-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
Background:
Infections during pregnancy have the potential to adversely impact birth outcomes. We evaluated the association between receipt of inactivated influenza vaccine during pregnancy and prematurity and small for gestational age (SGA) births.Methods and Findings:
We conducted a cohort analysis of surveillance data from the Georgia (United States) Pregnancy Risk Assessment Monitoring System. Among 4,326 live births between 1 June 2004 and 30 September 2006, maternal influenza vaccine information was available for 4,168 (96.3%). The primary intervention evaluated in this study was receipt of influenza vaccine during any trimester of pregnancy. The main outcome measures were prematurity (gestational age at birth <37 wk) and SGA (birth weight <10th percentile for gestational age). Infants who were born during the putative influenza season (1 October–31 May) and whose mothers were vaccinated against influenza during pregnancy were less likely to be premature compared to infants of unvaccinated mothers born in the same period (adjusted odds ratio [OR] = 0.60; 95% CI, 0.38–0.94). The magnitude of association between maternal influenza vaccine receipt and reduced likelihood of prematurity increased during the period of at least local influenza activity (adjusted OR = 0.44; 95% CI, 0.26–0.73) and was greatest during the widespread influenza activity period (adjusted OR = 0.28; 95% CI, 0.11–0.74). Compared with newborns of unvaccinated women, newborns of vaccinated mothers had 69% lower odds of being SGA (adjusted OR = 0.31; 95% CI, 0.13–0.75) during the period of widespread influenza activity. The adjusted and unadjusted ORs were not significant for the pre-influenza activity period.Conclusions:
This study demonstrates an association between immunization with the inactivated influenza vaccine during pregnancy and reduced likelihood of prematurity during local, regional, and widespread influenza activity periods. However, no associations were found for the pre-influenza activity period. Moreover, during the period of widespread influenza activity there was an association between maternal receipt of influenza vaccine and reduced likelihood of SGA birth.
: Please see later in the article for the Editors' Summary
Published in the journal: Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study. PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1000441
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000441Summary
Background:
Infections during pregnancy have the potential to adversely impact birth outcomes. We evaluated the association between receipt of inactivated influenza vaccine during pregnancy and prematurity and small for gestational age (SGA) births.Methods and Findings:
We conducted a cohort analysis of surveillance data from the Georgia (United States) Pregnancy Risk Assessment Monitoring System. Among 4,326 live births between 1 June 2004 and 30 September 2006, maternal influenza vaccine information was available for 4,168 (96.3%). The primary intervention evaluated in this study was receipt of influenza vaccine during any trimester of pregnancy. The main outcome measures were prematurity (gestational age at birth <37 wk) and SGA (birth weight <10th percentile for gestational age). Infants who were born during the putative influenza season (1 October–31 May) and whose mothers were vaccinated against influenza during pregnancy were less likely to be premature compared to infants of unvaccinated mothers born in the same period (adjusted odds ratio [OR] = 0.60; 95% CI, 0.38–0.94). The magnitude of association between maternal influenza vaccine receipt and reduced likelihood of prematurity increased during the period of at least local influenza activity (adjusted OR = 0.44; 95% CI, 0.26–0.73) and was greatest during the widespread influenza activity period (adjusted OR = 0.28; 95% CI, 0.11–0.74). Compared with newborns of unvaccinated women, newborns of vaccinated mothers had 69% lower odds of being SGA (adjusted OR = 0.31; 95% CI, 0.13–0.75) during the period of widespread influenza activity. The adjusted and unadjusted ORs were not significant for the pre-influenza activity period.Conclusions:
This study demonstrates an association between immunization with the inactivated influenza vaccine during pregnancy and reduced likelihood of prematurity during local, regional, and widespread influenza activity periods. However, no associations were found for the pre-influenza activity period. Moreover, during the period of widespread influenza activity there was an association between maternal receipt of influenza vaccine and reduced likelihood of SGA birth.
: Please see later in the article for the Editors' SummaryIntroduction
Infections during pregnancy have the potential to adversely impact birth outcomes and fetal growth and development. Respiratory infections—particularly those resulting in pneumonia—have been associated with low birth weight and increased risk of preterm birth [1],[2]. Influenza virus is an important respiratory pathogen that causes substantial burden of disease—including morbidity and mortality among pregnant women, with greater risk of influenza-related morbidity among pregnant women than among non-pregnant and postpartum women [3].
Vaccination against influenza is the most effective tool to prevent morbidity and mortality due to influenza. Influenza vaccination during pregnancy provides protection for the infant as well as the mother. A randomized controlled clinical trial in Bangladesh demonstrated that vaccination of pregnant women with the inactivated influenza vaccine had 63% effectiveness in reducing laboratory-confirmed influenza in their infants [4]. Since there is evidence of adverse fetal/newborn outcomes after infection during pregnancy [5],[6], including influenza infection [2], it is plausible that influenza vaccination in pregnancy could mitigate adverse birth outcomes such as prematurity and small for gestational age (SGA) births. The potential impact of maternal influenza immunization on birth outcomes could have important public health implications for developed as well as developing countries and may be of particular interest during influenza pandemics.
The objective of this study was to evaluate whether there is an association between receipt of inactivated influenza vaccine during pregnancy and birth outcomes using a multiyear, population-based, state-wide dataset from the state of Georgia (in the United States).
Methods
We conducted a retrospective cohort analysis of a large surveillance dataset. The primary exposure variable was receipt of inactivated influenza vaccine during any trimester of pregnancy by mothers of infants born between 1 June 2004 and 30 September 2006. The study period encompassed the 2004–2005 and the 2005–2006 influenza seasons (the two most recent seasons for which the data were available at the time of analysis). The main outcomes assessed were prematurity and SGA.
Data Sources and Study Population
We analyzed pregnancy - and birth-related data from the Georgia Pregnancy Risk Assessment Monitoring System (PRAMS) and influenza activity information compiled by Georgia for the Council of State and Territorial Epidemiologists (CSTE) reports. PRAMS is a multistate surveillance system managed by the US Centers for Disease Control and Prevention and state health departments, including the Georgia Department of Community Health [7],[8]. The PRAMS sample is drawn monthly from the state's birth file and includes resident women who have experienced a live birth. Georgia PRAMS oversamples women based on race (black) and birth weight (<2,500 g). We adjusted for the oversampling by using analysis weights described elsewhere in the methods section. PRAMS data are collected primarily by mailed questionnaires, with telephone follow-up among non-responders.
The Georgia PRAMS dataset contains information on maternal influenza vaccination (during any trimester); maternal attitudes, behaviors, and experiences before, during, and shortly after pregnancy; and newborn birth certificate data, including birth date and birth weight. Prematurity was defined as clinical estimate of gestational age (at birth) as less than 37 wk. Newborns with birth weight below the10th percentile for their gestational age were considered to be SGA. We used gender-specific reference values for the US published by Oken at al. [9] to assign SGA (yes/no) categories.
Definitions of Influenza Activity Periods
In order to model the impact of the intensity of influenza activity in Georgia on the association between maternal influenza vaccination and birth outcomes, we used a modified version of the CSTE report categories of influenza activity. CSTE reports assess the spread of influenza within each state for each week based on lab-confirmed and syndromic data [10]. The influenza activity is considered local if there are influenza outbreaks or an increase in cases of influenza-like illness in a single region of the state along with recent identification of laboratory-confirmed influenza from that region. In the case of influenza outbreaks or increases in influenza-like illness with recent laboratory-confirmed influenza in at least two but fewer than half the regions of the state, the influenza activity level is considered to be regional. If the influenza outbreaks or increased numbers of influenza-like illness cases (plus laboratory-confirmed cases) are reported in at least half of the regions of the state, the influenza activity is considered to be widespread (Figure 1). We defined the pre-influenza period as the period between the start of the putative influenza season (October 1) and the beginning of local influenza activity (per CSTE reports). The pre-influenza period is characterized by the availability of the vaccine and the absence of influenza activity (Figure 1). The definition for the pre-influenza period was similar to the one used by other authors [11],[12].
Fig. 1. Influenza activity/analysis periods. 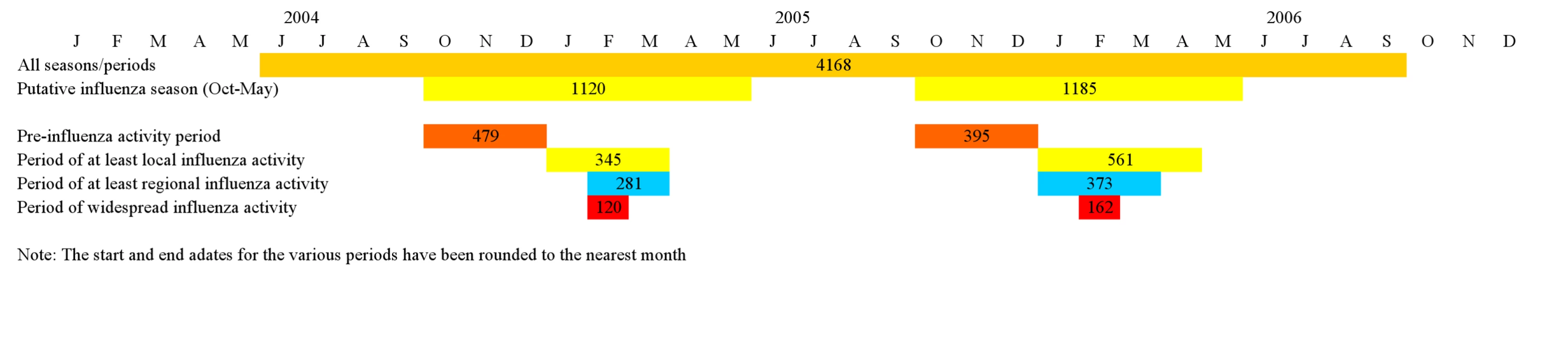
The numbers indicate births in each of the analysis periods. Stratified analysis was performed for the overall study period, the putative influenza season (1 October–31 May), the pre-influenza period (during the putative influenza season), the period of at least local activity, the period of regional activity, and the period of widespread activity (Figure 1).
Statistical Analysis and Confounder Assessment
In Georgia PRAMS, live births to black women and those that are low birth weight are oversampled in order to provide enough statistical power for stratified analyses for groups of interest relevant to PRAMS objectives. For combined analyses, in accordance with standard practice, analysis weights developed by the Centers for Disease Control and Prevention [13] were used to adjust for the sample design (e.g., to account for oversampling of the two risk groups) and differential response rate across groups.
We used logistic regression to evaluate the association of maternal influenza vaccine and (a) prematurity and (b) SGA. Linear regression was used to evaluate the statistical significance of differences between infants born to vaccinated and unvaccinated women in terms of mean gestational age at first antenatal visit and mean birth weight.
Confounding due to differences between the vaccinated and unvaccinated individuals is a recognized issue in observational studies of influenza vaccination—particularly studies evaluating vaccine effectiveness for reducing all-cause mortality in the elderly [12]. One approach to account for confounding is to choose a period when the vaccine was available but the influenza virus was not circulating as the “control” pre-influenza period [11],[12]. In the pre-influenza period, there should be no vaccine effect, and observed effects during this period are assumed to be due to confounding. Therefore, as our primary strategy for confounder adjustment, we identified a group of covariates that would move the odds ratios (ORs) of association between maternal influenza immunization and birth outcomes during the pre-influenza period to 1.0 (i.e., no effect), hence arriving at a set of covariates that could effectively control for confounding due to the differences between the vaccinated and the unvaccinated women in analyses of all influenza activity periods. We selected covariates for the separate multivariate models for each of the birth outcomes (i.e., prematurity and SGA) using a modified version of the approach described by Jackson et al. [11] and Nelson et al. [12]. Briefly, variables for each birth outcome were evaluated for potential confounding by selecting the covariates that, in bivariate models, modified the association between maternal influenza vaccine and the birth outcome and moved the OR towards 1. From this initial group of variables, we arrived at a parsimonious model by sequentially dropping each covariate and observing a change in OR of the association between maternal immunization and the birth outcome. We excluded the variable whose removal moved the OR the most towards 1. If dropping a covariate resulted in moving ORs away from 1 and a change in magnitude of less than 1%, we removed the covariate that caused the least change and then repeated the process. If the change was more than 1%, we considered the current set of covariates as the smallest group of variables required to account for confounding.
In order to address the possibility that the confounders in the pre-influenza period were different from the confounding factors in the influenza activity period, we also developed secondary multivariate models using a traditional analytic approach for confounder adjustment. In these secondary models, the covariate list (for both the pre-influenza-period-based and traditional adjustment) was based on evidence in the literature regarding associations with birth outcomes and availability of data in the Georgia PRAMS dataset. The covariate list included the following: gestational age at first antenatal visit, maternal age less than 19 y, maternal age more than 35 y, multiple births, maternal medical risk factors, labor/delivery complications, birth defects, maternal diabetes (gestational and/or non-gestational), hypertension (treated or untreated), mother insured, multivitamin use in pregnancy, history of smoking during pregnancy, history of alcohol use during pregnancy, black race, education less than 12th grade, mother's marital status, and maternal weight before pregnancy. The ORs for association between the different covariates and receipt of influenza vaccine were computed using logistic regression.
We also computed the population prevented fraction of prematurity for the various periods of influenza activity using the formula: where is the proportion of cases vaccinated and OR is the odds ratio approximating relative risk (we verified this assumption for our data). The formula, based on the approach described by Kleinbaum et al. [14], is suitable for computing the population prevented fraction when adjusted measures of association are used. The population prevented fraction for a vaccine estimates the reduction in an outcome given the efficacy/effectiveness of the vaccine and the specific vaccine coverage.
We used Stata version 10 (Stata Corporation) for statistical analysis. Identified associations were evaluated for statistical significance at α = 0.05 using two-tailed tests, and Taylor series linearization methods [15] were used to estimate variance.
Ethical Assessment and Institutional Review Boards
The study was reviewed and approved by the Emory University Institutional Review Board and the Georgia Department of Human Resources Institutional Review Board.
Results
A total of 4,326 women (and their newborns) were included in Georgia PRAMS during the 28-mo study period. Influenza vaccine information was available for 4,168 (96.3%) women in PRAMS (study population); of these, 578 women (14.9% [weighted]) had received the influenza vaccine during pregnancy. The vaccine coverage was 19.2% (weighted) among mothers of infants born during the putative influenza season. Out of the 122 wk of the study, at least local influenza activity was detected during 27 wk (22.1%)—including widespread activity in 8 wk (Figure 1). There were 1,547 premature newborns (10.6% [weighted]) and 1,186 newborns with SGA (11.2% [weighted]) in our study population.
The odds of having received influenza vaccine during pregnancy were lower (a) for black women than for all other ethnic groups (OR = 0.78; 95% CI, 0.62–0.98), (b) for women with diabetes (OR = 0.30, 95% CI, 0.10–0.95), and (c) for mothers who used multivitamins in pregnancy (OR = 0.64; 95% CI, 0.50–0.83) (Table 1). Insured women were more likely to have received the influenza vaccine (OR = 1.41; 95% CI, 1.09–1.81). Likelihood of having received an influenza vaccine during pregnancy was not associated with any of the other binary covariates (Table 1). Similarly, gestational age at first prenatal visit was similar for vaccinated women and unvaccinated women (mean: 5.2 wk versus 5.3 wk; p = 0.23), and maternal weight before pregnancy was similar for vaccine recipients and non-recipients (mean: 68.3 kg versus 68.1 kg; p = 0.88).
Tab. 1. Receipt of influenza vaccine during pregnancy categorized by maternal characteristics. 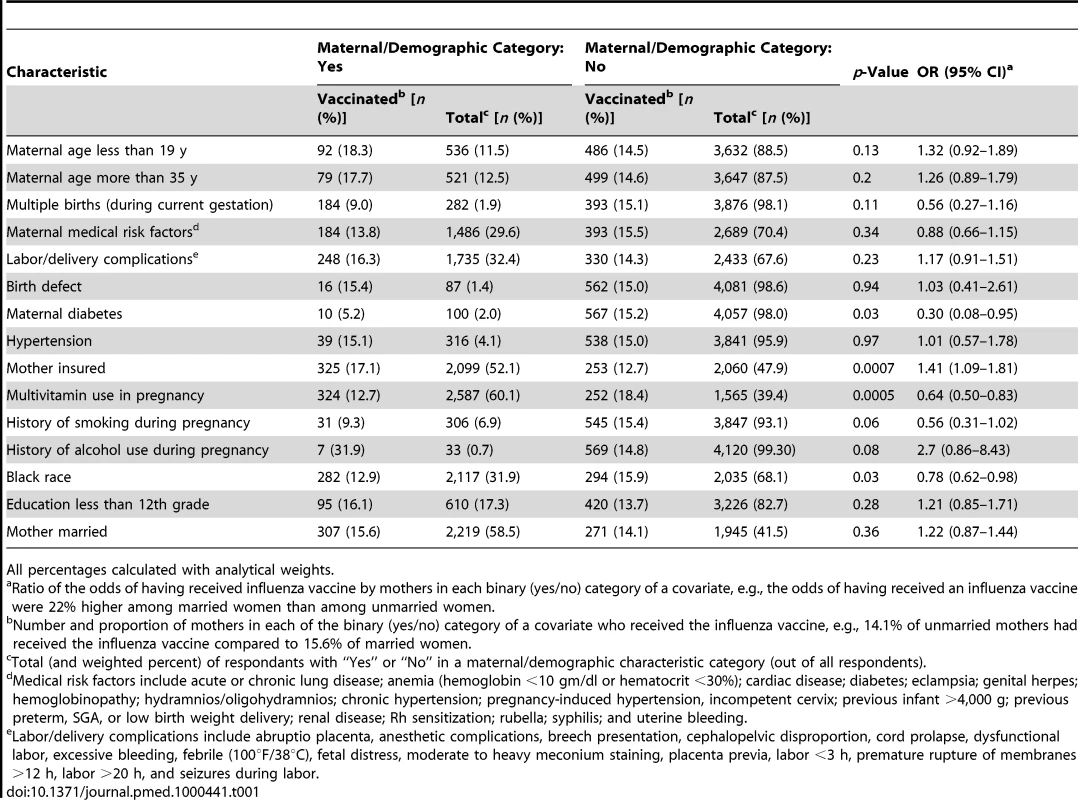
All percentages calculated with analytical weights. Based on the approach of identifying covariates that produce adjusted ORs of 1 during the pre-influenza period, the group of covariates in the prematurity multivariate models included gestational age for first antenatal visit, maternal diabetes (gestational and/or non-gestational), multivitamin use in pregnancy, history of alcohol use during pregnancy, education less than 12th grade, and mother married. The covariates in the primary multivariate models for SGA included maternal age less than 19 y, maternal medical risk factors, labor/delivery complications, hypertension (treated or untreated), birth defects, and history of alcohol use during pregnancy.
In unadjusted models, and in models with covariates based on lack of effects in the pre-influenza season, infants born during the putative influenza season (1 October–31 May) and whose mothers were vaccinated against influenza during pregnancy were less likely to be premature than infants of unvaccinated mothers born in the same period (adjusted OR = 0.60; 95% CI, 0.38–0.94). The magnitude of effect of maternal influenza vaccine on prematurity increased during the period when there was at least local influenza activity in any part of the state (adjusted OR = 0.44; 95% CI, 0.26–0.73) and was the highest for those born during the period of widespread influenza activity (adjusted OR = 0.28; 95% CI, 0.11–0.74) (Table 2). The adjusted and unadjusted ORs were not significant for the association between receipt of maternal influenza vaccine and prematurity for the pre-influenza activity period or for the analysis without consideration of influenza activity (Table 2).
Tab. 2. ORs of prematurity by maternal influenza vaccine status (ORs<1 imply a protective association of the vaccine). 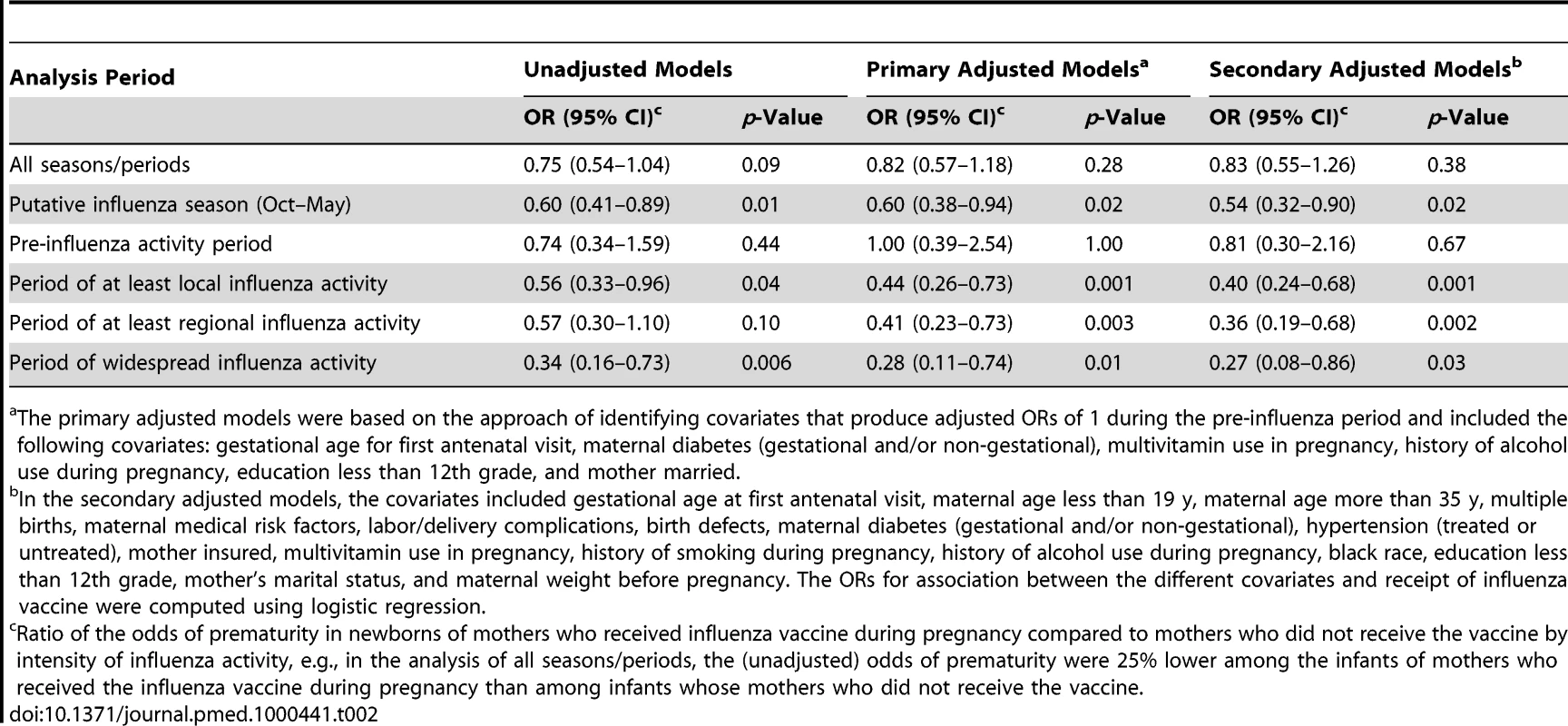
The primary adjusted models were based on the approach of identifying covariates that produce adjusted ORs of 1 during the pre-influenza period and included the following covariates: gestational age for first antenatal visit, maternal diabetes (gestational and/or non-gestational), multivitamin use in pregnancy, history of alcohol use during pregnancy, education less than 12th grade, and mother married. Compared with newborns of unvaccinated women, those born to vaccinated mothers had lower odds of SGA (adjusted OR = 0.31; 95% CI, 0.13–0.75) during the period of widespread influenza activity (Table 3). Although the magnitude of the ORs of the association between maternal influenza vaccine and SGA generally increased with the increase in the intensity of influenza activity, these associations were not statistically significant (other than for the period of widespread activity) (Table 3). The associations observed in the secondary multivariate models using the traditional approach were qualitatively similar to the associations in the primary multivariate models (Tables 2 and 3).
Tab. 3. ORs of being SGA by maternal influenza vaccine status (ORs<1 imply a protective association of the vaccine). 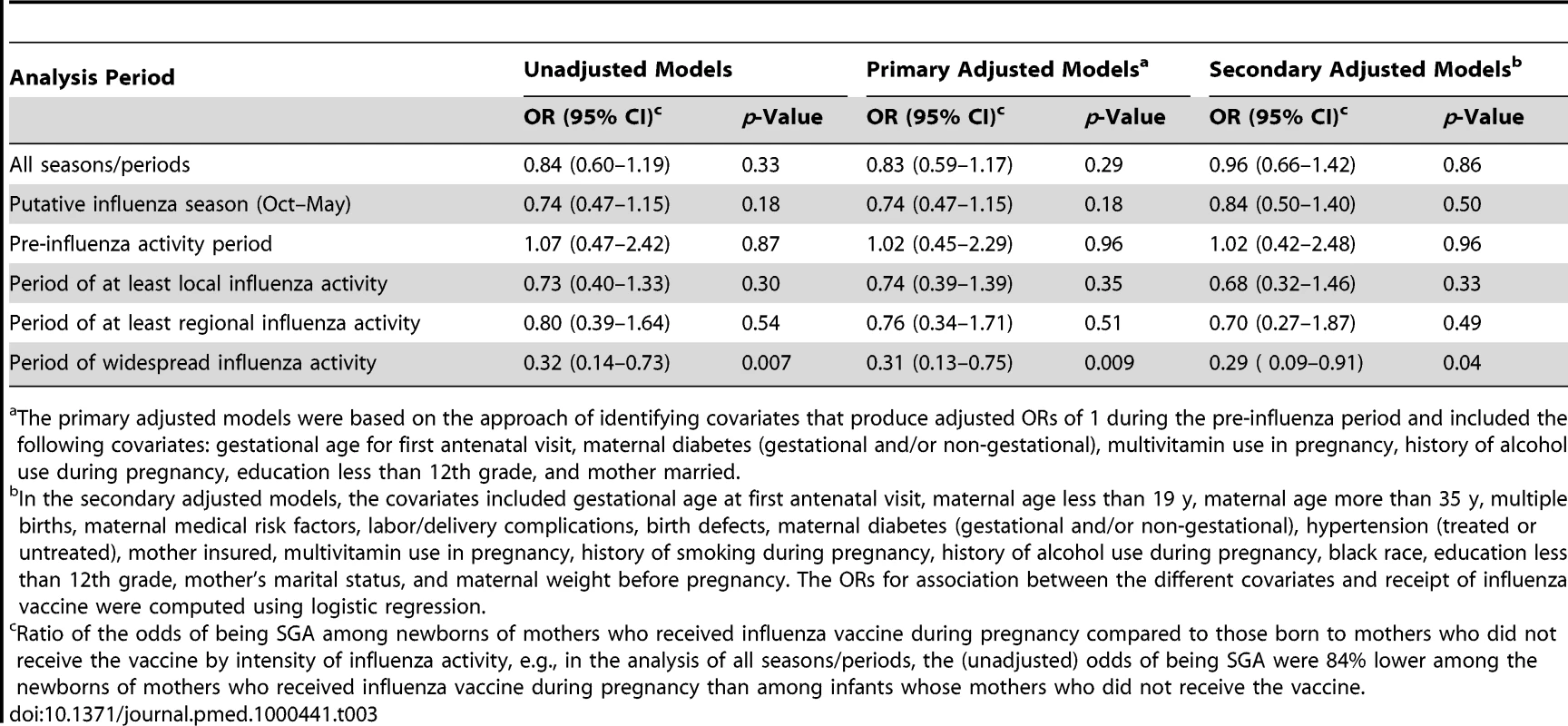
The primary adjusted models were based on the approach of identifying covariates that produce adjusted ORs of 1 during the pre-influenza period and included the following covariates: gestational age for first antenatal visit, maternal diabetes (gestational and/or non-gestational), multivitamin use in pregnancy, history of alcohol use during pregnancy, education less than 12th grade, and mother married. Newborns of vaccinated women were, on average, 96.7 g heavier than newborns of unvaccinated women (3,348 g versus 3,251 g; p = 0.002). During the putative influenza season, the difference between the two groups increased to 113 g (3,360 g for the vaccinated group versus 3,247 g for the unvaccinated group; p = 0.004). There were no significant differences in birth weights outside the putative influenza season (3,317 g [vaccinated] versus 3,255 g [unvaccinated]; p = 0.233).
There was no statistical interaction by specific influenza season (i.e., 2004–2005 and 2005–2006) for all analyses of prematurity, SGA, and birth weight (for all interaction terms: p>0.05—detailed data available on request). Moreover, the association between maternal influenza vaccine and birth outcomes was qualitatively similar for the two influenza seasons. For example, during the period of widespread influenza activity, the adjusted ORs for prematurity were 0.17 (95% CI, 0.03–0.86) for the 2004–2005 season and 0.32 (95% CI, 0.10–1.14) for the 2005–2006 season.
The fraction of prematurity prevented in the population during the study period (population prevented fraction of prematurity) was 0% for the pre-influenza activity period and 7.9% for the putative influenza season. The population prevented fraction increased during the periods of influenza activity (at least local activity, 15.7%; at least regional activity, 17.5%; widespread activity, 17.5%).
Discussion
This study demonstrates an association between immunization with the inactivated influenza vaccine during pregnancy and reduced likelihood of prematurity during local, regional, and widespread influenza activity periods. For births during the 8 wk of widespread influenza activity, the odds of prematurity were approximately 70% lower among the newborns of the vaccinated mothers compared to mothers who did not receive the influenza vaccine. During the period of widespread influenza activity there was also an association between maternal receipt of influenza vaccine and reduced likelihood of SGA. The magnitude of association between influenza vaccine and prematurity (as measured by the values of ORs) increased with the increase in the intensity of influenza activity and was higher for the 2004–2005 season than for the 2005–2006 season. Based on laboratory and epidemiologic criteria, the 2004–2005 influenza season was more intense than the 2005–2006 season in the US [16]. Although the SGA-related ORs were not statistically significant for influenza activity periods except for the period of widespread activity, the overall “gradient” of effect in the point estimates of the ORs was qualitatively similar to that of prematurity. The increase in the impact of maternal influenza vaccines on birth outcomes by influenza activity, both in terms of ORs and population prevented fractions, supports the validity of our findings.
The possibility of confounding due to differences between vaccinated and unvaccinated individuals included in observational studies of influenza immunization is well recognized [12]. The most significant type of confounding in influenza studies is due to a higher likelihood of individuals with high functional capacity (i.e., healthier individuals) to get vaccinated—a phenomenon often known as the “healthy user effect.” However, most observational studies where significant confounding has been documented were conducted in the elderly and included a relatively nonspecific end point of all-cause mortality. It is reasonable to assume that, compared to older individuals, women of reproductive age may be less likely to have significant functional limitation even in the presence of co-morbidities. Therefore, influenza vaccine studies in this age group may be less likely to suffer from bias due to the healthy user effect. Moreover, we found no statistically significant difference between the vaccinated women and the unvaccinated women in terms of gestational age at which they sought antenatal care. On the other hand, the possibility of other confounders cannot be discounted in studies involving pregnant women. In order to address confounding, we used the pre-influenza period (i.e., the season where vaccine was available but there was minimal circulation of influenza virus) as the “control” period. The use of the pre-influenza period for selecting confounders from a broad set of covariates is an approach suggested by Nelson et al. [12] and Jackson et al. [11] that takes advantage of the seasonality of influenza circulation. The associations observed in our study were robust to adjustment for confounders identified using this approach (and the more traditional approach)—supporting the validity of our findings.
Influenza virus, particularly seasonal influenza virus, rarely crosses the placenta [3],[17],[18]. However, the effect of infection on prematurity is thought be at least partially mediated through inflammatory pathways [5],[6]. Increase in pro-inflammatory cytokines (e.g., IL-1, IL-6 and TNF-α) and reduction in anti-inflammatory cytokines (e.g., IL-10) have been linked to preterm labor [6],[19],[20]. IL-1 stimulates the amnion and the decidua to produce prostaglandins and can stimulate myometrial contractions [20]. Prostaglandins are known to play an important role in the initiation and progression of labor [21]. Moreover, in animal models, administration of IL-1 results in preterm birth [20]. Similarly, TNF-α induces the amnion, the decidua, and the myometrium to produce prostaglandins, and administration of TNF-α to pregnant animals can induce preterm labor [19],[22]. Recent studies have shown that influenza virus infection induces gene expression of pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, interferon (IFN)-β, IFN-α, and granulocyte macrophage colony-stimulating factor (GM-CSF) [19].
In addition to biological plausibility, there is epidemiological evidence of an association between maternal infection and preterm birth [5]. The association is strongest for intrauterine viral infections and systemic and intrauterine bacterial infections [5],[6]. Viral infection outside the reproductive tract, including influenza infection, may also play a role in the etiology of prematurity. For example, in an analysis of 1957–1958 data, newborns of women who had serological evidence of “Asian” (pandemic) influenza during pregnancy were 50% more likely to be premature compared to newborns of uninfected women [2]. Moreover, a recent literature review found that SARS infection in the second or third trimester of pregnancy may be associated with spontaneous preterm delivery and early cesarean sections for deteriorating medical condition, although only 16 such cases were identified in the literature [23]. Moreover, in studies in China and Hungary, birth defects were associated with history of influenza [24],[25].
However, a few observational studies have failed to demonstrate an association between influenza infection and birth outcomes [26],[27]. The lack of observed effect in some studies could be due to a true lack of association, small or difficult to measure effect size, challenges related to the study population (e.g., administrative datasets), or non-differential misclassification due to challenges in retrospectively identifying influenza infection. Although less than ideal, modeling receipt of influenza vaccine as the exposure/independent variable reduces the likelihood and the intensity of non-differential misclassification bias.
Preterm births represent a significant burden to health care and society [5]. Like several developed countries, there has been an increase in the rate of preterm births in the US, which rose from 9.5% in 1981 to 12.8% in 2006 [28],[29]. Although the etiology of prematurity is complex [5] and not completely understood, our results suggest that at least a fraction of preterm births may be preventable through maternal influenza vaccination.
The association between maternal influenza vaccination and SGA was only statistically significant (and the highest in magnitude) for the period of widespread influenza activity. Possible reasons for the effect being limited to the period of highest influenza activity include the following: (a) in a developed country setting, the effect of maternal influenza infection on fetal growth is milder than the effect on prematurity; (b) SGA represents fetal compromise resulting from infection that is insufficient to trigger early parturition, but may result in the delayed observation of growth restriction (i.e., the observation in the widespread activity period may be the cumulative effect of previous periods). Moreover, in the vaccinated group, the birth weight distribution in the pre-influenza period was different from the distribution in the period of widespread activity (see Text S1). However, the difference in mean birth weights (in the vaccinated group) between these two periods was not statistically significant (p = 0.74). Since the ostensible increase in birth weight in the widespread activity period compared to the pre-influenza period in the vaccinated group cannot easily be explained by vaccine action, this difference—although non-significant—may suggest confounding vis-à-vis the birth weight outcome.
This study has a few limitations and strengths. Although we assessed and adjusted for many covariates, like any observational study, there is a possibility of residual confounding and selection bias. Moreover, data on influenza infection during pregnancy were not included in the PRAMS dataset. Although the primary explanation of the effects of influenza immunization in pregnancy on birth outcomes is through prevention of infection, having influenza infection data would have provided additional support for our findings. Another issue is that the information regarding maternal influenza immunization was based on recall and could be susceptible to information bias. However, the vaccination rates in our study are similar to the rates computed by other authors for Georgia, and to the United States national level coverage estimated by the National Health Interview Survey [30],[31].
The PRAMS dataset does not contain information regarding the precise trimester of vaccination. Therefore, the effect of vaccination in a specific trimester could not be evaluated. Moreover, it is possible that mothers of premature infants had less time to receive influenza vaccine than mothers of term infants (i.e., reverse causality). On the other hand, since this was a population-based study with a sampling strategy aimed at producing representative estimates, the temporal distribution of influenza vaccination in pregnancy would be similar to that of the general population, hence adding to the generalizability of our findings. The results of this study, nevertheless, need to be replicated in other populations as it is plausible that the impact of vaccines on birth outcomes would vary with the underlying influenza epidemiology and demographic characteristics.
Supporting Information
Zdroje
1. GoodnightWH
SoperDE
2005 Pneumonia in pregnancy. Crit Care Med 33 S390 S397
2. HardyJM
AzarowiczEN
ManniniA
MedearisDNJr
CookeRE
1961 The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957–1958. Am J Public Health Nations Health 51 1182 1188
3. MakTK
MangtaniP
LeeseJ
WatsonJM
PfeiferD
2008 Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 8 44 52
4. ZamanK
RoyE
ArifeenSE
RahmanM
RaqibR
2008 Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 359 1555 1564
5. GoldenbergRL
CulhaneJF
IamsJD
RomeroR
2008 Epidemiology and causes of preterm birth. Lancet 371 75 84
6. RomeroR
EspinozaJ
GoncalvesLF
KusanovicJP
FrielL
2007 The role of inflammation and infection in preterm birth. Semin Reprod Med 25 21 39
7. Centers for Disease Control and Prevention 2009 Pregnancy Risk Assessment Monitoring System (PRAMS): home. Available: http://www.cdc.gov/prams/. Accessed 29 April 2011
8. Georgia Department of Community Health 2009 What is Georgia PRAMS? Available: http://health.state.ga.us/epi/prams/index.asp. Accessed 29 April 2011
9. OkenE
KleinmanKP
Rich-EdwardsJ
GillmanMW
2003 A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3 6
10. Georgia Department of Community Health 2009 Influenza surveillance in Georgia. Available: http://health.state.ga.us/epi/flu/. Accessed 29 April 2011
11. JacksonML
NelsonJC
WeissNS
NeuzilKM
BarlowW
2008 Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet 372 398 405
12. NelsonJC
JacksonML
WeissNS
JacksonLA
2009 New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol 62 687 694
13. Centers for Disease Control and Prevention 2008 Pregnancy Risk Assessment Monitoring System (PRAMS): methodology. Available: http://www.cdc.gov/prams/methodology.htm. Accessed 29 April 2011
14. KleinbaumDG
KupperLL
MorgensternH
1982 Epidemiologic research: principles and quantitative methods New York John Wiley & Sons
15. RaoJNK
SottAJ
1984 On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 12 46 60
16. Centers for Disease Control and Prevention 2006 2005–06 U.S. influenza season summary. Available: http://www.cdc.gov/flu/weekly/weeklyarchives2005-2006/05-06summary.htm. Accessed 29 April 2011
17. IrvingWL
JamesDK
StephensonT
LaingP
JamesonC
2000 Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG 107 1282 1289
18. RasmussenSA
JamiesonDJ
MacfarlaneK
CraganJD
WilliamsJ
2009 Pandemic influenza and pregnant women: summary of a meeting of experts. Am J Public Health 99 Suppl 2 S248 S254
19. UchideN
OhyamaK
BesshoT
ToyodaH
2005 Induction of pro-inflammatory cytokine gene expression and apoptosis in human chorion cells of fetal membranes by influenza virus infection: possible implications for maintenance and interruption of pregnancy during infection. Med Sci Monit 11 RA7 16
20. RomeroR
DurumS
DinarelloCA
OyarzunE
HobbinsJC
1989 Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 37 13 22
21. ChallisJR
SlobodaDM
AlfaidyN
LyeSJ
GibbW
2002 Prostaglandins and mechanisms of preterm birth. Reproduction 124 1 17
22. FidelPLJr
RomeroR
WolfN
CutrightJ
RamirezM
1994 Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 170 1467 1475
23. RebmannT
2005 Severe acute respiratory syndrome: implications for perinatal and neonatal nurses. J Perinat Neonatal Nurs 19 332 345
24. LiZ
RenA
LiuJ
PeiL
ZhangL
2007 Maternal flu or fever, medication use, and neural tube defects: a population-based case-control study in Northern China. Birth Defects Res A Clin Mol Teratol 79 295 300
25. AcsN
BanhidyF
PuhoE
CzeizelAE
2005 Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol 73 989 996
26. HartertTV
NeuzilKM
ShintaniAK
MitchelEFJr
SnowdenMS
2003 Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 189 1705 1712
27. FranceEK
Smith-RayR
McClureD
HambidgeS
XuS
2006 Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med 160 1277 1283
28. HamiltonBE
MininoAM
MartinJA
KochanekKD
StrobinoDM
2007 Annual summary of vital statistics: 2005. Pediatrics 119 345 360
29. MartinJA
KungHC
MathewsTJ
HoyertDL
StrobinoDM
2008 Annual summary of vital statistics: 2006. Pediatrics 121 788 801
30. FioreAE
ShayDK
BroderK
IskanderJK
UyekiTM
2009 Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 58 1 52
31. Centers for Disease Control and Prevention 2009 Receipt of influenza vaccine during pregnancy among women with live births—Georgia and Rhode Island, 2004–2007. MMWR Morb Mortal Wkly Rep 58 972 975
Štítky
Interné lekárstvo
Článek The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in AfricaČlánek If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



