines augmenting the checklist refer to item numbers, we kept the existing items under their previous item numbers except for some renumbering of items 2 to 5. We added additional items either as a sub-item under an existing item, an entirely new item number at the end of the checklist, or (with item 3) an interjected item into a renumbered segment. We have summarised the noteworthy general changes in Box 1 and specific changes in Box 2. The CONSORT website contains a side by side comparison of the 2001 and 2010 versions.
Box 1. Noteworthy General Changes in CONSORT 2010 Statement
-
We simplified and clarified the wording, such as in items 1, 8, 10, 13, 15, 16, 18, 19, and 21.
-
We improved consistency of style across the items by removing the imperative verbs that were in the 2001 version.
-
We enhanced specificity of appraisal by breaking some items into sub-items. Many journals expect authors to complete a CONSORT checklist indicating where in the manuscript the items have been addressed. Experience with the checklist noted pragmatic difficulties when an item comprised multiple elements. For example, item 4 addresses eligibility of participants and the settings and locations of data collection. With the 2001 version, an author could provide a page number for that item on the checklist, but might have reported only eligibility in the paper, for example, and not reported the settings and locations. CONSORT 2010 relieves obfuscations and forces authors to provide page numbers in the checklist for both eligibility and settings.
Box 2. Noteworthy Specific Changes in CONSORT 2010 Statement
-
Item 1b (title and abstract)—We added a sub-item on providing a structured summary of trial design, methods, results, and conclusions and referenced the CONSORT for abstracts article [21].
-
Item 2b (introduction)—We added a new sub-item (formerly item 5 in CONSORT 2001) on “Specific objectives or hypotheses”.
-
Item 3a (trial design)—We added a new item including this sub-item to clarify the basic trial design (such as parallel group, crossover, cluster) and the allocation ratio.
-
Item 3b (trial design)—We added a new sub-item that addresses any important changes to methods after trial commencement, with a discussion of reasons.
-
Item 4 (participants)—Formerly item 3 in CONSORT 2001.
-
Item 5 (interventions)—Formerly item 4 in CONSORT 2001. We encouraged greater specificity by stating that descriptions of interventions should include “sufficient details to allow replication” [3].
-
Item 6 (outcomes)—We added a sub-item on identifying any changes to the primary and secondary outcome (endpoint) measures after the trial started. This followed from empirical evidence that authors frequently provide analyses of outcomes in their published papers that were not the prespecified primary and secondary outcomes in their protocols, while ignoring their prespecified outcomes (that is, selective outcome reporting). [4],[22] We eliminated text on any methods used to enhance the quality of measurements.
-
Item 9 (allocation concealment mechanism)—We reworded this to include mechanism in both the report topic and the descriptor to reinforce that authors should report the actual steps taken to ensure allocation concealment rather than simply report imprecise, perhaps banal, assurances of concealment.
-
Item 11 (blinding)—We added the specification of how blinding was done and, if relevant, a description of the similarity of interventions and procedures. We also eliminated text on “how the success of blinding (masking) was assessed” because of a lack of empirical evidence supporting the practice as well as theoretical concerns about the validity of any such assessment [23],[24].
-
Item 12a (statistical methods)—We added that statistical methods should also be provided for analysis of secondary outcomes.
-
Sub-item 14b (recruitment)—Based on empirical research, we added a sub-item on “Why the trial ended or was stopped” [25].
-
Item 15 (baseline data)—We specified “A table” to clarify that baseline and clinical characteristics of each group are most clearly expressed in a table.
-
Item 16 (numbers analysed)—We replaced mention of “intention to treat” analysis, a widely misused term, by a more explicit request for information about retaining participants in their original assigned groups [26].
-
Sub-item 17b (outcomes and estimation)—For appropriate clinical interpretability, prevailing experience suggested the addition of “For binary outcomes, presentation of both relative and absolute effect sizes is recommended” [27].
-
Item 19 (harms)—We included a reference to the CONSORT paper on harms [28].
-
Item 20 (limitations)—We changed the topic from “Interpretation” and supplanted the prior text with a sentence focusing on the reporting of sources of potential bias and imprecision.
-
Item 22 (interpretation)—We changed the topic from “Overall evidence.” Indeed, we understand that authors should be allowed leeway for interpretation under this nebulous heading. However, the CONSORT Group expressed concerns that conclusions in papers frequently misrepresented the actual analytical results and that harms were ignored or marginalised. Therefore, we changed the checklist item to include the concepts of results matching interpretations and of benefits being balanced with harms.
-
Item 23 (registration)—We added a new item on trial registration. Empirical evidence supports the need for trial registration, and recent requirements by journal editors have fostered compliance [29].
-
Item 24 (protocol)—We added a new item on availability of the trial protocol. Empirical evidence suggests that authors often ignore, in the conduct and reporting of their trial, what they stated in the protocol. [4],[22] Hence, availability of the protocol can instigate adherence to the protocol before publication and facilitate assessment of adherence after publication.
-
Item 25 (funding)—We added a new item on funding. Empirical evidence points toward funding source sometimes being associated with estimated treatment effects [30].
Implications and Limitations
We developed CONSORT 2010 to assist authors in writing reports of randomised controlled trials, editors and peer reviewers in reviewing manuscripts for publication, and readers in critically appraising published articles. The CONSORT 2010 Explanation and Elaboration provides elucidation and context to the checklist items. We strongly recommend using the explanation and elaboration in conjunction with the checklist to foster complete, clear, and transparent reporting and aid appraisal of published trial reports.
CONSORT 2010 focuses predominantly on the two group, parallel randomised controlled trial, which accounts for over half of trials in the literature. [2] Most of the items from the CONSORT 2010 Statement, however, pertain to all types of randomised trials. Nevertheless, some types of trials or trial situations dictate the need for additional information in the trial report. When in doubt, authors, editors, and readers should consult the CONSORT website for any CONSORT extensions, expansions (amplifications), implementations, or other guidance that may be relevant.
The evidence based approach we have used for CONSORT also served as a model for development of other reporting guidelines, such as for reporting systematic reviews and meta-analyses of studies evaluating interventions, [16] diagnostic studies, [17] and observational studies. [18] The explicit goal of all these initiatives is to improve reporting. The Enhancing the Quality and Transparency of Health Research (EQUATOR) Network will facilitate development of reporting guidelines and help disseminate the guidelines: http://www.equator-network.org provides information on all reporting guidelines in health research.
With CONSORT 2010, we again intentionally declined to produce a rigid structure for the reporting of randomised trials. Indeed, SORT [19] tried a rigid format, and it failed in a pilot run with an editor and authors. [20] Consequently, the format of articles should abide by journal style, editorial directions, the traditions of the research field addressed, and, where possible, author preferences. We do not wish to standardise the structure of reporting. Authors should simply address checklist items somewhere in the article, with ample detail and lucidity. That stated, we think that manuscripts benefit from frequent subheadings within the major sections, especially the methods and results sections.
CONSORT urges completeness, clarity, and transparency of reporting, which simply reflects the actual trial design and conduct. However, as a potential drawback, a reporting guideline might encourage some authors to report fictitiously the information suggested by the guidance rather than what was actually done. Authors, peer reviewers, and editors should vigilantly guard against that potential drawback and refer, for example, to trial protocols, to information on trial registers, and to regulatory agency websites. Moreover, the CONSORT 2010 Statement does not include recommendations for designing and conducting randomised trials. The items should elicit clear pronouncements of how and what the authors did, but do not contain any judgments on how and what the authors should have done. Thus, CONSORT 2010 is not intended as an instrument to evaluate the quality of a trial. Nor is it appropriate to use the checklist to construct a “quality score.”
Nevertheless, we suggest that researchers begin trials with their end publication in mind. Poor reporting allows authors, intentionally or inadvertently, to escape scrutiny of any weak aspects of their trials. However, with wide adoption of CONSORT by journals and editorial groups, most authors should have to report transparently all important aspects of their trial. The ensuing scrutiny rewards well conducted trials and penalises poorly conducted trials. Thus, investigators should understand the CONSORT 2010 reporting guidelines before starting a trial as a further incentive to design and conduct their trials according to rigorous standards.
CONSORT 2010 supplants the prior version published in 2001. Any support for the earlier version accumulated from journals or editorial groups will automatically extend to this newer version, unless specifically requested otherwise. Journals that do not currently support CONSORT may do so by registering on the CONSORT website. If a journal supports or endorses CONSORT 2010, it should cite one of the original versions of CONSORT 2010, the CONSORT 2010 Explanation and Elaboration, and the CONSORT website in their “Instructions to authors.” We suggest that authors who wish to cite CONSORT should cite this or another of the original journal versions of CONSORT 2010 Statement, and, if appropriate, the CONSORT 2010 Explanation and Elaboration. [13]. All CONSORT material can be accessed through the original publishing journals or the CONSORT website. Groups or individuals who desire to translate the CONSORT 2010 Statement into other languages should first consult the CONSORT policy statement on the website.
We emphasise that CONSORT 2010 represents an evolving guideline. It requires perpetual reappraisal and, if necessary, modifications. In the future we will further revise the CONSORT material considering comments, criticisms, experiences, and accumulating new evidence. We invite readers to submit recommendations via the CONSORT website.
Supporting Information
Zdroje
1. JüniP
AltmanDG
EggerM
2001 Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323 42 46
2. ChanAW
AltmanDG
2005 Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 365 1159 1162
3. GlasziouP
MeatsE
HeneghanC
ShepperdS
2008 What is missing from descriptions of treatment in trials and reviews? BMJ 336 1472 1474
4. DwanK
AltmanDG
ArnaizJA
BloomJ
ChanAW
2008 Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 3 e3081 doi:10.1371/journal.pone.0003081
5. BeggC
ChoM
EastwoodS
HortonR
MoherD
1996 Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 276 637 639
6. MoherD
SchulzKF
AltmanDG
2001 The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357 1191 1194
7. MoherD
SchulzKF
AltmanDG
2001 The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134 657 662
8. MoherD
SchulzKF
AltmanD
2001 The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 285 1987 1991
9. PlintAC
MoherD
MorrisonA
SchulzK
AltmanDG
2006 Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 185 263 267
10. HopewellS
DuttonS
YuL-M
ChanA-W
AltmanDG
2010 The quality of reports of randomised trials in 2000 and 2006: a comparative study of articles indexed by PubMed. BMJ 340 c723
11. CampbellMK
ElbourneDR
AltmanDG
2004 CONSORT statement: extension to cluster randomised trials. BMJ 328 702 708
12. PiaggioG
ElbourneDR
AltmanDG
PocockSJ
EvansSJ
2006 Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 295 1152 1160
13. MoherD
HopewellS
SchulzKF
MontoriV
GøtzschePC
2010 CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340 c869
14. SchulzKF
ChalmersI
HayesRJ
AltmanDG
1995 Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273 408 412
15. MoherD
PhamB
JonesA
CookDJ
JadadAR
1998 Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352 609 613
16. MoherD
LiberatiA
TetzlaffJ
AltmanDG
The PRISMA Group 2009 Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6 e1000097 doi:10.1371/journal.pmed.1000097
17. BossuytPM
ReitsmaJB
BrunsDE
GatsonisCA
GlasziouPP
2003 Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 326 41 44
18. von ElmE
AltmanDG
EggerM
PocockSJ
GøtzschePC
2007 Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335 806 808
19. Standards of Reporting Trials Group 1994 A proposal for structured reporting of randomized controlled trials. JAMA 272 1926 1931
20. RennieD
1995 Reporting randomized controlled trials. An experiment and a call for responses from readers. JAMA 273 1054 1055
21. HopewellS
ClarkeM
MoherD
WagerE
MiddletonP
2008 CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 371 281 283
22. ChanAW
HróbjartssonA
HaahrMT
GøtzschePC
AltmanDG
2004 Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 291 2457 2465
23. SackettDL
2007 Commentary: Measuring the success of blinding in RCTs: don't, must, can't or needn't? Int J Epidemiol 36 664 665
24. SchulzKF
GrimesDA
2002 Blinding in randomised trials: hiding who got what. Lancet 359 696 700
25. MontoriVM
DevereauxPJ
AdhikariNK
BurnsKE
EggertCH
2203-2209 Randomized trials stopped early for benefit: a systematic review. JAMA
26. HollisS
CampbellF
1999 What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319 670 674
27. NuovoJ
MelnikowJ
ChangD
2002 Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA 287 2813 2814
28. IoannidisJP
EvansSJ
GøtzschePC
O'NeillRT
AltmanDG
2004 Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 141 781 788
29. De AngelisC
DrazenJM
FrizelleFA
HaugC
HoeyJ
2004 Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet 364 911 912
30. LexchinJ
BeroLA
DjulbegovicB
ClarkO
2003 Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 326 1167 1170
31. HopewellS
ClarkeM
MoherD
WagerE
MiddletonP
2008 CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med 5 e20 doi:10.1371/journal.pmed.0050020
32. BoutronI
MoherD
AltmanDG
SchulzKF
RavaudP
2008 Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 148 295 309
33. GagnierJJ
BoonH
RochonP
MoherD
BarnesJ
2006 Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med 144 364 367
34. ZwarensteinM
TreweekS
GagnierJJ
AltmanDG
TunisS
2008 Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 337 a2390
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2010 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Nech brouka žít… Ať žije astma!
- Intermitentní hladovění v prevenci a léčbě chorob
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials
- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Preventing Road Deaths—Time for Data
- Essential Surgery at the District Hospital: A Retrospective Descriptive Analysis in Three African Countries
- Drivers of Inequality in Millennium Development Goal Progress: A Statistical Analysis
- Where Will the Next Generation of Stroke Treatments Come From?
- Unravelling the Genetics of Ischaemic Stroke
- Chronic Obstructive Pulmonary Disease: Effects beyond the Lungs
- The Promise of Prevention: The Effects of Four Preventable Risk Factors on National Life Expectancy and Life Expectancy Disparities by Race and County in the United States
- Can Animal Models of Disease Reliably Inform Human Studies?
- New Approaches to Preventing, Diagnosing, and Treating Neonatal Sepsis
- Protecting Vulnerable Road Users from Injury
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Accelerating Policy Decisions to Adopt Type b Vaccine: A Global, Multivariable Analysis
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- BMI and Risk of Serious Upper Body Injury Following Motor Vehicle Crashes: Concordance of Real-World and Computer-Simulated Observations
- Unravelling the Genetics of Ischaemic Stroke
- Providing Alcohol-Related Screening and Brief Interventions to Adolescents through Health Care Systems: Obstacles and Solutions
- Human Resource and Funding Constraints for Essential Surgery in District Hospitals in Africa: A Retrospective Cross-Sectional Survey
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



