-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
How Has the Age-Related Process of Overweight or Obesity Development Changed over Time? Co-ordinated Analyses of Individual Participant Data from Five United Kingdom Birth Cohorts
Background:
There is a paucity of information on secular trends in the age-related process by which people develop overweight or obesity. Utilizing longitudinal data in the United Kingdom birth cohort studies, we investigated shifts over the past nearly 70 years in the distribution of body mass index (BMI) and development of overweight or obesity across childhood and adulthood.Methods and Findings:
The sample comprised 56,632 participants with 273,843 BMI observations in the 1946 Medical Research Council National Survey of Health and Development (NSHD; ages 2–64 years), 1958 National Child Development Study (NCDS; 7–50), 1970 British Cohort Study (BCS; 10–42), 1991 Avon Longitudinal Study of Parents and Children (ALSPAC; 7–18), or 2001 Millennium Cohort Study (MCS; 3–11). Growth references showed a secular trend toward positive skewing of the BMI distribution at younger ages. During childhood, the 50th centiles for all studies lay in the middle of the International Obesity Task Force normal weight range, but during adulthood, the age when a 50th centile first entered the overweight range (i.e., 25–29.9 kg/m2) decreased across NSHD, NCDS, and BCS from 41 to 33 to 30 years in males and 48 to 44 to 41 years in females. Trajectories of overweight or obesity showed that more recently born cohorts developed greater probabilities of overweight or obesity at younger ages. Overweight or obesity became more probable in NCDS than NSHD in early adulthood, but more probable in BCS than NCDS and NSHD in adolescence, for example. By age 10 years, the estimated probabilities of overweight or obesity in cohorts born after the 1980s were 2–3 times greater than those born before the 1980s (e.g., 0.229 [95% CI 0.219–0.240] in MCS males; 0.071 [0.065–0.078] in NSHD males). It was not possible to (1) model separate trajectories for overweight and obesity, because there were few obesity cases at young ages in the earliest-born cohorts, or (2) consider ethnic minority groups. The end date for analyses was August 2014.Conclusions:
Our results demonstrate how younger generations are likely to accumulate greater exposure to overweight or obesity throughout their lives and, thus, increased risk for chronic health conditions such as coronary heart disease and type 2 diabetes mellitus. In the absence of effective intervention, overweight and obesity will have severe public health consequences in decades to come.
Published in the journal: How Has the Age-Related Process of Overweight or Obesity Development Changed over Time? Co-ordinated Analyses of Individual Participant Data from Five United Kingdom Birth Cohorts. PLoS Med 12(5): e32767. doi:10.1371/journal.pmed.1001828
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001828Summary
Background:
There is a paucity of information on secular trends in the age-related process by which people develop overweight or obesity. Utilizing longitudinal data in the United Kingdom birth cohort studies, we investigated shifts over the past nearly 70 years in the distribution of body mass index (BMI) and development of overweight or obesity across childhood and adulthood.Methods and Findings:
The sample comprised 56,632 participants with 273,843 BMI observations in the 1946 Medical Research Council National Survey of Health and Development (NSHD; ages 2–64 years), 1958 National Child Development Study (NCDS; 7–50), 1970 British Cohort Study (BCS; 10–42), 1991 Avon Longitudinal Study of Parents and Children (ALSPAC; 7–18), or 2001 Millennium Cohort Study (MCS; 3–11). Growth references showed a secular trend toward positive skewing of the BMI distribution at younger ages. During childhood, the 50th centiles for all studies lay in the middle of the International Obesity Task Force normal weight range, but during adulthood, the age when a 50th centile first entered the overweight range (i.e., 25–29.9 kg/m2) decreased across NSHD, NCDS, and BCS from 41 to 33 to 30 years in males and 48 to 44 to 41 years in females. Trajectories of overweight or obesity showed that more recently born cohorts developed greater probabilities of overweight or obesity at younger ages. Overweight or obesity became more probable in NCDS than NSHD in early adulthood, but more probable in BCS than NCDS and NSHD in adolescence, for example. By age 10 years, the estimated probabilities of overweight or obesity in cohorts born after the 1980s were 2–3 times greater than those born before the 1980s (e.g., 0.229 [95% CI 0.219–0.240] in MCS males; 0.071 [0.065–0.078] in NSHD males). It was not possible to (1) model separate trajectories for overweight and obesity, because there were few obesity cases at young ages in the earliest-born cohorts, or (2) consider ethnic minority groups. The end date for analyses was August 2014.Conclusions:
Our results demonstrate how younger generations are likely to accumulate greater exposure to overweight or obesity throughout their lives and, thus, increased risk for chronic health conditions such as coronary heart disease and type 2 diabetes mellitus. In the absence of effective intervention, overweight and obesity will have severe public health consequences in decades to come.Introduction
The obesity epidemic is a daunting public health threat, even in high-income countries with good infrastructure for education and health care. In the United Kingdom, the projected total cost to the National Health Service (NHS) is estimated to be £22.9 billion per year by 2050 [1]. The 2012 Health Survey for England (HSE) reported that 25% of adults were obese and a further 42% of males and 32% of females were overweight, according to body mass index (BMI) [2]. Similar results have been published for Scotland, Wales, and Northern Ireland [3–5], and worryingly high prevalence rates exist in most other high-income countries outside of the UK [6]. Children in such settings have not been exempt from the epidemic [6]. The 2012–2013 National Child Measurement Programme for England (NCMP) reported that 9.3% of 4–5 year olds and 18.9% of 10–11 year olds were obese, for example [7]. This is particularly concerning given that childhood obesity tends to lead to adulthood obesity, and this tracking is often accompanied by the development of other cardiovascular risk factors, such as dyslipidaemia, hyperglycaemia, hypertension, and vascular inflammation [8]. Obesity rates are, therefore, associated with rates of adiposity-related conditions, such as coronary heart disease (CHD) and type 2 diabetes mellitus [9–11].
Cross-sectional surveys in the UK and elsewhere estimate the scale of the problem, but longitudinal data are needed to provide information on the age-related process of obesity development, such as the ages when risk is increasing rapidly. Such trajectories are related to disease processes [12–18]. Some surveys in the UK have been repeated over time, thereby allowing demonstration of relatively recent (since 1991 in HSE and 2005 in NCMP) increases in prevalence rates [2,7], while longer-term secular trends have been published in other high-income countries, for example, using data from the National Health and Nutrition Examination Surveys (NHANES) in the United States [19]. Such publications do not, however, tell us how the process of obesity development across childhood and adulthood has changed over time in response to environment changes, including those in the behavioural, sociocultural, and economic landscape. This information would be useful to identify appropriate ages and patterns of overweight or obesity development for targeted prevention or intervention and to provide insight into the possible aetiological factors responsible.
The UK has uniquely invested in a series of four nationally representative birth cohort studies that could be used to investigate the development of obesity across age and time [20]. Li et al. published age-related mean BMI trajectories in the two oldest studies, the 1946 Medical Research Council National Survey of Health and Development (1946 NSHD) and the 1958 National Child Development Study (1958 NCDS); the trajectories were similar in childhood but diverged in early adulthood such that the more recently born cohort gained an additional 0.06 kg/m2 per year [21]. The rest of our knowledge is based on studies in which data have been treated cross-sectionally or studies that are (1) not representative of the UK population, (2) span only a small part of the life course, and/or (3) describe only a short-term trend [18,22–24]. Studies conducted outside of the UK, mainly in the US, are similarly limited [25–27]. Furthermore, all of these published cohort studies have focused on mean BMI trajectories, despite the predominant discourse based on studies in diverse settings around the world being that the obesity epidemic reflects shifts at the upper end of the distribution [28–33].
The present study utilises the extensive longitudinal data on BMI in the UK birth cohort studies, with the aims to investigate (1) shifts over time in the distribution of BMI across age and (2) shifts over time in the development of overweight or obesity across age.
Methods
Study Samples
The 1946 NSHD is based on a sample (n = 5,362) of all singleton births in one week in March 1946 in England, Scotland, and Wales [34,35]. The 1958 NCDS is based on 17,638 people born in one week in March 1958 in England, Scotland, and Wales; 920 immigrants born in the same week were incorporated during childhood [36]. A similar strategy was used in the 1970 British Cohort Study (1970 BCS), which is based on 17,287 people born in one week in April 1970, with the addition of 1,814 individuals who were (1) born in Northern Ireland and included only in the birth sweep, (2) immigrants who were incorporated into the study in childhood, or (3) never took part in any sweep [37]. The 1991 Avon Longitudinal Study of Parents and Children (1991 ALSPAC) is based on 15,444 births to women living in the defunct county of Avon in England with an expected delivery date between April 1991 and December 1992; this number includes 713 people who were enrolled during childhood recruitment drives [38–40]. A fully searchable data dictionary is available for the 1991 ALSPAC study at http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary. This study was used to fill a 30-year gap in which no nationally representative study was initiated. Finally, the 2001 Millennium Cohort Study (2001 MCS) is based on 18,818 people born between September 2000 and January 2002 who were living in England, Scotland, Wales, or Northern Ireland at age 9 months [41]. All of the studies have received ethical approval and obtained informed parental and/or participant consent; this information is available from the study websites and/or cohort profiles [34–41].
The inclusion criteria for the present study excluded groups likely to have particularly different BMI values, thereby ensuring each study sample comprised a comparable composition of people. The criteria were (1) part of the original cohort (i.e., not an immigrant), (2) white race, (3) singleton birth, and (4) survival to at least age 9 months in the 2001 MCS; age 1 year in the 1946 NSHD, 1958 NCDS, and 1991 ALSPAC; and age 5 years in the 1970 BCS. Each study determined race/ethnicity differently and not always consistently across all sweeps; we use “white” as it best captures everyone in our sample (i.e., everyone was white British in the 1946 NSHD, while in the 1958 NCDS, 1970 BCS, 1991 ALSPAC, and 2001 MCS the samples were restricted to the responses “European/Caucasian,” “European/UK,” “White,” and “White,” respectively). Participants were not excluded on the basis of missing data for these variables, but were required to have sex recorded and at least one observation of BMI. Sample selection is shown in S1 Fig and sample sizes are shown in Table 1.
Tab. 1. Description of BMI data in the five UK birth cohort studies. 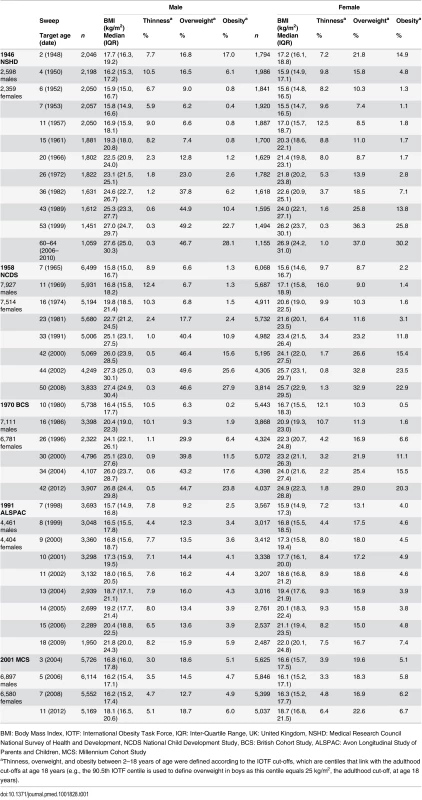
BMI: Body Mass Index, IOTF: International Obesity Task Force, IQR: Inter-Quartile Range, UK: United Kingdom, NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study, ALSPAC: Avon Longitudinal Study of Parents and Children, MCS: Millennium Cohort Study Anthropometry
Weight and height were assessed at data collection sweeps at target ages of 2, 4, 6, 7, 11, 15, 20, 26, 36, 43, 53, and 60–64 years in the 1946 NSHD; 7, 11, 16, 23, 33, 42, 44, and 50 years in the 1958 NCDS; 10, 16, 26, 30, 34, and 42 years in the 1970 BCS; 7, 8, 9, 10, 11, 13, 14, 15, and 18 years in the 1991 ALSPAC; and 3, 5, 7, and 11 years in the 2001 MCS. S1 Table summarises the main differences in measurement protocols and S2 Table describes the main steps used to make the anthropometry as comparable as possible.
Statistical Analysis
WJ and RH determined which analyses to perform and include in the present paper in July 2013 after discussing options with all co-authors.
BMI was computed as weight (kg)/height (m)2, and thinness, overweight, and obesity were defined according to International Obesity Task Force (IOTF) cut-offs during childhood and standard cut-offs of 18.5, 25, and 30 kg/m2 during adulthood [42,43]. The IOTF cut-offs are centiles spanning 2–18 years of age that link with the adulthood cut-offs at age 18 years (e.g., the 90.5th IOTF centile is used to define overweight in boys as this centile equals 25 kg/m2, the adulthood cut-off, at age 18 years), thereby avoiding artificial change in prevalence during the transition to adulthood.
The Lambda-Mu-Sigma (LMS) method was used to summarise the distribution of BMI across age in sex, study, and childhood (ages 2–18 years) versus adulthood (ages 20–64 years) stratified models [44]. Briefly, the LMS method models variation in size across age as a function of three curves: (1) the lambda (L) curve describes the Box-Cox power needed to remove skewness, (2) the mu (M) curve describes the median, and (3) the sigma (S) curve describes the coefficient of variation. With these three curves it is possible to compute any centile, thereby allowing investigation of secular trends at different centiles of the BMI distribution. The three curves are fitted as cubic splines, with Equivalent Degrees of Freedom (EDF) governing their complexity. Models were built by choosing the EDF for M, then S, then L, with the aim to make EDF for M > EDF for S > EDF for L, before checking whether or not to use rescaled age. A change in the Bayesian Information Criterion (BIC) greater than 10 indicated improved model fit [45]. Model choice was also guided by visual inspection of the centiles against the observed data. Differences between the expected and observed percentage of participants with BMI above select centiles were investigated in the finals models. Common centiles used in the UK (98th, 91st, 50th, 9th, and 2nd) for each study were overlaid in centile and sex-specific plots.
Sex - and study-stratified binary logistic multilevel models (observations at level one nested within individuals at level two) were used to describe weight status trajectories, assuming missing data were at random. Overweight and obesity were combined as there were few cases of overweight or obesity at early sweeps in older studies (e.g., there were only 8 obese boys in the 1946 NSHD at age 7 years). This was necessary because a multilevel logistic regression model may not converge and, if it does converge, is likely to produce unstable estimates when one of the responses (e.g., obesity) includes only a few cases. Thinness could not be considered as a separate group for the same reason, and was recoded as missing because it is a risk factor for health outcomes and thus should not be combined with the normal weight group [46]. The referent group was, therefore, normal weight. The age scale was centred about the mean, and the shape of the trajectory was specified as a restricted cubic spline [47], with knots at equally spaced percentiles of the age distribution [48]. Models were tested with increasingly greater number of knots, with a minimum of three and a maximum equal to the number of sweeps minus one. The best model was selected based on a balance between the lowest BIC, the smallest differences between estimated probabilities and observed prevalence rates (divided by 100) of overweight or obesity, and a trajectory that provided the right degree of smoothing. In some instances it was necessary to remove random effects for the level two parameters (with little between-person variation) to achieve model convergence; no constraints were applied to the random effects variance-covariance matrix. The models were fitted using iterative generalized least squares and first order marginal quasi-likelihood. Trajectories and their 95% confidence intervals (CI) for each study were produced with probability of overweight or obesity on the y-axis and age on the x-axis in sex-specific plots.
The only age when all cohorts had sweeps was at 10 or 11 years, and additional analyses focusing on these data were conducted. Weight, height, and BMI were converted to sex - and age-specific Z-scores according to the UK—World Health Organisation (WHO) chart [49], and cohort-stratified box plots were produced. Because the Z-scores were skewed, Kruskal-Wallis tests with Bonferroni correction were used to test between-study differences.
LMSgrowth was used to compute childhood weight status and Z-scores and LMSchartmaker was used to fit the LMS models (http://www.healthforallchildren.com/). The Stata command runmlwin, which calls on MLwiN for model fitting, was used for the multilevel models [50]. All other analyses were performed in Stata 13 (StataCorp LP: College Station, TX, US).
Results
In total, there were 273,843 BMI observations on 56,632 participants in studies spanning births between 1946–2001 and ages from 2–64 years (Table 1). Median BMI and the prevalence of overweight and obesity generally increased with age after mid-childhood in each study.
Shifts in the Distribution of BMI
The 98th, 91st, and 50th centiles are shown for childhood (ages 2–18 years) in Fig 1 and adulthood (ages 20–64 years) in Fig 2; model estimates and diagnostics can be found in S3–S7 Tables. During childhood, the 50th centiles for all studies lay in the middle of the normal weight range. There was little distinction in each of the centiles between the 1946 NSHD, 1958 NCDS, and 1970 BCS at overlapping ages, but large upward shifts at the 98th and 91st centiles and small upward shifts at the 50th centile were apparent for the 1991 ALSPAC and 2001 MCS. The exception to this pattern is the suggestive evidence that before 4 years of age, the 98th and 91st centiles were actually highest in the 1946 NSHD. During adulthood, separation between the 1946 NSHD, 1958 NCDS, and 1970 BCS centiles occurred, again with the greatest differences observed at the upper end of the BMI distribution. The age when a 50th centile first entered the overweight range was computed to the nearest year, and across the 1946 NSHD, 1958 NCDS, and 1970 BCS this age decreased from 41 to 33 to 30 years in males and 48 to 44 to 41 years in females. During childhood and adulthood, between-study differences in the 9th and 2nd centiles were negligible (S2 Fig and S3 Fig).
Fig. 1. The 98th, 91st, and 50th childhood BMI centiles from sex- and study-stratified LMS models plotted against the IOTF cut-offs. 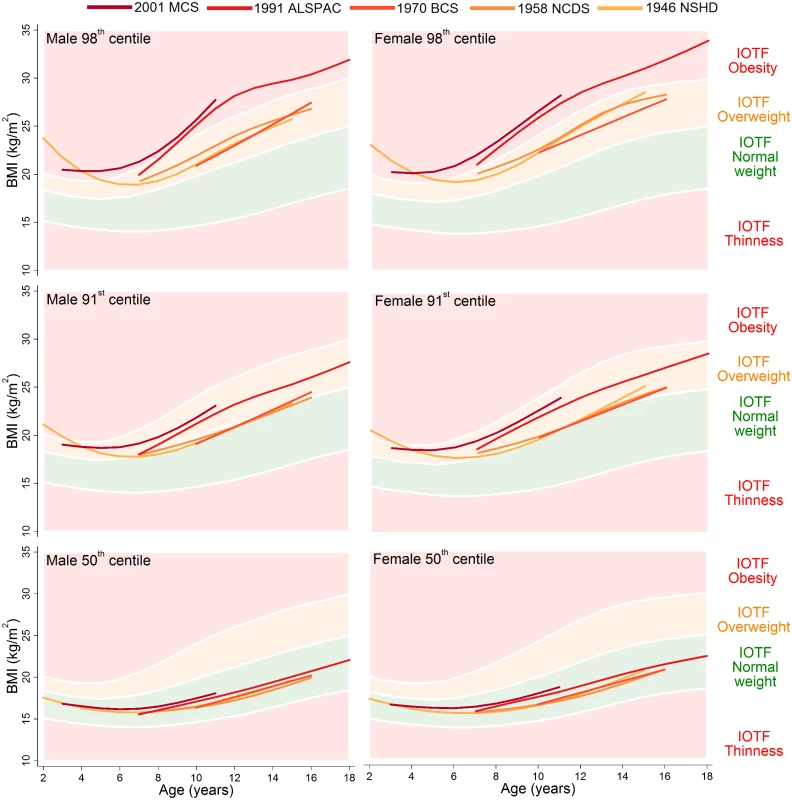
BMI: Body Mass Index, IOTF: International Obesity Task Force, LMS: Lambda-Mu-Sigma, NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study, ALSPAC: Avon Longitudinal Study of Parents and Children, MCS: Millennium Cohort Study. Fig. 2. The 98th, 91st, and 50th adulthood BMI centiles from sex- and study-stratified LMS models plotted against the normal cut-offs. 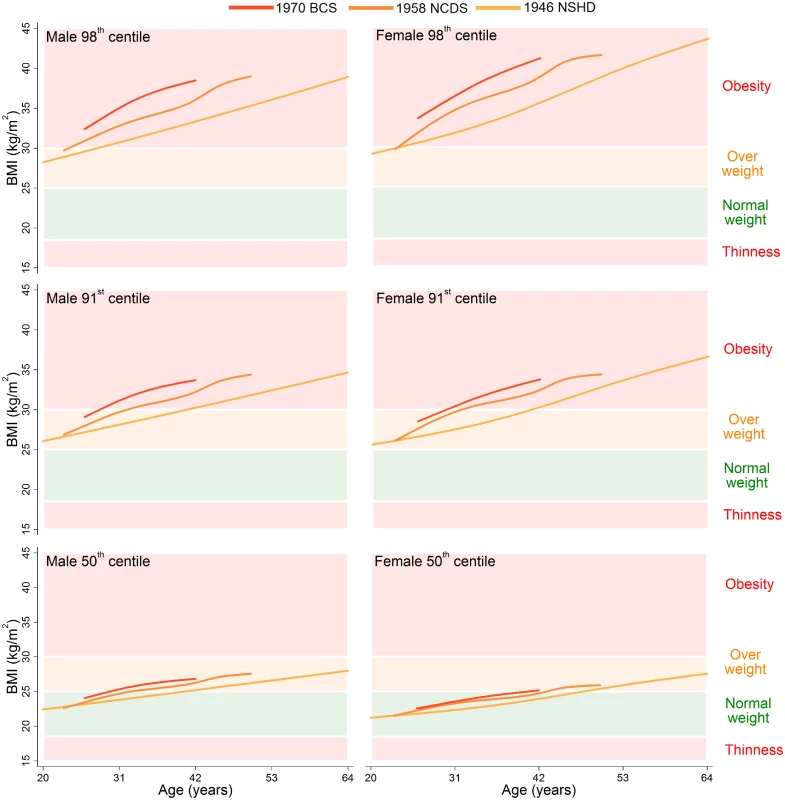
BMI: Body Mass Index, LMS: Lambda-Mu-Sigma, NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study. Shifts in the Development of Overweight or Obesity
Trajectories describing the probability (or multiplied by 100 to get prevalence) of overweight or obesity are shown in Fig 3; model estimates and diagnostics etc. can be found in S8 Table and S9 Table. In the 1946 NSHD, 1958 NCDS, and 1970 BCS, the trajectories were initially similar in childhood at overlapping ages, after which they became steeper and diverged from one another. The probability of overweight or obesity in the 1958 NCDS diverged upward from that in the 1946 NSHD in early adulthood, as indicated by non-overlapping 95% CI (e.g., males at age 27 years: 0.294 [95% CI 0.283, 0.305] in 1958 NCDS; 0.255 [0.238, 0.273] in 1946 NSHD). However, the probability of overweight or obesity in the 1970 BCS diverged upward from that in the 1958 NCDS and 1946 NSHD in late adolescence (e.g., males at age 18 years: 0.138 [0.126, 0.151] in 1970 BCS; 0.113 [0.107, 0.121] in 1958 NCDS). Table 2 provides estimated probabilities at every decade of life in each study; even by 10 years of age, the probabilities in the 1991 ALSPAC and 2001 MCS were between two to three times those in the three older studies (e.g., males: 0.229 [0.219, 0.240] in 2001 MCS; 0.071 [0.065, 0.078] in 1946 NSHD). As shown in Fig 4, box plots revealed that observed median weight and height Z-scores at 10 or 11 years of age, as well as BMI Z-scores, were greater in the 1991 ALSPAC and 2001 MCS compared to the older three cohorts; these comparisons were nominally significant at p < 0.05 (S10 Table). Before age 4 years, the trajectories in Fig 3 suggest that children in the 1946 NSHD actually had the highest risks of overweight or obesity. Sensitivity analyses shown in S4 Fig found similar trajectories for participants with BMI data at all ages.
Tab. 2. Estimated probabilities of overweight or obesity (versus normal weight) from sex- and study-stratified multilevel logistic regression models. 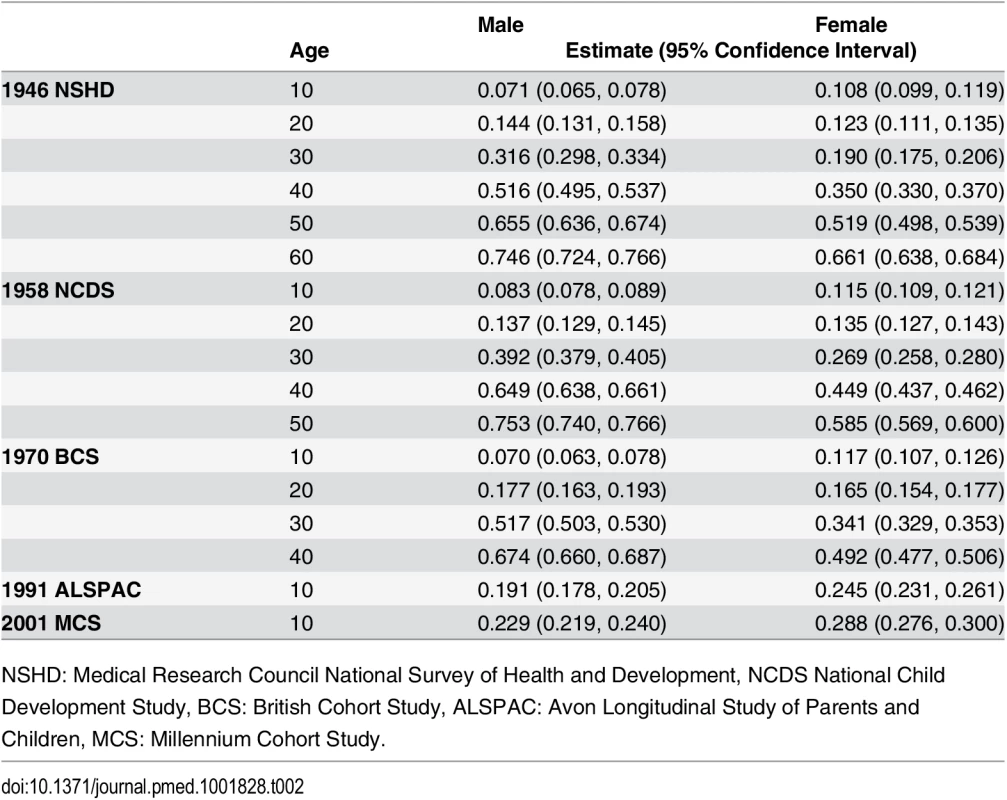
NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study, ALSPAC: Avon Longitudinal Study of Parents and Children, MCS: Millennium Cohort Study. Fig. 3. Trajectories of the probability of overweight or obesity (versus normal weight) from sex- and study-stratified multilevel logistic regression models. 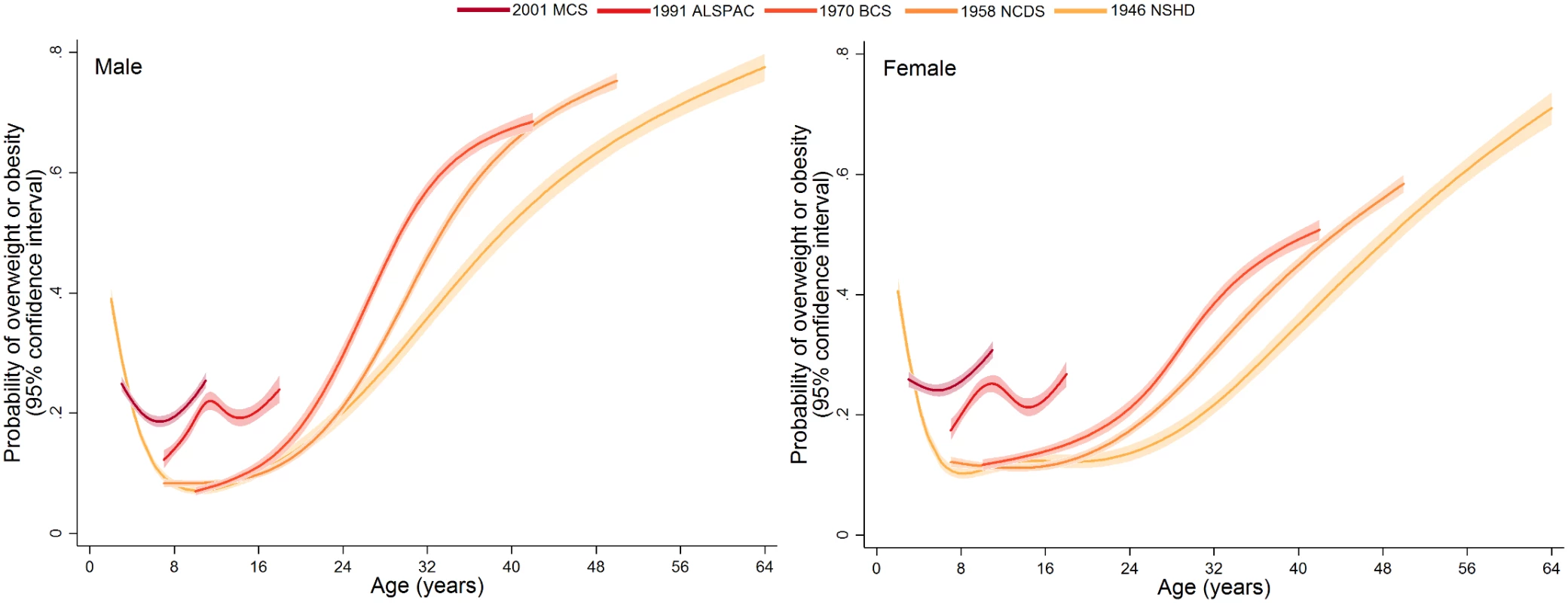
NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study, ALSPAC: Avon Longitudinal Study of Parents and Children, MCS: Millennium Cohort Study. Fig. 4. Study-stratified box plots for height, weight, and BMI Z-scores according to the UK-WHO chart at 10 or 11 years of age. 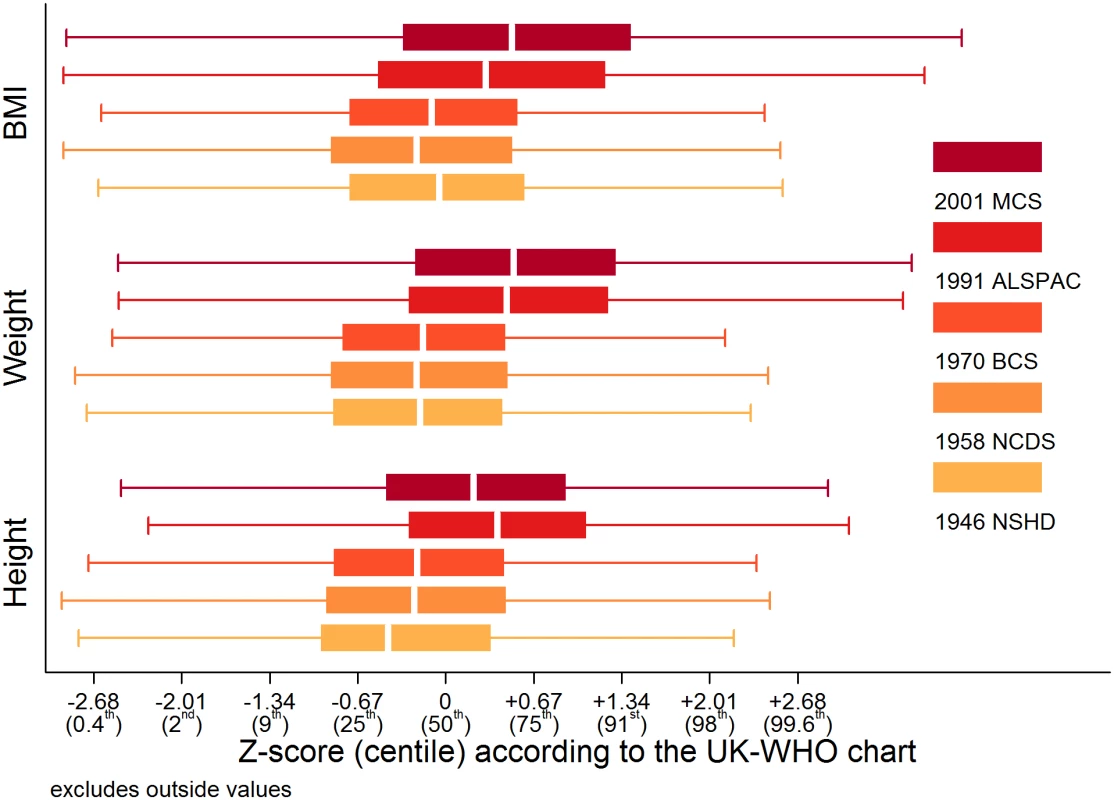
BMI: Body Mass Index, UK-WHO: United Kingdom-World Health Organisation, NSHD: Medical Research Council National Survey of Health and Development, NCDS National Child Development Study, BCS: British Cohort Study, ALSPAC: Avon Longitudinal Study of Parents and Children, MCS: Millennium Cohort Study. Discussion
This study utilised the extensive longitudinal BMI data in the four national representative UK birth cohort studies, plus the 1991 ALSPAC, to investigate shifts over time in the BMI distribution and trajectories of overweight or obesity in a high-income country. The key findings were that (1) between-study differences in BMI occurred at the upper end of the distribution; (2) the median child in each study was normal weight, but the median adult was first overweight at increasingly younger ages; (3) cohorts born before the 1980s demonstrated increases in the probability of overweight or obesity across adulthood, with cohorts born more recently showing rapid increases at earlier ages; and (4) cohorts born after the 1980s already had probabilities of overweight or obesity in childhood that were two to three times greater than those for cohorts born before the 1980s. The childhood data may be reassuring in the sense that BMI values have remained relatively stable over time and within the normal range for the majority of the population. The UK is still, however, in a situation in which many more children are overweight or obese than in previous generations and, if the observed trends in adulthood BMI continue, the majority of children are likely to develop overweight or obesity at some point in their lives and at younger ages than did previous generations.
The main strength of the paper is the thorough analysis of 273,843 BMI observations on 56,632 participants in studies spanning births between 1946–2001 and ages from 2–64 years. No other study has such extensive serial data covering such a wide range of ages and birth years. In terms of weaknesses, (1) it was not possible to model separate trajectories for overweight and obesity; (2) the trajectories were smoothed over age periods in which no sweep took place and thus did not capture local traits, such as a peak during puberty, for some studies; (3) we assume our findings are due to changes in adiposity more so than fat-free mass, but this might not always be the case [51,52]; and (4) by excluding non-white participants, we were not able to consider the extent to which secular trends in obesity might be driven by the changing ethnic composition of the UK. This topic is perhaps more relevant to studies of more recent secular trends, at times when the ethnic composition of the UK has been less stable. Data from the HSE between 1998–2009 suggest that secular trends in overweight or obesity in ethnic minority groups do not follow those of white English children (e.g., obesity rates appear to have plateaued in white English children, but are continuing to rise in black Caribbean children) [53], thereby suggesting that our results are unlikely to be generalizable to other ethnic groups. The measurement protocols for weight and height were not consistent within and between-studies, which could have introduced bias if, for example, self-reported measurements were systemically under or over-reported. The tendency of people with greater BMIs to under-report weight suggests that our results are conservative, if anything [54,55]. The multilevel models were not conditional on any other covariates and therefore made the assumption that trajectories for individuals with missing BMI observations were similar to those whose BMI was observed at all ages. Sensitivity analyses found similar trajectories (compared to those reported in the paper) for participants with BMI data at all ages, thereby providing no evidence to suggest that (1) BMI was not missing at random or (2) there was a “healthy survivor effect” (i.e., those with healthy BMI values were surviving longer and contributing more data at older ages). We cannot be certain that our results are representative of secular trends in obesity development in other settings, although we imagine that similar processes have occurred in most high-income countries.
The observed prevalence rates of overweight or obesity in the 1946 NSHD, 1958 NCDS, and 1970 BCS at the most recent sweeps approximately matched those reported in the 2012 HSE at comparable adulthood ages [2], and the prevalence rates in the 2001 MCS approximately matched those reported in the 2012–2013 NCMP at age 11 years [7]. This suggests that our results are representative of the current epidemic at the national level. While the cross-sectional surveys have been able to stratify results by different divisions of society, such as ethnic group, the present paper was able to describe the age-related processes that have led to the current obesity epidemic. This work builds on the publication of Li et al. that investigated mean BMI trajectories in the 1946 NSHD and 1958 NCDS [21]. Both studies provide evidence that changes in the environment, largely starting in the 1980s, were responsible for increased risk of overweight or obesity mostly starting at 20–30 years of age. By the inclusion of more recently born cohorts, the present paper further demonstrates how children born after the 1980s have increased risk of overweight or obesity (compared to those born before the 1980s) because of earlier exposure to the obesogenic environment. Outside of the UK, this work adds to the large body of literature from studies in diverse settings around the world reporting secular trends toward positive skewing of the BMI distribution at cross-sectional ages [28–33] and the much smaller body of literature, mainly in the US, reporting secular trends in mean BMI trajectories [25–27].
There are two findings that warrant brief discussion in the context of public health implications. The first, that children in the 1946 NSHD appeared to have the highest risks of overweight or obesity before age 4 years, is in agreement with (1) other secular trend studies showing a decline in maximum infant BMI over time and (2) epidemiological studies showing that low rather than high infant BMI is deleterious in older cohorts [27,56,57]. The second, that at 10 or 11 years of age modern-day children appeared to be taller as well as heavier than children in previous generation, is in agreement with literature showing obese children to be temporarily taller than their non-obese peers at pubertal ages due to an advanced pace of development [58–60]. Clinical assessment of overweight or obesity risk in puberty might, therefore, consider height as well as BMI.
Since the inception of the obesity epidemic, the UK has not matched gains made in childhood and young adulthood mortality by comparable European Union member states [61]. Our results suggest that more recently born people accumulate greater exposure to overweight or obesity across their lives, and there is a large body of literature linking such “accumulation of risk” to a wide range of health outcomes, including CHD, type 2 diabetes, hypertension, and osteoarthritis [14–17,62]. Understanding the characteristics of individuals who maintain a normal BMI throughout their lives may also help with designing novel interventions and clinical recommendations on weight maintenance. Currently, there is a dearth of research and trials in this area [63]. It is obviously important to intervene early to prevent overweight or obesity, but there is also evidence to suggest that losing weight at any age in early to mid-adulthood is beneficial, for example to vascular health [64]. Despite the importance of life-course adiposity in disease processes being well-documented, future research is warranted to investigate the extent to which the documented shifts in the BMI distribution and overweight or obesity trajectories, which have resulted in greater accumulation of risk, have contributed to changes over time in adiposity-related morbidity and mortality. The plateauing of adulthood type 2 diabetes rates in the US between 2008–2012 might reflect a lagged effect of there being no change in the prevalence of obesity since 2003–2004, for example [65,66]. Changes in obesity prevalence in low - and middle-income countries may impact differently on rates on adiposity-related diseases than those in high-income countries because of contextual differences like access to health care and medication. Indeed, the availability of medication in high-income countries is one explanation for why CHD rates have declined in most countries over the past 30 years, in spite of adulthood obesity rates continuing to rise [67]. Nevertheless, being overweight or obese is bad for health at the individual level. Investigation of which changes over time in the environment have caused the secular trends documented in the present paper is also needed to further knowledge on the aetiology of overweight or obesity. In the absence of a series of national birth cohort studies, comparable work in other settings may be possible through exploitation of routine anthropometric data and record linkage, as demonstrated by publications using the Copenhagen School Health Records Register [68,69].
In conclusion, our results demonstrate how a population in a high-income country is experiencing greater risk of overweight or obesity due to secular trends at increasingly younger ages toward (1) positive skewing of the BMI distribution and (2) life-course overweight or obesity trajectories that are generally steeper and higher (i.e., more deleterious). If the secular trends persist, then modern-day and successive generations of children will accumulate greater overweight or obesity exposure across their lives than previous generations. Given our knowledge that such accumulation of exposure increases risk for diseases like CHD and type 2 diabetes, overweight and obesity will have severe public health consequences in decades to come, in the absence of effective intervention.
Supporting Information
Zdroje
1. McPherson K, Marsh T, Brown M (2007) Foresight. Tackling obesities: Future choices—Modelling future trends in obesity and their impact on health. London, UK: Government Office for Science. doi: 10.1093/heapro/dat007 23392756
2. Craig R, Mindell J (2013) Health Survey for England 2012. London, UK: The Information Centre.
3. Department of Health Social Services and Public Safety (2012) Health Survey Northern Ireland: First Results from the 2011/12 Survey. Belfast, Northern Ireland: Public Health Information and Research Branch.
4. The Scottish Government (2013) The Scottish Health Survey 2012, Volume 1: Main Report. Edinburgh, Scotland: The Scottish Government.
5. Welsh Government (2013) The Welsh Health Survey, 2012. Cardiff, Wales: The Welsh Government. http://gov.wales/docs/statistics/2013/130911-welsh-health-survey-2012-en.pdf
6. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384 : 766–781. doi: 10.1016/S0140-6736(14)60460-8 24880830
7. The Health and Social Care Information Centre (2013) The National Child Measurement Programme: England, 2012/13 school year. London, UK: The Health and Social Care Information Centre.
8. Herouvi D, Karanasios E, Karayianni C, Karavanaki K (2013) Cardiovascular disease in childhood: the role of obesity. Eur J Pediatr 172 : 721–732. doi: 10.1007/s00431-013-1932-8 23340698
9. Adair LS, Gordon-Larsen P, Du SF, Zhang B, Popkin BM (2014) The emergence of cardiometabolic disease risk in Chinese children and adults: consequences of changes in diet, physical activity and obesity. Obes Rev 15 Suppl 1 : 49–59. doi: 10.1111/obr.12123 24341758
10. Basu S, Vellakkal S, Agrawal S, Stuckler D, Popkin B, et al. (2014) Averting obesity and type 2 diabetes in India through sugar-sweetened beverage taxation: an economic-epidemiologic modeling study. PLoS Med 11: e1001582. doi: 10.1371/journal.pmed.1001582 24409102
11. Eisenmann JC (2003) Secular trends in variables associated with the metabolic syndrome of North American children and adolescents: a review and synthesis. Am J Hum Biol 15 : 786–794. 14595870
12. Howe LD, Chaturvedi N, Lawlor DA, Ferreira DL, Fraser A, et al. (2014) Rapid increases in infant adiposity and overweight/obesity in childhood are associated with higher central and brachial blood pressure in early adulthood. J Hypertens 32 : 1789–1796. doi: 10.1097/HJH.0000000000000269 25023150
13. Howe LD, Tilling K, Benfield L, Logue J, Sattar N, et al. (2010) Changes in ponderal index and body mass index across childhood and their associations with fat mass and cardiovascular risk factors at age 15. PLoS ONE 5: e15186. doi: 10.1371/journal.pone.0015186 21170348
14. Silverwood RJ, Pierce M, Hardy R, Thomas C, Ferro C, et al. (2013) Early-life overweight trajectory and CKD in the 1946 British birth cohort study. American Journal of Kidney Diseases 62 : 276–284. doi: 10.1053/j.ajkd.2013.03.032 23714172
15. Silverwood RJ, Pierce M, Thomas C, Hardy R, Ferro C, et al. (2013) Association between younger age when first overweight and increased risk for CKD. J Am Soc Nephrol 24 : 813–821. doi: 10.1681/ASN.2012070675 23559581
16. Wills AK, Black S, Cooper R, Coppack RJ, Hardy R, et al. (2012) Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis 71 : 655–660. doi: 10.1136/ard.2011.154021 21979003
17. Wills AK, Hardy RJ, Black S, Kuh DJ (2010) Trajectories of overweight and body mass index in adulthood and blood pressure at age 53: the 1946 British birth cohort study. Journal of Hypertension 28 : 679–686. doi: 10.1097/HJH.0b013e328335de7b 20042875
18. Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, et al. (2011) Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 8: e1000440. doi: 10.1371/journal.pmed.1000440 21695075
19. Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311 : 806–814. doi: 10.1001/jama.2014.732 24570244
20. Power C, Kuh D, Morton S (2013) From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu Rev Public Health 34 : 7–28. doi: 10.1146/annurev-publhealth-031912-114423 23514315
21. Li L, Hardy R, Kuh D, Lo Conte R, Power C (2008) Child-to-adult body mass index and height trajectories: a comparison of 2 British birth cohorts. Am J Epidemiol 168 : 1008–1015. doi: 10.1093/aje/kwn227 18801885
22. Hulman A, Tabak AG, Nyari TA, Vistisen D, Kivimaki M, et al. (2014) Effect of secular trends on age-related trajectories of cardiovascular risk factors: the Whitehall II longitudinal study 1985–2009. Int J Epidemiol 43 : 866–877. doi: 10.1093/ije/dyt279 24464190
23. Peckham CS, Stark O, Simonite V, Wolff OH (1983) Prevalence of obesity in British children born in 1946 and 1958. Br Med J (Clin Res Ed) 286 : 1237–1242. 6404405
24. Shaw RJ, Green MJ, Popham F, Benzeval M (2014) Differences in adiposity trajectories by birth cohort and childhood social class: evidence from cohorts born in the 1930s, 1950s and 1970s in the west of Scotland. J Epidemiol Community Health 68 : 550–556. doi: 10.1136/jech-2013-203551 24502886
25. Demerath EW, Li J, Sun SS, Chumlea WC, Remsberg KE, et al. (2004) Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. American Journal of Clinical Nutrition 80 : 441–446. 15277168
26. Jaacks LM, Gordon-Larsen P, Mayer-Davis EJ, Adair LS, Popkin B (2013) Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: the China Health and Nutrition Survey. International Journal of Epidemiology 42 : 828–837. doi: 10.1093/ije/dyt052 23771721
27. Johnson W, Soloway LE, Erickson D, Choh AC, Lee M, et al. (2012) A changing pattern of childhood BMI growth during the 20th century: 70 y of data from the Fels Longitudinal Study. Am J Clin Nutr 95 : 1136–1143. doi: 10.3945/ajcn.111.022269 22418089
28. Bjornelv S, Lydersen S, Mykletun A, Holmen TL (2007) Changes in BMI-distribution from 1966–69 to 1995–97 in adolescents. The Young-HUNT study, Norway. BMC Public Health 7 : 279. 17916233
29. Eriksson M, Rasmussen F, Nordqvist T (2005) Changes in shape and location of BMI distributions of Swedish children. Acta Paediatrica 94 : 1558–1565. 16303694
30. Komlos J, Brabec M (2011) The trend of BMI values of US adults by deciles, birth cohorts 1882–1986 stratified by gender and ethnicity. Econ Hum Biol 9 : 234–250. doi: 10.1016/j.ehb.2011.03.005 21561815
31. Staub K, Ruhli FJ, Woitek U, Pfister C (2010) BMI distribution/social stratification in Swiss conscripts from 1875 to present. European Journal of Clinical Nutrition 64 : 335–340. doi: 10.1038/ejcn.2010.7 20160753
32. Walls HL, Wolfe R, Haby MM, Magliano DJ, de Courten M, et al. (2010) Trends in BMI of urban Australian adults, 1980–2000. Public Health Nutr 13 : 631–638. doi: 10.1017/S1368980009991455 19772687
33. Razak F, Corsi DJ, Subramanian SV (2013) Change in the body mass index distribution for women: analysis of surveys from 37 low - and middle-income countries. PLoS Med 10: e1001367. doi: 10.1371/journal.pmed.1001367 23335861
34. Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, et al. (2011) Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol 40: e1–9. doi: 10.1093/ije/dyq231 21345808
35. Wadsworth M, Kuh D, Richards M, Hardy R (2006) Cohort Profile: The 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 35 : 49–54. 16204333
36. Power C, Elliott J (2006) Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol 35 : 34–41. 16155052
37. Elliott J, Shepherd P (2006) Cohort profile: 1970 British Birth Cohort (BCS70). Int J Epidemiol 35 : 836–843. 16931528
38. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, et al. (2013) Cohort Profile: the 'children of the 90s'—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 42 : 111–127. doi: 10.1093/ije/dys064 22507743
39. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, et al. (2013) Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 42 : 97–110. doi: 10.1093/ije/dys066 22507742
40. Golding J, Pembrey M, Jones R (2001) ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 15 : 74–87. 11237119
41. Hansen K (2012) Millennium Cohort Study first, second, third and fourth surveys: a guide to the datasets 6th edn. London, UK: Centre for Longitudinal Studies, University of London.
42. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320 : 1240–1243. 10797032
43. Cole TJ, Flegal KM, Nicholls D, Jackson AA (2007) Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 335 : 194. 17591624
44. Cole TJ, Green PJ (1992) Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11 : 1305–1319. 1518992
45. Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6 : 461–464.
46. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373 : 1083–1096. doi: 10.1016/S0140-6736(09)60318-4 19299006
47. Gasser T, Engel J, Seifert B (1993) Nonparametric function estimation. In: Rao C, editor. Handbook of statistics. Amsterdam, The Netherlands: Elsevier Science. pp. 423–465.
48. Harrell FJ (2001) Regression modeling strategies. New York, NY, USA: Springer Publishing Company.
49. Wright CM, Williams AF, Elliman D, Bedford H, Birks E, et al. (2010) Using the new UK-WHO growth charts. BMJ 340: c1140. doi: 10.1136/bmj.c1140 20231247
50. Leckie G, Charlton C (2012) runmlwin: a program to run the MLwiN multilevel modeling software from within Stata. Journal of Statistical Software 52 : 1–40.
51. Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, et al. (2006) Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 117: e487–495. 16510627
52. Johnson W, Chumlea WC, Czerwinski SA, Demerath EW (2013) Secular trends in the fat and fat-free components of body mass index in children aged 8–18 years born 1958–1995. Ann Hum Biol 40 : 107–110. doi: 10.3109/03014460.2012.720710 23013058
53. Karlsen S, Morris S, Kinra S, Vallejo-Torres L, Viner RM (2014) Ethnic variations in overweight and obesity among children over time: findings from analyses of the Health Surveys for England 1998–2009. Pediatr Obes 9 : 186–196. doi: 10.1111/j.2047-6310.2013.00159.x 23554401
54. Crawley HF, Portides G (1995) Self-reported versus measured height, weight and body mass index amongst 16–17 year old British teenagers. Int J Obes Relat Metab Disord 19 : 579–584. 7489030
55. Shields M, Connor Gorber S, Tremblay MS (2008) Estimates of obesity based on self-report versus direct measures. Health Rep 19 : 61–76. 18642520
56. Johnson W, Choh AC, Lee M, Towne B, Czerwinski SA, et al. (2013) Characterization of the infant BMI peak: sex differences, birth year cohort effects, association with concurrent adiposity, and heritability. Am J Hum Biol 25 : 378–388. doi: 10.1002/ajhb.22385 23606227
57. Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, et al. (2009) Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 33 : 866–877. doi: 10.1038/ijo.2009.102 19506565
58. Ahmed ML, Ong KK, Dunger DB (2009) Childhood obesity and the timing of puberty. Trends Endocrinol Metab 20 : 237–242. doi: 10.1016/j.tem.2009.02.004 19541497
59. Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, et al. (2012) Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes (Lond) 36 : 535–541. doi: 10.1038/ijo.2011.238 22124455
60. Metcalf BS, Hosking J, Fremeaux AE, Jeffery AN, Voss LD, et al. (2011) BMI was right all along: taller children really are fatter (implications of making childhood BMI independent of height) EarlyBird 48. Int J Obes (Lond) 35 : 541–547. doi: 10.1038/ijo.2010.258 21304486
61. Viner RM, Hargreaves DS, Coffey C, Patton GC, Wolfe I (2014) Deaths in young people aged 0–24 years in the UK compared with the EU15+ countries, 1970–2008: analysis of the WHO Mortality Database. Lancet 384 : 880–892. doi: 10.1016/S0140-6736(14)60485-2 24929452
62. Park MH, Sovio U, Viner RM, Hardy RJ, Kinra S (2013) Overweight in childhood, adolescence and adulthood and cardiovascular risk in later life: pooled analysis of three british birth cohorts. PLoS ONE 8: e70684. doi: 10.1371/journal.pone.0070684 23894679
63. Peirson L, Douketis J, Ciliska D, Fitzpatrick-Lewis D, Ali MU, et al. (2014) Prevention of overweight and obesity in adult populations: a systematic review. CMAJ Open 2: E268–272. doi: 10.9778/cmajo.20140019 25485253
64. Charakida M, Khan T, Johnson W, Finer N, Woodside J, et al. (2014) Lifelong patterns of BMI and cardiovascular phenotype in individuals aged 60–64 years in the 1946 British birth cohort study: an epidemiological study. Lancet Diabetes Endocrinol 2 : 648–654. doi: 10.1016/S2213-8587(14)70103-2 24856161
65. Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, et al. (2014) Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 312 : 1218–1226. doi: 10.1001/jama.2014.11494 25247518
66. Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307 : 491–497. doi: 10.1001/jama.2012.39 22253363
67. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, et al. (2014) Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation 129 : 1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042 24573352
68. Ajslev TA, Angquist L, Silventoinen K, Gamborg M, Allison DB, et al. (2012) Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Front Genet 3 : 125. doi: 10.3389/fgene.2012.00125 23056005
69. Bua J, Olsen LW, Sorensen TI (2007) Secular trends in childhood obesity in Denmark during 50 years in relation to economic growth. Obesity (Silver Spring) 15 : 977–985. 17426333
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2015 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- The Tobacco Endgame: Is It Possible?
- Screening and Treating UN Peacekeepers to Prevent the Introduction of Artemisinin-Resistant Malaria into Africa
- The Health System and Population Health Implications of Large-Scale Diabetes Screening in India: A Microsimulation Model of Alternative Approaches
- Seasonal Influenza Vaccination for Children in Thailand: A Cost-Effectiveness Analysis
- Healthy Eating and Risks of Total and Cause-Specific Death among Low-Income Populations of African-Americans and Other Adults in the Southeastern United States: A Prospective Cohort Study
- An Unsupported Preference for Intravenous Antibiotics
- Use of Fixed Dose Combination (FDC) Drugs in India: Central Regulatory Approval and Sales of FDCs Containing Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Metformin, or Psychotropic Drugs
- A Global Biomedical R&D Fund and Mechanism for Innovations of Public Health Importance
- Setting International Standards for the Management of Public Health Pesticides
- How Has the Age-Related Process of Overweight or Obesity Development Changed over Time? Co-ordinated Analyses of Individual Participant Data from Five United Kingdom Birth Cohorts
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Use of Fixed Dose Combination (FDC) Drugs in India: Central Regulatory Approval and Sales of FDCs Containing Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Metformin, or Psychotropic Drugs
- How Has the Age-Related Process of Overweight or Obesity Development Changed over Time? Co-ordinated Analyses of Individual Participant Data from Five United Kingdom Birth Cohorts
- A Global Biomedical R&D Fund and Mechanism for Innovations of Public Health Importance
- An Unsupported Preference for Intravenous Antibiotics
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



