-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
Background:
Acute bronchiolitis treatment in children and infants is largely supportive, but chest physiotherapy is routinely performed in some countries. In France, national guidelines recommend a specific type of physiotherapy combining the increased exhalation technique (IET) and assisted cough (AC). Our objective was to evaluate the efficacy of chest physiotherapy (IET + AC) in previously healthy infants hospitalized for a first episode of acute bronchiolitis.Methods and Findings:
We conducted a multicenter, randomized, outcome assessor-blind and parent-blind trial in seven French pediatric departments. We recruited 496 infants hospitalized for first-episode acute bronchiolitis between October 2004 and January 2008. Patients were randomly allocated to receive from physiotherapists three times a day, either IET + AC (intervention group, n = 246) or nasal suction (NS, control group, n = 250). Only physiotherapists were aware of the allocation group of the infant. The primary outcome was time to recovery, defined as 8 hours without oxygen supplementation associated with minimal or no chest recession, and ingesting more than two-thirds of daily food requirements. Secondary outcomes were intensive care unit admissions, artificial ventilation, antibiotic treatment, description of side effects during procedures, and parental perception of comfort. Statistical analysis was performed on an intent-to-treat basis. Median time to recovery was 2.31 days, (95% confidence interval [CI] 1.97–2.73) for the control group and 2.02 days (95% CI 1.96–2.34) for the intervention group, indicating no significant effect of physiotherapy (hazard ratio [HR] = 1.09, 95% CI 0.91–1.31, p = 0.33). No treatment by age interaction was found (p = 0.97). Frequency of vomiting and transient respiratory destabilization was higher in the IET + AC group during the procedure (relative risk [RR] = 10.2, 95% CI 1.3–78.8, p = 0.005 and RR = 5.4, 95% CI 1.6–18.4, p = 0.002, respectively). No difference between groups in bradycardia with or without desaturation (RR = 1.0, 95% CI 0.2–5.0, p = 1.00 and RR = 3.6, 95% CI 0.7–16.9, p = 0.10, respectively) was found during the procedure. Parents reported that the procedure was more arduous in the group treated with IET (mean difference = 0.88, 95% CI 0.33–1.44, p = 0.002), whereas there was no difference regarding the assessment of the child's comfort between both groups (mean difference = −0.07, 95% CI −0.53 to 0.38, p = 0.40). No evidence of differences between groups in intensive care admission (RR = 0.7, 95% CI 0.3–1.8, p = 0.62), ventilatory support (RR = 2.5, 95% CI 0.5–13.0, p = 0.29), and antibiotic treatment (RR = 1.0, 95% CI 0.7–1.3, p = 1.00) was observed.Conclusions:
IET + AC had no significant effect on time to recovery in this group of hospitalized infants with bronchiolitis. Additional studies are required to explore the effect of chest physiotherapy on ambulatory populations and for infants without a history of atopy.Trial registration:
ClinicalTrials.gov NCT00125450
: Please see later in the article for the Editors' Summary
Published in the journal: Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial. PLoS Med 7(9): e32767. doi:10.1371/journal.pmed.1000345
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000345Summary
Background:
Acute bronchiolitis treatment in children and infants is largely supportive, but chest physiotherapy is routinely performed in some countries. In France, national guidelines recommend a specific type of physiotherapy combining the increased exhalation technique (IET) and assisted cough (AC). Our objective was to evaluate the efficacy of chest physiotherapy (IET + AC) in previously healthy infants hospitalized for a first episode of acute bronchiolitis.Methods and Findings:
We conducted a multicenter, randomized, outcome assessor-blind and parent-blind trial in seven French pediatric departments. We recruited 496 infants hospitalized for first-episode acute bronchiolitis between October 2004 and January 2008. Patients were randomly allocated to receive from physiotherapists three times a day, either IET + AC (intervention group, n = 246) or nasal suction (NS, control group, n = 250). Only physiotherapists were aware of the allocation group of the infant. The primary outcome was time to recovery, defined as 8 hours without oxygen supplementation associated with minimal or no chest recession, and ingesting more than two-thirds of daily food requirements. Secondary outcomes were intensive care unit admissions, artificial ventilation, antibiotic treatment, description of side effects during procedures, and parental perception of comfort. Statistical analysis was performed on an intent-to-treat basis. Median time to recovery was 2.31 days, (95% confidence interval [CI] 1.97–2.73) for the control group and 2.02 days (95% CI 1.96–2.34) for the intervention group, indicating no significant effect of physiotherapy (hazard ratio [HR] = 1.09, 95% CI 0.91–1.31, p = 0.33). No treatment by age interaction was found (p = 0.97). Frequency of vomiting and transient respiratory destabilization was higher in the IET + AC group during the procedure (relative risk [RR] = 10.2, 95% CI 1.3–78.8, p = 0.005 and RR = 5.4, 95% CI 1.6–18.4, p = 0.002, respectively). No difference between groups in bradycardia with or without desaturation (RR = 1.0, 95% CI 0.2–5.0, p = 1.00 and RR = 3.6, 95% CI 0.7–16.9, p = 0.10, respectively) was found during the procedure. Parents reported that the procedure was more arduous in the group treated with IET (mean difference = 0.88, 95% CI 0.33–1.44, p = 0.002), whereas there was no difference regarding the assessment of the child's comfort between both groups (mean difference = −0.07, 95% CI −0.53 to 0.38, p = 0.40). No evidence of differences between groups in intensive care admission (RR = 0.7, 95% CI 0.3–1.8, p = 0.62), ventilatory support (RR = 2.5, 95% CI 0.5–13.0, p = 0.29), and antibiotic treatment (RR = 1.0, 95% CI 0.7–1.3, p = 1.00) was observed.Conclusions:
IET + AC had no significant effect on time to recovery in this group of hospitalized infants with bronchiolitis. Additional studies are required to explore the effect of chest physiotherapy on ambulatory populations and for infants without a history of atopy.Trial registration:
ClinicalTrials.gov NCT00125450
: Please see later in the article for the Editors' SummaryIntroduction
Acute viral bronchiolitis is generally a self-limiting condition and is most commonly associated with respiratory syncytial virus (RSV) infection. It is the most common lower respiratory tract infection in the first years of life, and it is usually a mild to moderate disease. However, some infants develop severe disease and hospitalization is necessary in about 1% of previously healthy infected infants [1]–[3]. Recent population-based studies have shown that mean annual hospitalization rates for conditions related to RSV infection were between 5 and 17 per 1,000 children under 12 mo of age [4],[5].
The treatment of bronchiolitis in infants is largely supportive, with oxygen supplementation, minimal handling of the infant, and the use of enteral feeding, intravenous fluids, or ventilatory support when necessary. Respiratory symptoms result from bronchiolar obstruction due to inflammatory edema in the small airways, with an accumulation of mucus and cellular debris secondary to the epithelial necrosis caused by the virus [6]–[8].
Chest physiotherapy is routinely used in children with chronic respiratory diseases, such as cystic fibrosis and primary ciliary dyskinesia, or in children with neuromuscular disease, to facilitate the clearance of tracheobronchial secretions. Chest physiotherapy aims to clear airway obstruction, thereby decreasing airway resistance, improving gas exchange, and making breathing easier. International recommendations do not recommend chest physiotherapy for the management of bronchiolitis [9],[10]. These recommendations are based on a recent Cochrane review analyzing three clinical trials evaluating chest physiotherapy in infants with bronchiolitis [11]; however, these studies have several limitations. Most importantly, these studies were based on percussion and vibration techniques, which are very different from the increased exhalation technique (IET) with assisted cough (AC) that might be more appropriate in this context. IET is designed to clear the distal airways, whereas AC is known to facilitate large-airway clearance [12],[13]. During IET, the manual compression of the infant's thorax is aimed at achieving distal airways flow limitation at low lung volume (as during the rapid thoracic compression lung function technique [14]–[16]) to facilitate mucus clearance.
A French consensus panel recommended the use of IET + AC in infants with bronchiolitis [17]. Despite the very large use of IET + AC in outpatients with bronchiolitis in France (82.5%–99%) [18], the efficacy of this technique has so far not been evaluated.
Thus, we conducted a multicenter, randomized, outcomes assessor-blind and parent-blind trial to evaluate the efficacy of chest physiotherapy by IET + AC in previously healthy infants, hospitalized for a first episode of acute bronchiolitis.
Methods
Ethics Statement
The Saint Germain en Laye ethics committee approved the study.
Patients
We conducted the study in seven French pediatrics departments in the Parisian area (Antoine Béclère, Clamart; Jean Verdier, Bondy; Ambroise Paré, Boulogne; Kremlin Bicêtre, Le Kremlin Bicêtre; and Paris, Robert Debré, Necker Enfants Malades, and Armand Trousseau) during bronchiolitis outbreaks from October 2004 through January 2008 (Texts S1 and S2). Planned start and end dates were identical for all centers.
We included infants between the ages of 15 d and 24 mo hospitalized with a first episode of wheezing diagnosed as bronchiolitis. Bronchiolitis was diagnosed on the basis of a history of upper respiratory tract infection and clinical findings consistent with bronchiolitis, including wheezing or wheezing with crackles and respiratory distress. Infants were eligible within 24 h of hospitalization if they presented at least one of the following on admission: toxic aspect; history of apnea or cyanosis; respiratory rate >60/min; pulse oxymetry <95%; alimentary intake <2/3 of needs. A maximum of two chest physiotherapy procedures since admission was allowed before inclusion.
Infants with severe respiratory distress necessitating immediately admission to the pediatric intensive care unit (PICU), with cardiac disease, with a previous significant respiratory condition, or premature (<34 wk) were not eligible. Infants were not included if they had contraindications for the intervention (IET + AC): thrombocytopenia, prolonged corticosteroid treatment, rickets, bone diseases, known rib fracture.
The parents were informed about the study, its aims, and design. In particular, they were informed that they could not stay with their children during treatment (to respect blinding). Both parents gave written informed consent.
Clinical bronchiolitis was confirmed at enrollment and clinicians determined the duration of symptoms before hospitalization, clinical respiratory score, and clinical variables (respiratory and heart rates, temperature, and oxygen saturation whilst breathing ambient air). Medical history was obtained from parents or guardians, on a standardized data-collection form including questions about personal history of eczema, family history of asthma or eczema in parents and siblings, and tobacco smoke in the home environment.
Randomization
Randomization involved the chest physiotherapist opening a sealed sequentially numbered envelope containing a random allocation computer generated with SAS (SAS Inc.) software packages in advance by the biostatistician. Randomization was stratified according to center and according to age (<2 mo, ≥2 mo) at each center, using permutation blocks with a block size of four that was not mentioned to the physicians involved in the patient recruitment.
All pediatric department staff, parents, and guardians were blind to treatment assignment. Randomization codes were kept secure until data entry was complete. Thus, those involved in the evaluation of primary outcome or in the decision of the cointerventions were blinded to group assignment.
Study Intervention
The treatment, either intervention or control, was performed by the physiotherapist staying alone with the infant, in a room with a covered window pane, to ensure that clinicians and parents could not observe treatment, thus preserving blinding in the trial. All infants received treatment three times daily. In each center, four to six physiotherapists, specially trained to carry out chest physiotherapy in children, participated in the study. The therapists were not involved in the evaluation of time to recovery.
Intervention group
The intervention was defined as the IET followed by AC, with gentle nasal suction (NS).
Just before the start of the study, a senior physiotherapist presented this technique at each center and all physiotherapists received formal training in these techniques. During the study, a referent physiotherapist at each center ensured that chest physiotherapy was consistent and standardized.
IET involved the generation of synchronized thoracic-abdominal movement by the hands of the physiotherapist at the beginning of expiration with one hand on the thorax, meanwhile, with the other on the abdomen, centered on the umbilicus, the physiotherapist applied an abdominal counter-weight. This maneuver allowed to create a passive expiratory flow. The maneuver began at the end of the inspiratory plateau and was pursued until the end of expiration, according to the infant's thoraco-pulmonary compliance and up to his or her chest wall and lung resistance limits. The resulting dynamic compression of the respiratory system increased expiratory airflow. The procedure was repeated until meeting auscultation-efficacy criteria (decrease or disappearance of wheezing and/or increase of ronchi), but did not last longer than 10 to 15 min. The procedure was stopped in the case of respiratory status aggravation. If no spontaneous coughing occurred, coughing could be triggered by pressure on the suprasternal notch. Gentle NS with a flexible probe was used to remove mucous secretions at the end of the procedure. All patients were closely monitored by continuous pulse oxymetry during chest physiotherapy.
Control group
Similarly to the intervention group, the control group also spent 10–15 min in a room alone with the therapist three times daily. In this group of infants, the physiotherapists performed only gentle NS to remove mucous secretions for few minutes and stayed inside the room for the remaining time without performing any maneuver on the infants.
Other Treatments
All children enrolled in this study followed the same clinical treatment pathway to ensure consistent care and minimal variability of the results. Guidelines for the use and termination of oxygen supplementation and orogastric feeding or intravenous fluids were followed.
Oxygen supplementation was administered if oxygen saturation was below 95% when awake and 92% when asleep. It was stopped when oxygen saturation was consistently above 95% when awake and 92% when asleep. Nurses interrupted oxygen supplementation three times per day to assess saturation in room air.
Enteral feeding was administered when possible, with orogastric feeding offered to infants spontaneously ingesting less than two-thirds of their daily needs or with significant signs of chest recession, tachypnea (>60/min) or hypoxemia, or a worsening of respiratory signs during feeding. Intravenous fluids were preferred over oral feeding if respiratory conditions did not improve with orogastric feeding or oral feeding was insufficient (particularly if vomiting occurred during orogastric feeding). The use of intravenous fluids stopped when the infant was able to tolerate oral feeding. A physician and nurses reevaluated the need for orogastric feeding and intravenous fluids twice daily.
Other treatments, such as bronchodilators, corticosteroids, and antibiotics, are not recommended in national and international guidelines [2],[3],[10],[19], but could be prescribed freely if physicians felt it appropriate. Data concerning deviations from the clinical treatment pathway, including drug treatments, were recorded.
Outcome Measure
Primary outcome
The primary outcome was time from randomization to recovery. An infant was considered to be cured if no oxygen supplementation had been given for 8 h and the child had minimal or no chest recession and was ingesting more than two-thirds of daily needs. The nursing staff recorded respiratory and heart rates, oxygen saturation, and signs of chest recession when the patient was quiet, at least once every 8 h. Evaluation was based on a clinical score that could be recorded reliably, every 8 h, by any doctor, nurse, or physiotherapist [20].
Secondary outcomes
First, physiotherapists reported side effects during procedures: bradycardia (<80/min) without desaturation, bradycardia with desaturation (SpO2<85%), vomiting, transient respiratory destabilization, or bouts of hypotonia requiring the interruption of the procedure.
Upon discharge from the hospital, parents answered a questionnaire regarding their perception of their child's comfort and were invited to give their opinion on the efficiency of physiotherapy for their own child. As parents were not allowed to be present during the physiotherapy procedure, their opinion was a global one, based on their observations before and after each procedure, and on their global evaluation of their child. Parents answered several questions: (1) During hospitalization, how would you evaluate the comfort of your child? Analogical visual scale ranging from 0 (very bad) to 10 (excellent); (2) How would you evaluate how arduous the physiotherapy procedure was for your child? Analogical visual scale ranging from 0 (not arduous at all) to 10 (very arduous); (3) Do you think that the physiotherapy procedure has really worsened, rather worsened, not changed anything, rather improved, or really improved the comfort of your child (choose one answer)?; and (4) Do you think that the physiotherapy procedure has really worsened, rather worsened, not changed anything, rather improved, or really improved your child's breathing (choose one answer).
Finally, secondary PICU admission and artificial ventilation, antibiotic treatment were recorded. The parents were contacted by telephone within 30 d of discharge to identify cases of relapse and rehospitalization.
Computation of Sample Size
No accurate data for mean time to recovery were available from the literature. Therefore, to determine the sample size, we used the duration of hospitalization for bronchiolitis recorded in the study hospitals during previous years (mean duration of hospitalization, 6.5 d, and standard deviation [SD], 3.5 d). For a type I error of 0.05 and a power of 0.80, for detecting a 20% decrease in time to recovery in the IET + AC group, we needed to include 228 infants (114 infants in each group). One of the aims of this trial was to investigate possible interactions with age. We therefore set up two groups of 228 children (under and over the age of 2 mo), giving 456 children in total. We planned to include an additional 10% of patients to ensure that we had sufficient subjects for analysis (due to potential study dropouts or consent withdrawals). We therefore planned to enrol 500 infants in this trial.
Statistical Analysis
Analysis was performed on an intent-to-treat basis and all patients included in the study were analyzed, including the two lost to follow-up (one in each group).
Baseline demographic data were expressed as number and percentage for binary or ordinary data, and means ±SD for continuous data unless skewed, where median and interquartile range (IQR) were reported. Time failure data were summarized as medians and 95% confidence interval (CI) [21].
We first tested treatment by age group (<2 mo and ≥2 mo) interaction on the primary outcome by fitting Cox models in each age group, then testing for quantitative interaction with the Gail and Simon test [22]. No treatment by age interaction was found (p = 0.97), making it possible to perform the analysis on the pooled sample. Thus, survival curves for time to recovery were estimated on the whole cohort using the Kaplan-Meier method, then compared across randomized groups by using the log-rank test stratified by age group.
We additionally adjusted survival analyses for prognostic baseline covariates (personal eczema or history of atopy, age in months, hypoxemia at randomization, need for IV fluids at randomization, atelectasia at randomization, duration of symptoms, use of mucolytics before randomization, RSV infection), using a Cox model. The center effect, that is, difference in baseline risk between centers, was analyzed using frailty models [23].
For secondary outcomes, we compared adverse events frequency using the Fisher test. The need for PICU admission or ventilation, lung atelectasia, relapse, and the need for antibiotic treatment or secondary hospitalization were compared between the two groups using the chi square test stratified on age. Data from analogical visual scales were compared using Wilcoxon test.
Finally, we tested treatment by covariate interactions on the primary outcome with personal eczema or history of atopy (history of atopy was defined as eczema or asthma in first-degree relatives), hypoxemia (SpO2<95%) at randomization, and RSV infection. These analyses were not prespecified and were identified by post hoc analysis. All these quantitative interactions were tested with the Gail and Simon test [22].
Measures of treatment effect were either hazard ratio (HR) for survival data, relative risk (RR) for binary data, mean differences for continuous data, all given with 95% CIs. For continuous skewed variables, 95% CIs were obtained by the bootstrap method [24]. All tests were two-tailed, with p-values of 0.05 or less considered as statistically significant.
Statistical analysis was carried out with R version 2.10.11 (The R Foundation for Statistical Computing; http://www.R-project.org) and SAS version 9.2 (SAS Inc.).
Results
Patients and Baseline Characteristics
496 infants were included from October 2004 to January 2008 (Figure 1): 246 (49.6%) were assigned to the IET + AC group and 250 (50.4%) to the NS group. For153 additional infants whose parents were invited to participate, the parents refused participation. The reasons for their refusal were the desire to stay with their infant, and therefore the impossibility to maintain the blind, and the desire for their children to receive IEC + AC treatment.
Fig. 1. Eligibility, randomization, and follow-up of study participants. 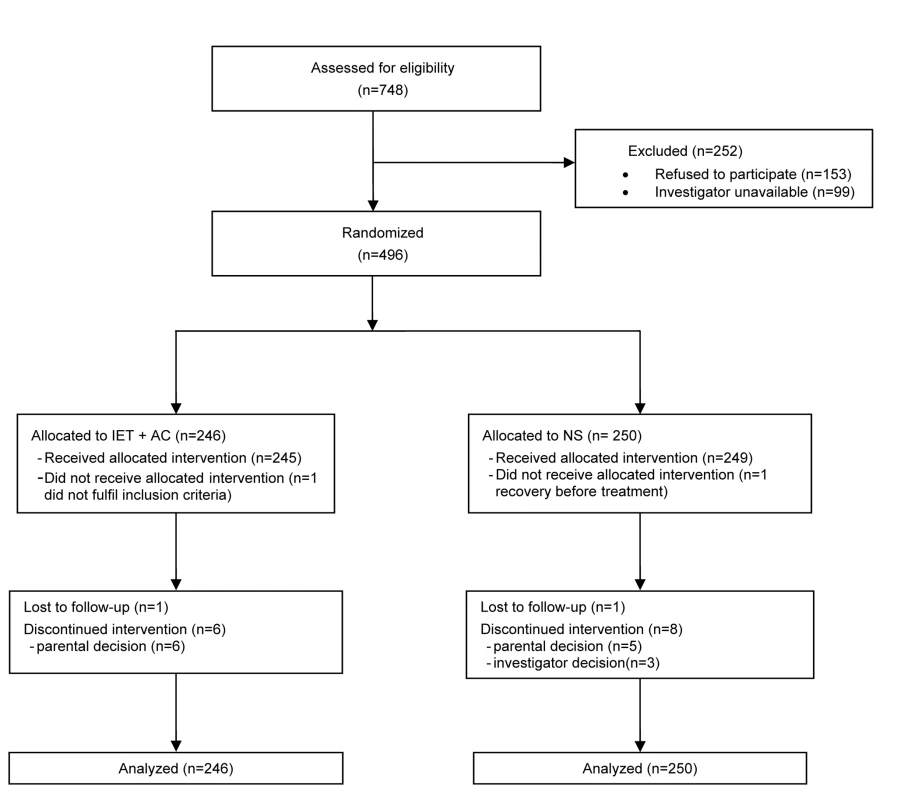
At randomization, there were no marked differences between the two randomized groups for demographic variables, percentage of infants with hypoxemia or feeding difficulties, percentage of infants with nasal aspirate positive for RSV, and duration of respiratory symptoms before hospitalization (Table 1). The proportion of cases of lung atelectasia diagnosis on X-ray was higher in the NS group (12.9% versus 7.6%).
Tab. 1. Demographic characteristics of the infants on admission to the hospital. 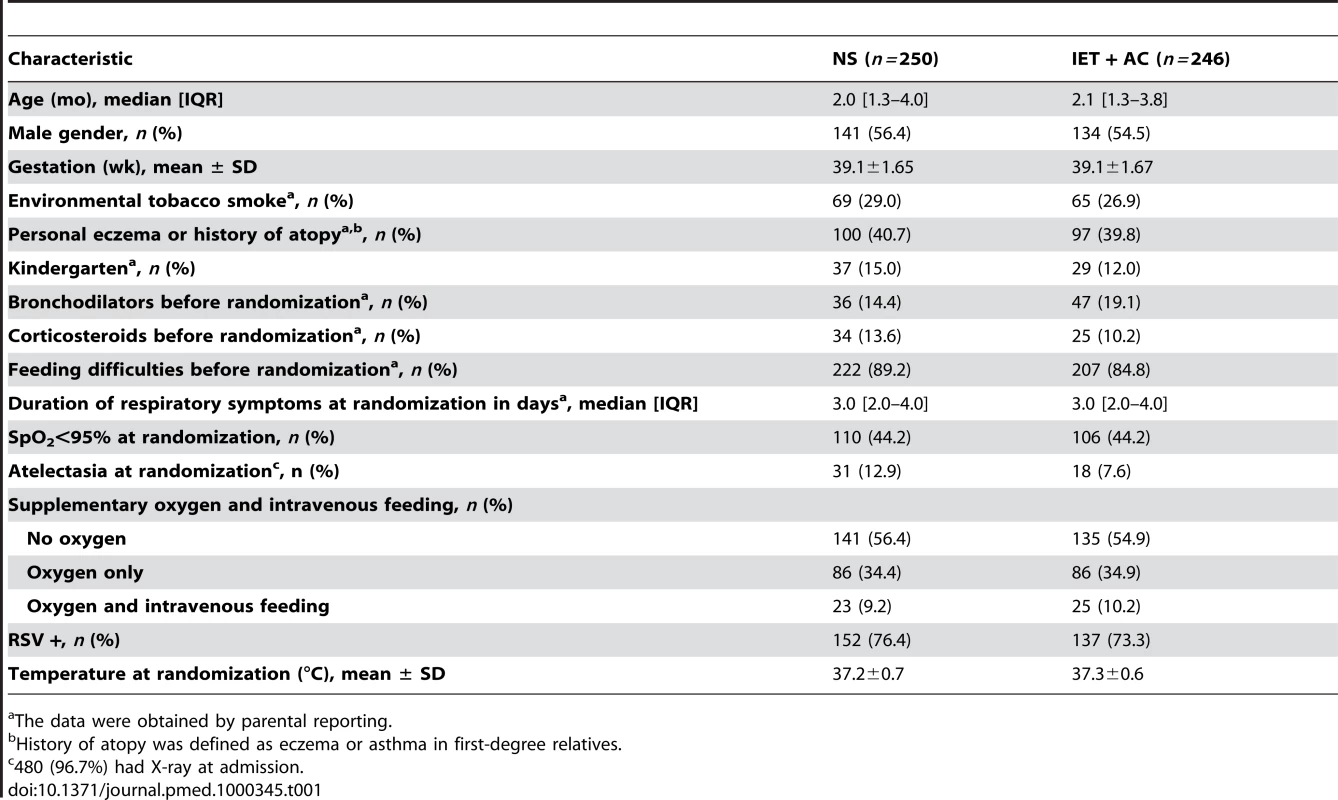
The data were obtained by parental reporting. Before randomization, 36 (14.4%) infants in the NS group and 47 (19.1%) infants in the IET + AC group received bronchodilator inhalations (salbutamol). 34 (13.6%) infants in the NS group and 25 (10.2%) infants in the IET + AC group were treated with oral corticosteroids (betamethasone).
Primary End Point
The physiotherapy intervention (IET + AC) had no significant effect on time to recovery. The median (95% CI) time to recovery was 2.31 (1.97–2.73) d for the NS group and 2.02 (1.96–2.34) d for the IET + AC group (HR [95% CI] = 1.09, 0.91–1.31, p = 0.33; Figure 2; Table 2). Significant heterogeneity across centers in baseline hazards was observed (p = 0.001), reflecting a difference between centers in time to recovery independently of the treatment effect. After controlling for prognostic baseline covariates, the random effect Cox regression model, that takes into account the center effect, showed that the effect of IET + AC on time to recovery remained not significant (HR = 1.21, 0.97–1.49, p = 0.09). Of note, the center effect persisted, though erased by the handling of baseline prognostic covariate differences (p = 0.03).
Fig. 2. Kaplan-Meier estimates of the proportion of children not recovering. 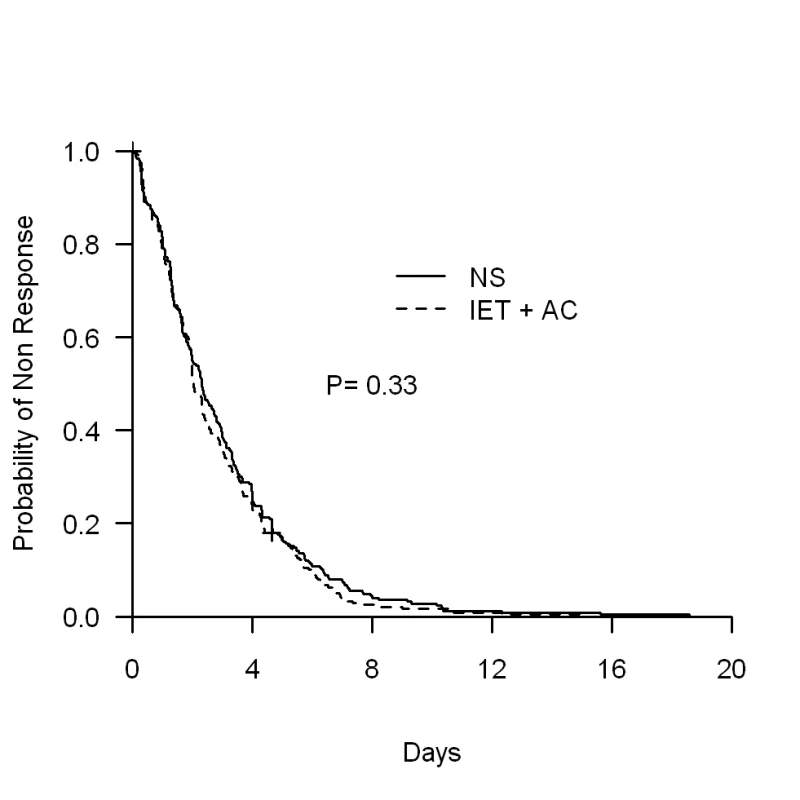
Tab. 2. Estimated effect of increased exhalation technique on time to recovery (d) as a function of age (univariate analyses). 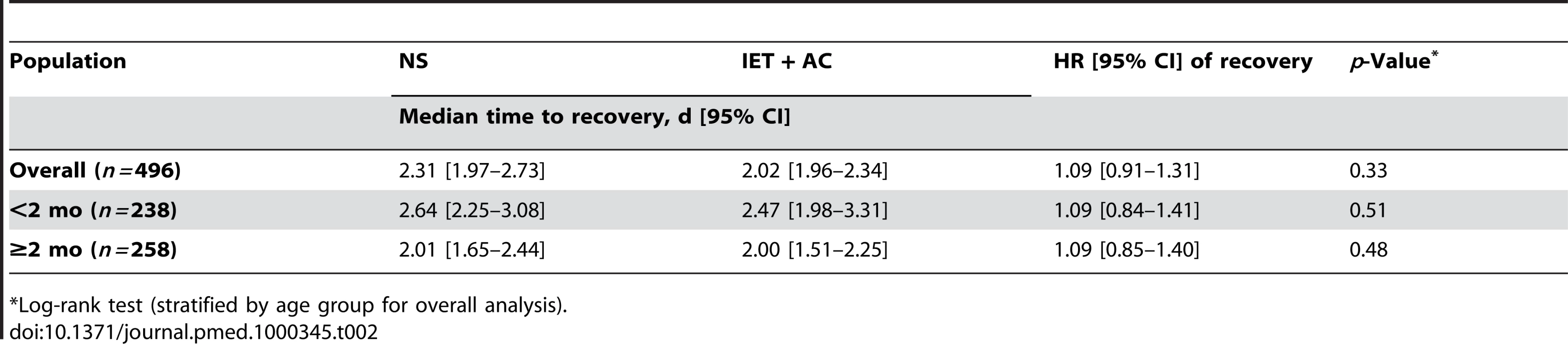
*Log-rank test (stratified by age group for overall analysis). Secondary End Points
Table 3 summarizes side effects during procedures reported by physiotherapists in both groups: there were no significant difference between groups in the proportion of children who experienced one episode of bradycardia with desaturation (RR [95% CI] = 1.0, 0.2–5.0, p = 1.00) or without desaturation (RR = 3.6, 0.7–16.9, p = 0.10). Conversely, the proportion of children who either had transient respiratory destabilization or vomited during the procedure was significantly higher in the group treated with IET + AC than in the group treated with NS (RR = 10.2, 1.3–78.8, p = 0.005 and RR = 5.4, 1.6–18.4, p = 0.002, respectively).
Tab. 3. Side effects reported by physiotherapists during procedures. 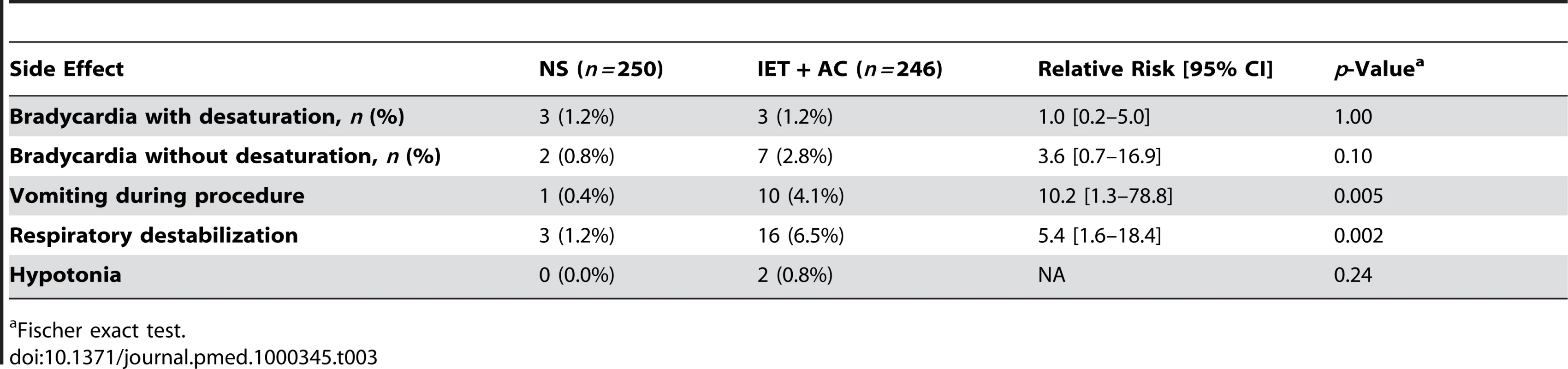
Fischer exact test. We obtained 371 (74.8%) responses to parental questionnaire (187 in the NS group and 184 in the IET + AC group). Table 4 summarizes parental answers regarding the influence of the physiotherapist visit on both the child's comfort and respiratory status. According to the parents, the procedure was significantly more arduous in the group treated with IET (mean difference [95% CI] = 0.88, 0.33–1.44, p = 0.002), whereas there was no significant difference regarding the comfort of the child between both groups (Difference of mean = −0.07, −0.53 to 0.38, p = 0.40). Finally, there was no significant difference in the way the parents rated the influence of physiotherapy on respiratory status (RR = 0.99, 0.90–1.08, p = 0.89 or comfort (RR = 0.99, 0.94–1.05, p = 0.84).
Tab. 4. Parental opinions regarding the comfort of their child and the consequences of the procedure on this parameter and on the respiratory status. 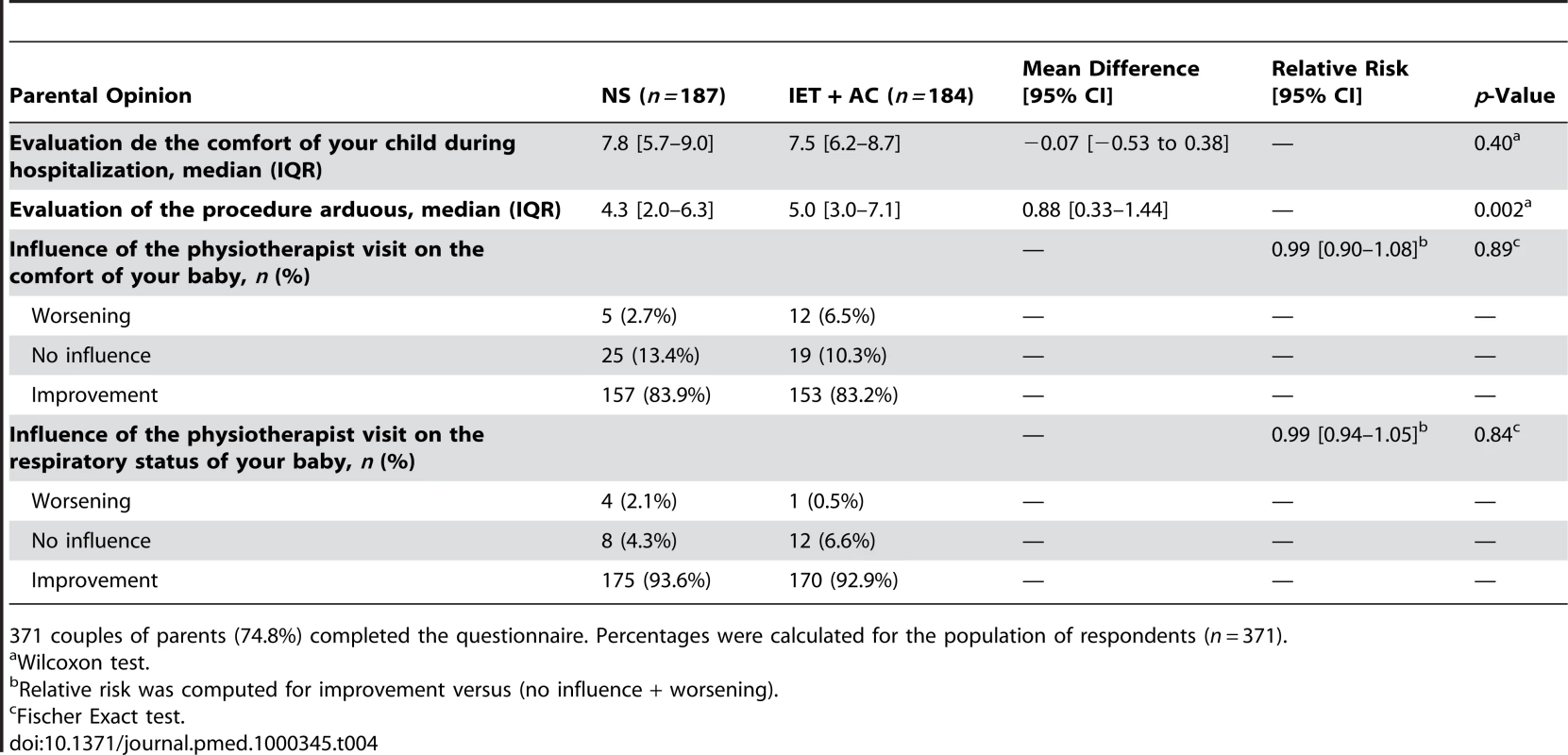
371 couples of parents (74.8%) completed the questionnaire. Percentages were calculated for the population of respondents (n = 371). 17 (3.4%) infants, including seven (1.4%) requiring ventilatory support, were admitted to the PICU. There was no evidence of any difference between groups in the number of infants requiring intensive care admission (RR = 0.7, 0.3–1.8, p = 0.62) or ventilatory support (RR = 2.5, 0.5–13.0, p = 0.29). 69 (28.5%) infants in the NS group and 67 (28.6%) in the IET + AC group received antibiotics (RR = 1.0, 0.7–1.3, 1.00). 53 infants in each group (31.0% in the IET + AC group) relapsed within 1 mo after discharge (RR = 1.1, 0.8–1.5, p = 0.73). 26 patients were admitted to the hospital within 1 mo after discharge (12 in the NS group and 14 in the IET + AC group; RR = 1.2, 0.6–2.6, p = 0.68) (Table 5). Respiratory symptoms were responsible for rehospitalization in 16 (six in the NS group and ten in the IET + AC group) out of 26 cases. Gastroenteritis was the other main cause (seven out of 26) of secondary hospitalization; the three remaining infants were hospitalized for miscellaneous nonrespiratory disorders.
Tab. 5. Secondary outcomes. 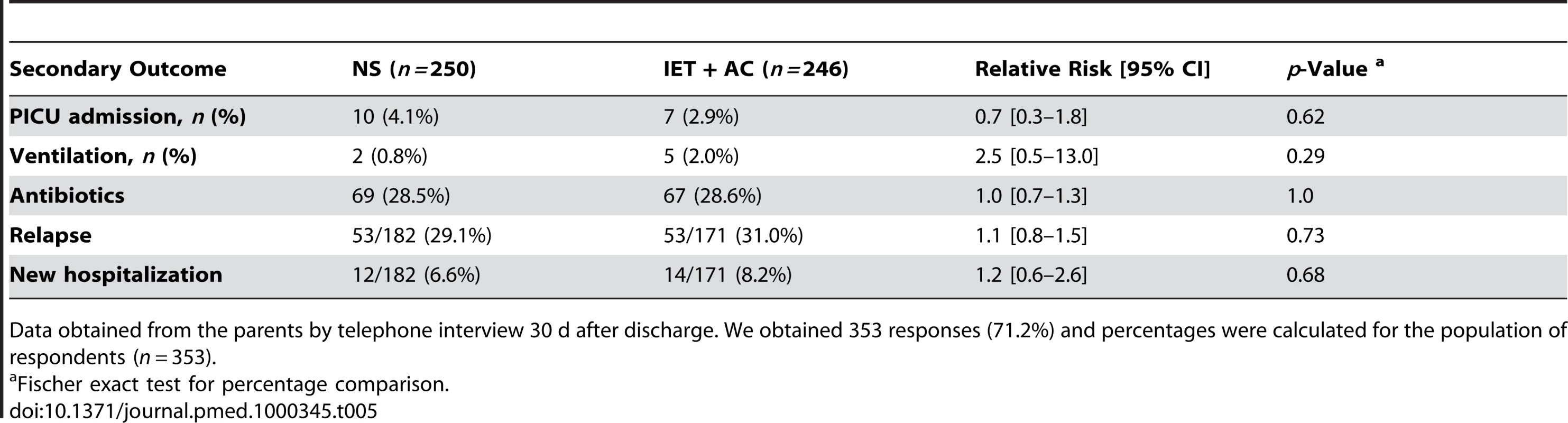
Data obtained from the parents by telephone interview 30 d after discharge. We obtained 353 responses (71.2%) and percentages were calculated for the population of respondents (n = 353). Post Hoc Interaction Test and Subset Analyses
No statistically significant quantitative interaction on time to recovery between treatment and eczema or atopy history was found (p = 0.06) (Figure 3; Table 6). HR (95% CI) was equal to 1.25 (0.99–1.58) for infants without personal eczema or history of atopy and 0.88 (0.66–1.17) for infants with personal eczema or history of atopy. Similarly, there were no differences in HRs as a function of RSV (HR = 1.01, 0.79–1.28 for infants with and 1.43, 0.94–2.16 for infants without RSV infection, p = 0.15), or of hypoxemia at randomization (HR = 1.23, 0.96–1.56 for infants without and 0.99, 0.75–1.30 for infants with hypoxemia at randomization, p = 0.25).
Fig. 3. HRs and 95% CIs for healing in the group receiving IET + AC, as compared with the NS group, as a function of baseline prognostic factors. 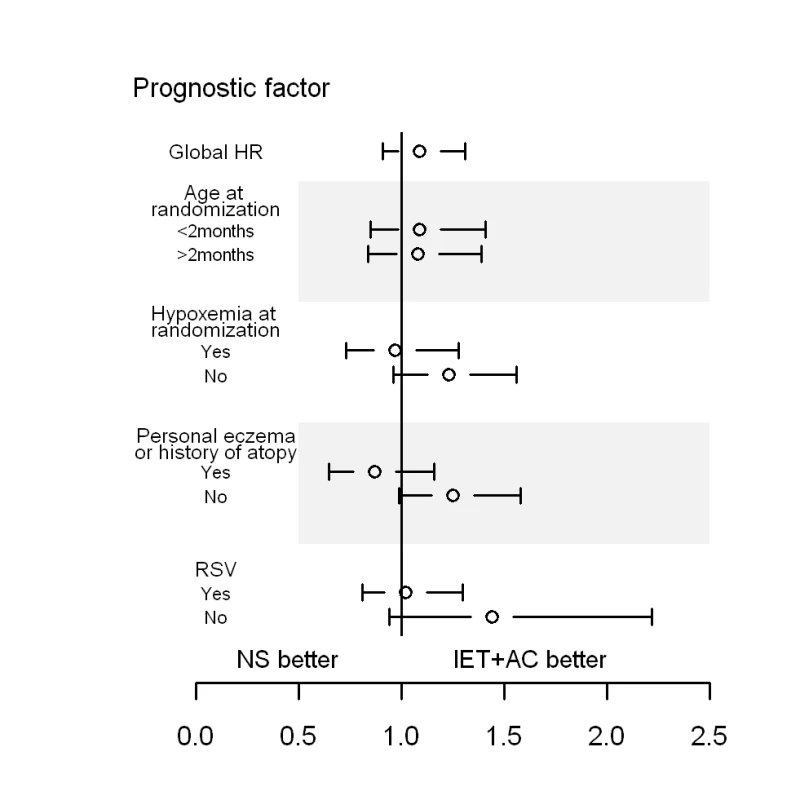
History of atopy was defined as the presence of eczema or asthma in first-degree relatives. Tab. 6. Search for treatment by covariate interactions on the main outcome measure, time to recovery. 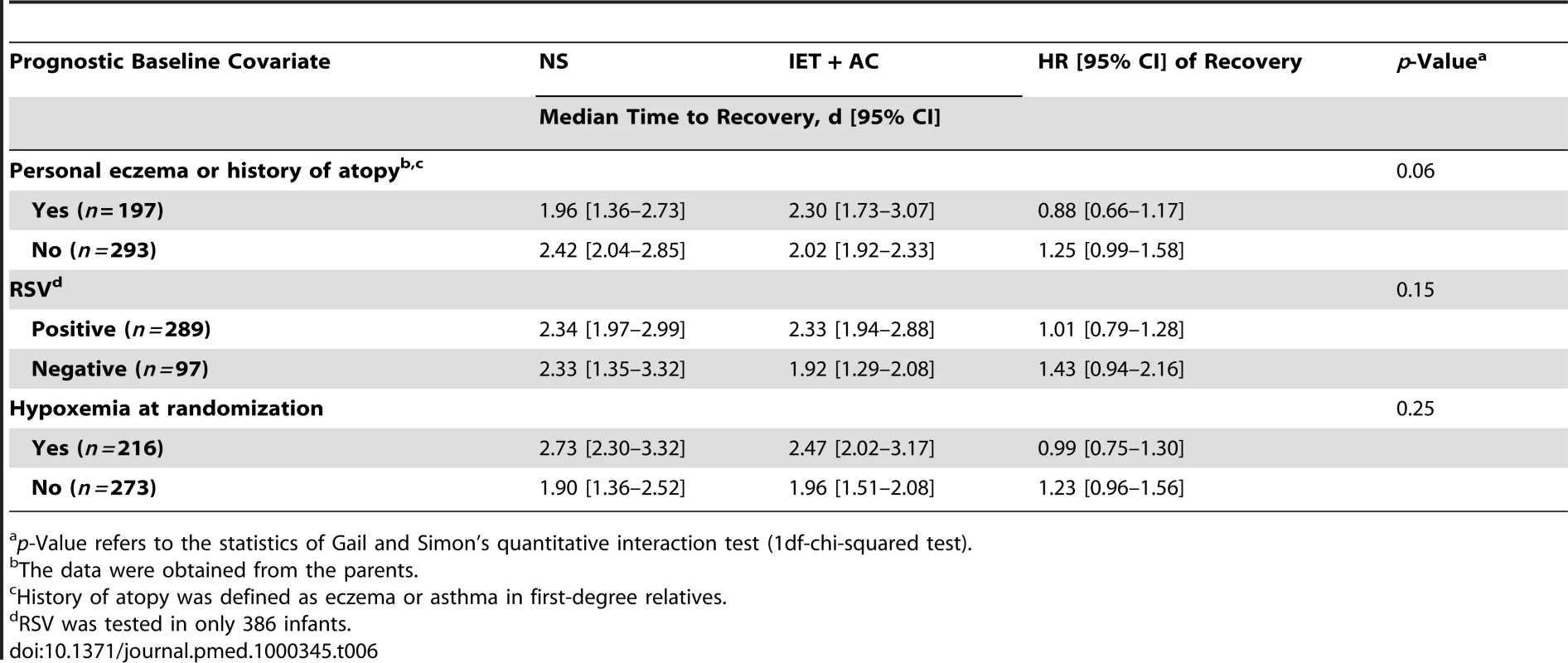
p-Value refers to the statistics of Gail and Simon's quantitative interaction test (1df-chi-squared test). Discussion
This study provides an evaluation of chest physiotherapy with IET + AC in a large population of infants hospitalized for a first episode of bronchiolitis with time to recovery as the primary endpoint. In this seven-center trial, no evidence of any difference in time to recovery between the IET + AC group and the NS group was found, with no interaction with age group. The CIs around estimates of effect excluded a clinically meaningful difference in time to recovery between groups in the whole population.
Otherwise, there was no evidence of any difference in secondary outcomes between children with and without IET + AC except for vomiting and transient respiratory destabilization during the procedure. This significant difference of vomiting and respiratory destabilization frequency was expected and in accordance with our physiotherapists'experiences. However, it is worth noting that these side effects of IET + AC were transient and resolved as soon as the procedure was interrupted. Although not statistically significant, the percentage of bradycardia during a procedure was three times higher in the group EIT + AC. This side effect of IET is rare but known, and it is important to note that bradycardia quickly disappeared in all children.
For parents, predictably, the perception of the arduousness of the procedure was significantly higher for IET + AC as compared to NS. Indeed, IET + AC is a tiring procedure for children even though the recovery is quick once the procedure is over. This perception may explain the absence of difference between both groups for the evaluation of the global comfort.
Although we preplanned an economic analysis, it was not conducted given the negative findings on efficacy outcomes.
Chest physiotherapy is recommended in infants with bronchiolitis by the French national consensus guidelines [17]. The rationale for such a recommendation is that physiotherapy could enhance the clearance of bronchial secretions, thereby decreasing respiratory distress and chest recession and increasing oxygenation. Airflow has not been assessed in infants undergoing IET, but the feasibility of achieving maximal flow (i.e., flow limitation at low lung volumes) has been demonstrated in sleeping healthy infants wearing an inflatable jacket compressing the thorax at the end of normal inspiration [16],[25] or after lung inflation to total pulmonary capacity [14],[15]. When the thorax was compressed from total pulmonary capacity to residual volume, flow limitation at low lung volumes (distal airways) was achieved for lower transpulmonary pressure than flow limitation at high lung volume (large airways) [14],[15], but further increase in transpulmonary pressure could have a negative effect on flow limitation at low lung volumes. These studies conducted in healthy sleeping infants do not mimic IET + AC applied in infants with bronchiolitis. In the present study, pulmonary distension could alter chest wall compliance and therefore transpulmonary pressure transmission. Infants were awake with the possibility to decreased thoraco-pulmonary compliance in response to manipulations, and most of all interindividual variability in IET + AC application could not be avoided despite the optimal training and continuous survey of IET + AC during the study. However, IET + AC is routine practice in France and physiotherapists were substantially experienced and trained. Therefore, the study reflects the effectiveness of physiotherapy in infants with bronchiolitis in real life practice as recommended by guidelines.
Previous studies on chest physiotherapy included smaller numbers of subjects, with physician-assessed clinical scores as the primary outcome and nonrelevant variables as secondary outcomes (durations of oxygen supplementation and hospitalization duration stay) [26]–[28]. Duration of oxygen supplementation was clearly not a relevant outcome because it was required in only 56.5% of the infants studied. The results of any study including only infants requiring oxygen supplementation should therefore not be extrapolated to infants not requiring oxygen.
We tried to determine the time to recovery as accurately as possible. It was therefore necessary to assess the conditions of the infants around the clock, and not only when physicians were present. Nurses recorded respiratory signs, heart rate, and SpO2 at least every 8 h, as previously performed [29]. Thus, the 0.3-d (which is almost equivalent to 8 h) difference observed between the two randomized groups in median time to recovery could be related to the study design, which hampered the detection of a smaller difference. However, a smaller gain of healing time would not have any clinical relevance. Otherwise, we have previously shown that the clinical signs used for monitoring (respiratory rates and retraction signs) were recorded similarly by all physicians, nurses, and physiotherapists, with a high level of interobserver reproducibility [20]. We compared IET + AC with NS, because NS provides temporary relief for nasal congestion and is routinely used.
Age, which was thought to be a possible determinant of IET efficacy, did not influence treatment effect. We used a cut-off point of 2 mo to allow stratifying randomization according to previous recommendations that set the age limit for severe bronchiolitis between 6 and 8 wk. Indeed, although such a dichotomization achieved some loss of information, the effectiveness of chest physiotherapy could have been decreased in the youngest owing to their highest risk of severe disease. Airway size increases with age, and we thought that it would be difficult to increase the clearance of bronchial secretions from very small airways by physiotherapy. The lack of documented specific mechanisms of pulmonary change after IET makes it difficult to draw firm conclusions concerning the age-related efficacy of physiotherapy.
Post hoc analyses showed no significant quantitative interaction between IET + AC effect on the main endpoint and personal atopy or history of atopy in first-degree relatives, the type of virus, or the presence of baseline hypoxemia. Although exploratory, our findings for infants without personal atopy or history of atopy in first-degree relatives and for those without hypoxemia were close to statistical significance as shown by the CIs around estimate of effect for these subgroups (respectively [0.99–1.58] and [0.96–1.56]) and deserve further investigations.
Infants included in the present study represent a spectrum of bronchiolitis phenotype including infants prone or not prone to experience repeated wheezing episodes. Reynolds et al. [30] suggested that there might be two different groups of patients: those with obstructive disease resulting entirely from infection, thickening of the bronchiolar walls, and intrabronchial secretions, and those with a predisposition to asthma, who develop obstruction because of both inflammation and bronchospasm. It is still currently impossible to determine which of the infants hospitalized for bronchiolitis will subsequently develop asthma, even though family history of atopy and atopic dermatitis in the infant have been shown to be predictive of asthma [31]. It is possible that future studies will lead to recommendations for bronchiolitis treatment according to the phenotype of the infant, rather than merely being based on current disease diagnosis alone [32]. Our study was not designed to address this issue and atopic status was not found to influence the effect of IET + AC.
The physiopathology of bronchiolitis remains unclear, but the need for oxygen supplementation is considered as a marker of considerable ventilation heterogeneity, due to a large number of units with poor ventilation (obstructed) and good perfusion or overdistension and poor perfusion. In the absence of segmental or lobar lung atelectasia, ventilation heterogeneity is a peripheral phenomenon unlikely to be affected by physiotherapy. Infants requiring oxygen supplementation may not benefit at all from physiotherapy, but the benefits of physiotherapy for nonhypoxemic infants did not reach significance (Figure 3; Table 5). Studying a mixture of patients may provide unclear or misleading results. This perspective raises questions about limiting studies to homogeneous populations, making it impossible to extrapolate the results obtained to patients with a different clinical status. The heterogeneity in concerned patient populations may contribute to the difficulty of definitively evaluating physiotherapy in clinical trials and in making clear recommendations on its benefit in practice.
The “interphysiotherapist” variability is an important issue in our study. Despite having taken great care in optimizing the training, some variability in the way compression was applied may remain and is difficult to evaluate. We deliberately chose to have more than one physiotherapist by center to mimic as closely as possible real-life conditions, and to be able to extrapolate our results, should they have been positive. The problem of practitioner variability for complex interventions is common in nonpharmacological trials. We standardized the cessation of the intervention on the same auscultation-efficacy criteria in order to reduce interphysiotherapist variability.
In summary, this large, randomized, controlled, outcomes assessor-blind and parent-blind trial of three daily chest physiotherapy sessions with the IET technique in infants hospitalized for a first episode of bronchiolitis provided no evidence that this treatment shortened time to recovery. Our results did not support the recommendation that chest physiotherapy be routinely performed in hospitalized infants with acute bronchiolitis. Our conclusions in infants with severe bronchiolitis cannot necessarily be extrapolated to infants with mild or moderate bronchiolitis. Further studies would be required to assess chest physiotherapy in outpatients and for infants without history of atopy.
Supporting Information
Zdroje
1. GlezenP
DennyFW
1973 Epidemiology of acute lower respiratory disease in children. N Engl J Med 288 498 505
2. BushA
ThomsonAH
2007 Acute bronchiolitis. BMJ 335 1037 1041
3. SmythRL
OpenshawPJM
2006 Bronchiolitis. Lancet 368 312 322
4. HallCB
WeinbergGA
IwaneMK
BlumkinAK
EdwardsKM
2009 The burden of respiratory syncytial virus infection in young children. N Engl J Med 360 588 598
5. KoehoornM
KarrCJ
DemersPA
LencarC
TamburicL
2008 Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics 122 1196 1203
6. WohlME
ChernickV
1978 State of the art: bronchiolitis. Am Rev Respir Dis 118 759 781
7. AherneW
BirdT
CourtSD
AherneW
BirdTM
1970 Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol 23 7 18
8. HallCB
1998 Respiratory syncytial virus.
FeiginRD
CherryJD
Textbook of pediatric infectious diseases Philadelphia WB Saunders 2084 2111
9. SIGN 2006 Bronchiolitis in children: a National Clinical Guideline. Available: http://www.sign.ac.uk/pdf/sign91.pdf. Accessed 8 August 2010.
10. Subcommittee on Diagnosis and Management of Bronchiolitis 2006 Diagnosis and management of bronchiolitis. Pediatrics 118 1774 1793
11. PerrottaC
OrtizZ
RoqueM
2007 Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev CD004873
12. KingM
BrockG
LundellC
1985 Clearance of mucus by simulated cough. J Appl Physiol 58 1776 1782
13. SivasothyP
BrownL
SmithIE
ShneersonJM
2001 Effect of manually assisted cough and mechanical insufflation on cough flow of normal subjects, patients with chronic obstructive pulmonary disease (COPD), and patients with respiratory muscle weakness. Thorax 56 438 444
14. FeherA
CastileR
KislingJ
AngelicchioC
FilbrunD
1996 Flow limitation in normal infants: a new method for forced expiratory maneuvers from raised lung volumes. J Appl Physiol 80 2019 2025
15. HaydenMJ
SlyPD
DevadasonSG
GurrinLC
WildhaberJH
1997 Influence of driving pressure on raised-volume forced expiration in infants. Am J Respir Crit Care Med 156 1876 1883
16. MarshMJ
FoxGF
HoskynsEW
MilnerAD
1994 The Hering-Breuer deflationary reflex in the newborn infant. Pediatr Pulmonol 18 163 169
17. 2001 Consensus conference on the management of infant bronchiolitis. Paris, France, 21 September 2000. Proceedings. Arch Pediatr 8 Suppl 1 1s 196s
18. BeauvoisE
2001 Role of respiratory therapy in the treatment acute bronchiolitis in infants. Arch Pediatr 8 Suppl 1 128S 131S
19. YanneyM
VyasH
2008 The treatment of bronchiolitis. Arch Dis Child 93 793 798
20. GajdosV
BeydonN
BommenelL
PellegrinoB
de PontualL
2009 Inter-observer agreement between physicians, nurses, and respiratory therapists for respiratory clinical evaluation in bronchiolitis. Pediatr Pulmonol 44 754 762
21. BlandM
2000 An introduction to medical statistics. Oxford Oxford University Press
22. GailM
SimonR
1985 Testing for qualitative interactions between treatment effects and patient subsets. Biometrics 41 361 372
23. AalenOO
1988 Heterogeneity in survival analysis. Stat Med 7 1121 1137
24. EfronB
TibshiraniR
1998 An introduction to the bootstrap. Boca Raton xvi London Chapman & Hall/CRC 436
25. HoskynsEW
MilnerAD
HopkinIE
1987 Validity of forced expiratory flow volume loops in neonates. Arch Dis Child 62 895 900
26. BoheL
FerreroME
CuestasE
PolliottoL
GenoffM
2004 [Indications of conventional chest physiotherapy in acute bronchiolitis]. Medicina (B Aires) 64 198 200
27. NicholasKJ
DhouiebMO
MarshallTG
EdmundsAT
GrantMB
1999 An evaluation of chest physiotherapy in the management of acute bronchiolitis. Physiotherapy 85 669 674
28. WebbMS
MartinJA
CartlidgePH
NgYK
WrightNA
1985 Chest physiotherapy in acute bronchiolitis. Arch Dis Child 60 1078 1079
29. WainwrightC
AltamiranoL
CheneyM
CheneyJ
BarberS
2003 A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med 349 27 35
30. ReynoldsEO
CookCD
1963 The treatment of bronchiolitis. J Pediatr 63 1205 1207
31. Castro-RodriguezJA
HolbergCJ
WrightAL
MartinezFD
2000 A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 162 1403 1406
32. FreyU
von MutiusE
2009 The challenge of managing wheezing in infants. N Engl J Med 360 2130 2133
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2010 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Examining the “Urban Advantage” in Maternal Health Care in Developing Countries
- Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: Raising the Bar
- Does It Matter Who Writes Medical News Stories?
- Drug Companies Should Be Held More Accountable for Their Human Rights Responsibilities
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Merck Perspective
- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
- Combined Impact of Lifestyle-Related Factors on Total and Cause-Specific Mortality among Chinese Women: Prospective Cohort Study
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Perspective of the Former United Nations Special Rapporteur (2002-2008)
- AIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
- Are Drug Companies Living Up to Their Human Rights Responsibilities? Moving Toward Assessment
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
- Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Cost-Effectiveness of Pooled Nucleic Acid Amplification Testing for Acute HIV Infection after Third-Generation HIV Antibody Screening and Rapid Testing in the United States: A Comparison of Three Public Health Settings
- Community Case Management of Fever Due to Malaria and Pneumonia in Children Under Five in Zambia: A Cluster Randomized Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



