-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
All gammaherpesviruses encode a major glycoprotein homologous to the Epstein-Barr virus gp350. These glycoproteins are often involved in cell binding, and some provide neutralization targets. However, the capacity of gammaherpesviruses for long-term transmission from immune hosts implies that in vivo neutralization is incomplete. In this study, we used Bovine Herpesvirus 4 (BoHV-4) to determine how its gp350 homolog - gp180 - contributes to virus replication and neutralization. A lack of gp180 had no impact on the establishment and maintenance of BoHV-4 latency, but markedly sensitized virions to neutralization by immune sera. Antibody had greater access to gB, gH and gL on gp180-deficient virions, including neutralization epitopes. Gp180 appears to be highly O-glycosylated, and removing O-linked glycans from virions also sensitized them to neutralization. It therefore appeared that gp180 provides part of a glycan shield for otherwise vulnerable viral epitopes. Interestingly, this O-glycan shield could be exploited for neutralization by lectins and carbohydrate-specific antibody. The conservation of O-glycosylation sites in all gp350 homologs suggests that this is a general evasion mechanism that may also provide a therapeutic target.
Published in the journal: Antibody Evasion by a Gammaherpesvirus O-Glycan Shield. PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002387
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002387Summary
All gammaherpesviruses encode a major glycoprotein homologous to the Epstein-Barr virus gp350. These glycoproteins are often involved in cell binding, and some provide neutralization targets. However, the capacity of gammaherpesviruses for long-term transmission from immune hosts implies that in vivo neutralization is incomplete. In this study, we used Bovine Herpesvirus 4 (BoHV-4) to determine how its gp350 homolog - gp180 - contributes to virus replication and neutralization. A lack of gp180 had no impact on the establishment and maintenance of BoHV-4 latency, but markedly sensitized virions to neutralization by immune sera. Antibody had greater access to gB, gH and gL on gp180-deficient virions, including neutralization epitopes. Gp180 appears to be highly O-glycosylated, and removing O-linked glycans from virions also sensitized them to neutralization. It therefore appeared that gp180 provides part of a glycan shield for otherwise vulnerable viral epitopes. Interestingly, this O-glycan shield could be exploited for neutralization by lectins and carbohydrate-specific antibody. The conservation of O-glycosylation sites in all gp350 homologs suggests that this is a general evasion mechanism that may also provide a therapeutic target.
Introduction
Epstein-Barr virus (EBV) and Kaposi’s Sarcoma Associated Herpesvirus (KSHV) are DNA tumor viruses that provide risk factors for Burkitt's lymphoma, Hodgkin's lymphoma, nasopharyngeal carcinoma, Kaposi's Sarcoma and post-transplant lymphoproliferative disease [1]–[2]. EBV infection has also been associated with multiple sclerosis [3]–[4]. Healthy carriers consistently shed virus in saliva [5] that infects naïve individuals [6]–[7] despite being exposed to virus-specific antibody [8]–[9]. This lack of neutralization contrasts completely with non-persistent mucosal infections such as that of poliovirus [10]–[11], and implies that gammaherpesviruses have evolved specific antibody evasion mechanisms.
Neutralizing antibodies generally target epitopes involved in virion binding or membrane fusion [12]. Targeting of the gB/gH/gL [13]–[16] fusion machinery [17]–[18] seems to be limited by a paucity of good targets [19] and poor immunogenicity [20]. Therefore most studies have looked at binding. The EBV gp350 is an abundant component of the virion envelope that binds to CD21 on B cells [21]–[22] and is a target for antibodies that neutralize B cell infection [23]. However, while EBV lacking gp350 is poorly infectious for B cells [24]–[25], it infects CD21-negative epithelial cells better than the wild-type [25], and these may provide a primary target for virions entering naive hosts. Epithelial infection can even be enhanced by gp350-specific antibodies [26]. Therefore the relationship between EBV transmission, gp350, and gp350-specific antibodies needs further exploration, particularly as gp350 is a candidate EBV vaccine [27]–[28].
Our understanding of EBV and KSHV is limited by their narrow species tropisms. Related animal viruses are therefore an important source of information. Two of the best established experimental models are provided by Murid herpesvirus 4 (MuHV-4) [29] and Bovine herpesvirus 4 (BoHV-4) [30]–[31]. Their homologs of gp350 are gp150 in MuHV-4 [32], encoded by M7, and gp180 in BoHV-4 [33], encoded by Bo10. While these proteins are diverse in sequence, they seem to be related in function, being involved in both binding to a cellular receptor and in blocking the infection of cells that do not express this receptor [25], [32]–[33]. It has been proposed that the receptor interaction displaces each homolog to reveal other glycoproteins involved in entry. Thus, a non-essential glycoprotein [24], [32]–[33] could hide from neutralization some critical epitopes on cell-free virions.
To date, the in vivo function of gp350 homologs has only been investigated with MuHV-4. Surprisingly, gp150-deficient viruses showed only a transient lag in lytic replication in vivo and established normal levels of latency [32]. Gp150 is the most immunogenic MuHV-4 glycoprotein and anti-gp150 antibodies play a predominant role in driving Fc receptor-dependent infection [20]. While gp150 does not have an obvious direct role in cell-binding, BoHV-4 lacking gp180 displays a binding deficit [33]. Therefore this protein may be more closely analogous to gp350 and the KSHV K8.1 than is gp150. Here we investigated the consequences of gp180 deletion for BoHV-4 replication in vivo and neutralization. An important gp180 function seemed to be to block the binding to virions of antibodies that would otherwise neutralize.
Results
Generation of a Bo10 nonsense BoHV-4 mutant
We previously described a BoHV-4 strain in which the entire Bo10 ORF was replaced by an eGFP expression cassette [33]. Since expression cassettes can cause in vivo attenuation, we also generated a second Bo10 mutant virus, in which stop codons terminated Bo10 translation 7 amino acids before the end of its predicted signal sequence without any associated deletion (Figure 1A). A revertant strain, called Bo10 STOP Rev, was finally constructed to validate the Bo10 STOP mutant. The predicted molecular structures of the recombinant strains were confirmed by EcoRI restriction mapping and Southern blotting (Figure 1B), and further by DNA sequencing. Immunoblotting with an anti-Bo10-c15 rabbit polyserum [33] established that the Bo10 mutant virions lacked gp180 (Figure 1C).
Fig. 1. Generation of a Bo10 STOP BoHV-4 mutant. 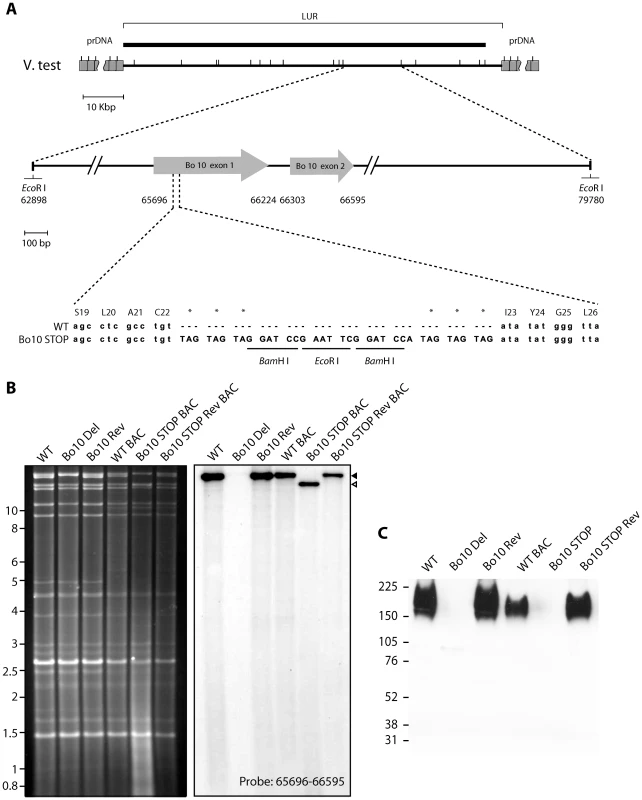
A. Schematic representation of the strategy followed to produce the recombinant BoHV-4 strains. The Bo10 STOP BoHV-4 mutant was derived from a cloned BoHV-4 BAC by galK-counterselection method. The Bo10 coding sequence was disrupted by inserting stop codons near the end of the coding sequence for its predicted signal peptide (Bo10 STOP). The mutation incorporated two BamHI and one EcoRI restriction sites. B. Verification of the molecular structure. Viral DNA was digested with EcoRI, resolved by agarose gel electrophoresis, and hybridized with a 32P-labeled probe, corresponding to nucleotides 65,696–66,595 of the BoHV-4 V.test strain genome. Black triangle shows the restriction fragment that contains the WT Bo10 gene. The Bo10 Del fragment is higher due to eGFP insertion [33], however, it is much less visible due to deletion of most of the ORF. The 16,882-bp wild-type (WT) band becomes 14,033-bp (open triangle) for the Bo10 STOP mutant. The 2,885 bp band is not visible because it only hybridizes with a few nucleotides of the probe. In WT BAC, Bo10 STOP BAC and Bo10 STOP BAC Rev strains, the 4,870 bp band becomes 9,964 bp and 3,416 bp due to BAC cassette insertion as described previously [30]. Marker sizes in Kbp are indicated on the left. C. Detection of the Bo10 encoded gp180 protein by the anti-Bo10-c15 serum. Purified virions (5*105 virions per lane) were subjected to western blotting with anti-Bo10-c15 serum as described in the Material and Methods. The position of a molecular mass (MM) standard (in kDa) is shown on the left. Dissemination of Bo10 - mutants in vivo
To investigate the importance of Bo10 in vivo, we infected rabbits with the different viral strains as described in the Material and Methods. No rabbit showed clinical disease or noticeable pathology at necropsy 64 days post-inoculation. Host colonization was assayed by quantitative PCR of DNA from peripheral blood mononuclear cells (PBMC) over time (Figure 2A and B) and from the spleens at 64 days post-inoculation (Figure 2C and D). The Bo10 mutants showed no deficit. We further performed infectious center assays on spleen cells from the WT and Bo10 STOP infected rabbits. Viral plaques were observed in all samples (Figure 2E). No preformed infectious virus was detected in the equivalent freeze-thawed samples (data not shown), so this was latent infection. Thus, we detected no difference in acute replication, latency establishment or reactivation of Bo10-deficient mutants compared to WT or revertant strains.
Fig. 2. In vivo persistence of Bo10- mutants. 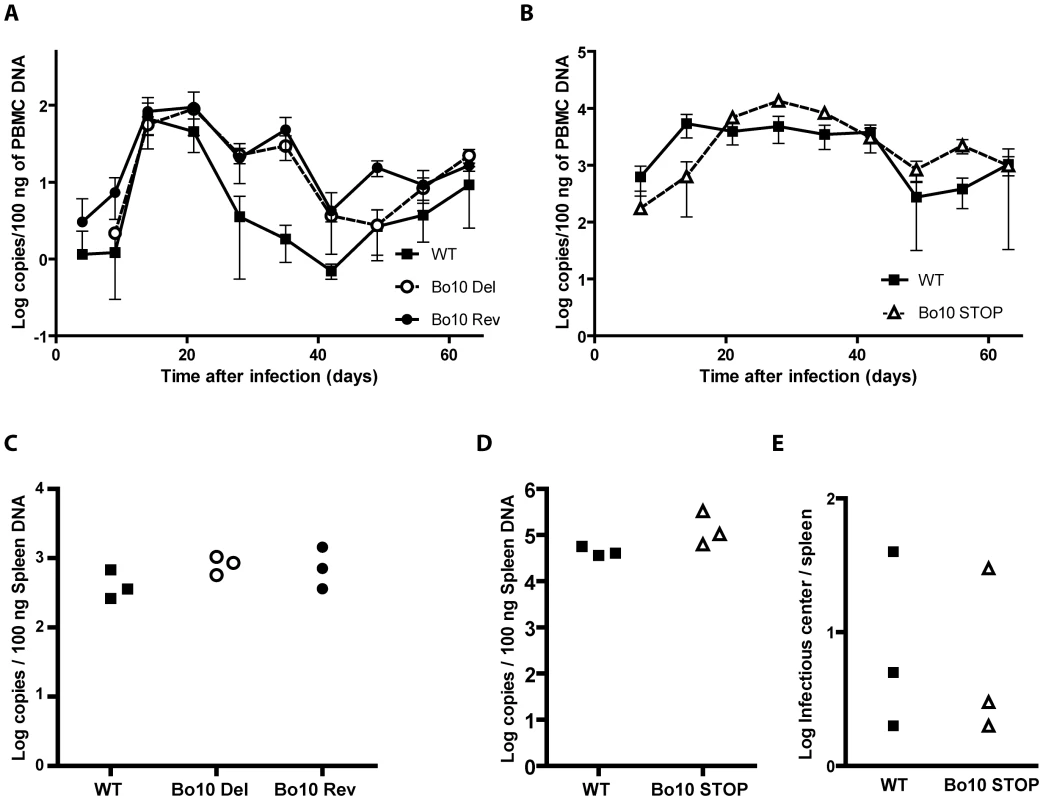
Groups consisting of 3 rabbits were mock-infected or infected with 108 PFU of BoHV-4 WT V.test, Bo10 Del and Bo10 Rev strains (A and C) or infected with 107 PFU of BoHV-4 WT V.test and Bo10 STOP excised strains (B, D and E). A–D. Real-Time PCR relative quantification of BoHV-4 genomes. DNA was extracted from the PBMC (A and B) at the different times post-inoculation and from the spleen (C and D) 64 days post-inoculation. Data are expressed as the number of BoHV-4 ORF8 gene copies per 100 ng of total DNA. In A and B, the data presented are the average ± SEMs and were analyzed by 2ways ANOVA and Bonferroni posttests. In C and D, each point shows the genome copies for one rabbit. The data were analyzed by 1way ANOVA and Bonferroni posttests or Student t-test. No significative difference was observed. E. Spleens from the different rabbits were analyzed individually for reactivable BoHV-4 by infectious-center assay. Each point shows the infectious centers for one rabbit. The data were analyzed by Student t-test. No significative difference was observed. Increased susceptibility of Bo10 - BoHV-4 to serum neutralization
While pathogenesis assays are a useful measure of viral fitness, they do not measure all viral functions. In particular, virion susceptibility to neutralization [33] might not be measured because intra-host dissemination depends mainly on cell/cell virus spread and latency-associated cell proliferation. We therefore further compared the sensitivity of BoHV-4 WT, Bo10 Del, Bo10 Rev and Bo10 STOP strains to neutralization by sera of rabbits infected with the BoHV-4 V.test strain (Figure 3). WT and Bo10 Rev virions were poorly neutralized. Bo10 Del and Bo10 STOP virions were neutralized much better. In particular, complete neutralization was now possible. Neutralization experiments with eGFP expressing viruses on different cell types confirmed this result, with gp180-deficient virions showing increased sensitivity to neutralization by anti-BoHV-4 serum compared to WT virions (Figure S1). Thus gp180 seemed to limit virion neutralization.
Fig. 3. Neutralization of the BoHV-4 Bo10 mutants. 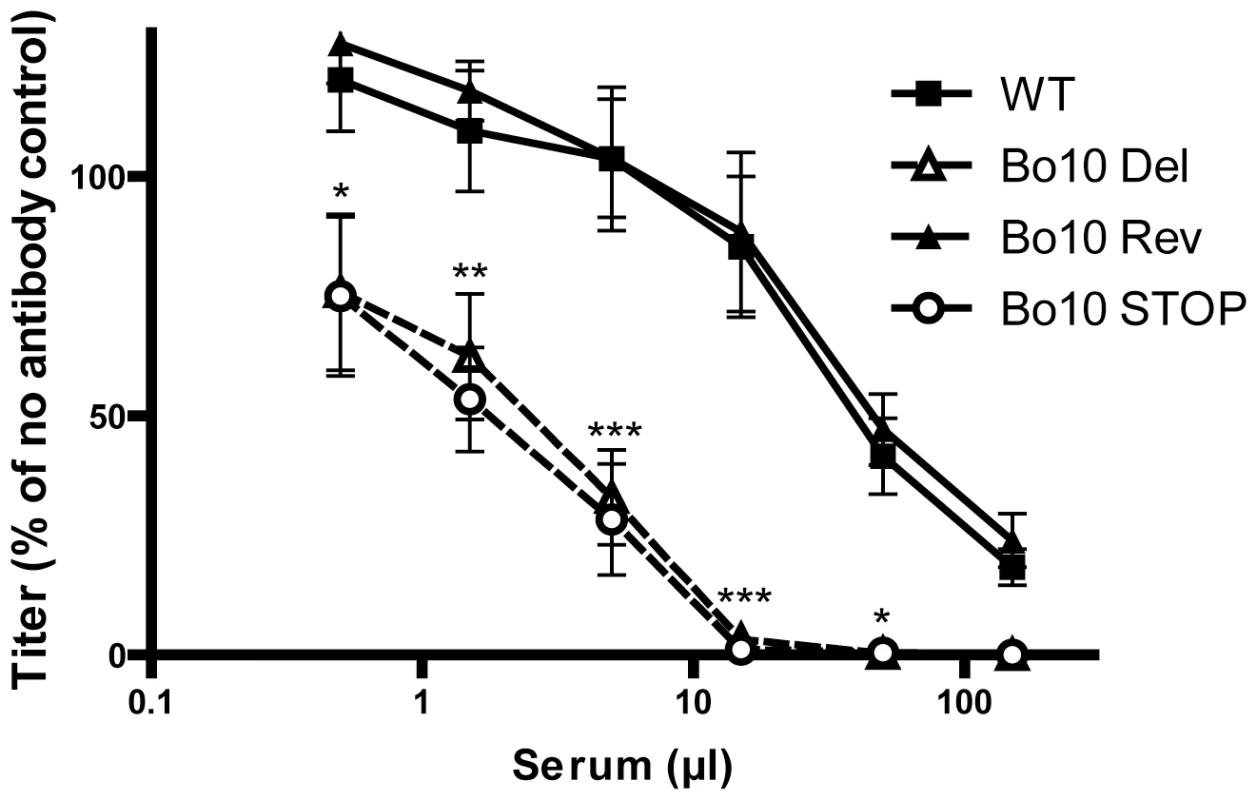
BoHV-4 WT V.test, Bo10 Del, Bo10 Rev and Bo10 STOP virions were incubated with sera of 4 different rabbits infected with BoHV-4 V.test strain. After incubation (2 h, 37°C) the viruses were plaque assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. The data presented are the average ± SEMs for 4 measurements and were analyzed by 2way ANOVA and Bonferroni posttests, * p<0.05, ** p<0.01, *** p<0.001. Bo10 sequence variation
In order to understand how gp180 might protect virion against neutralization, we compared the Bo10 genes of different BoHV-4 isolates [34]. All showed consensus splice donor and acceptor sites that are used in the BoHV-4 V.test strain to generate gp180 [33]. Nucleotide sequences comparison across the entire open reading frames revealed up to 15% inter-strain divergence (Table S1). Amino acid divergence between American-European and African strains reached 39%, mostly in the N-terminal half of the protein ectodomain (Figure 4A). By comparison, gB, gH and gL differ by <2% between KSHV strains [35]; gB differs by <2% between OHV-2 strains [36]; and ORF71 differs by only 5% between BoHV-4 strains. All gp180s were extremely rich in serine and threonine residues, which accounted for 54.3 +/ − 0.6% of each mature ectodomain (Figure 4A). Asparagine residues accounted for a further 8.0 +/ − 0.6%. Therefore, a conserved feature seemed to be extensive O - and N-linked glycosylation (Figure 4A–C) [37].
Fig. 4. Sequence analysis of the Bo10 encoded proteins of nine BoHV-4 strains. 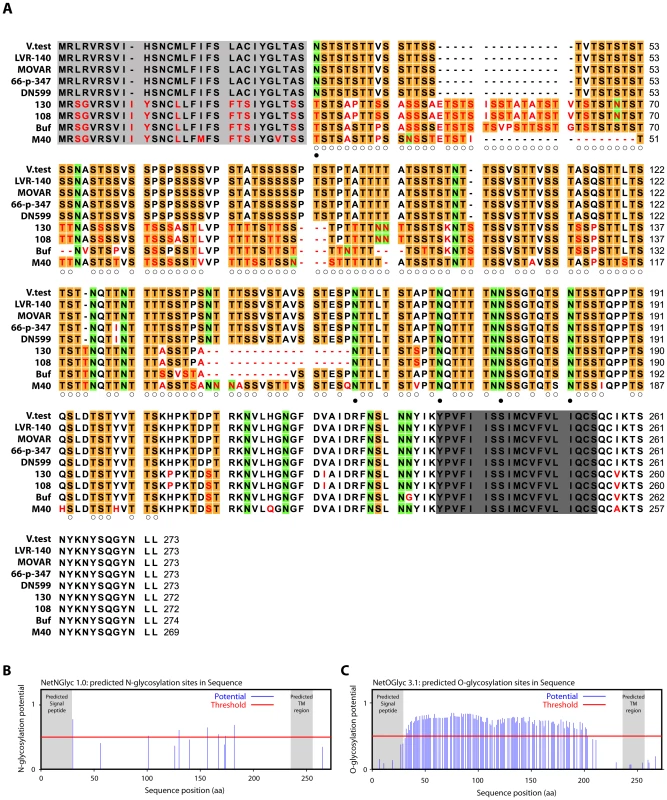
A. BoHV-4 Bo10 encoded proteins alignment. Predicted Bo10 transmembrane protein encoded by nine different BoHV-4 strains were aligned (ClustalX; [88]). Predicted peptide signals [89] and transmembrane regions were highlighted in pale and dark grey respectively. Non-conserved residues were printed in red. Serine (S) and threonine (T) residues of the N-terminal ectodomain were highlighted in orange, asparagine (N) residues of the N-terminal ectodomain were highlighted in green. Open and filled circles indicate potential O- and N-glycosylation sites respectively (using NetNglyc 1.0 and NetOglyc 3.1 algorithms [37]) that are predicted for each of these residues in all the different strains that display such residue at that position. B. Prediction of N-glycosylation sites for the complete BoHV-4 V.test gp180 protein sequence using the NetNglyc 1.0 algorithm. The shaded regions indicate the signal peptide and transmembrane region. The red line indicates significative threshold. C. Prediction of O-glycosylation sites for the complete BoHV-4 V.test gp180 protein sequence using the NetOglyc 3.1 algorithm. The shaded regions indicate the signal peptide and transmembrane region. The red line indicates significative threshold. Gp180 is O-glycosylated
The Bo10 gene product of BoHV-4 V.test has 122 and 7 potential O - and N-glycosylation sites respectively (Figure 4) [37]. This protein has a predicted molecular mass (MM) of 25 kDa but an apparent MM of 180 kDa [33]. To establish the contribution of glycans to the apparent MM, we digested virions lysates with glycanases. We removed high mannose, hybrid and complex N-glycans [38] with PNGase F. We removed O-glycans successively with sialidase A, β1-4 Galactosidase and O-glycanase. While PNGase F did not affect the apparent MM of gp180 (Figure 5A), removing O-glycans reduced it to approximately 20 kDa, consistent with its predicted unglycosylated MM. Therefore, gp180 was extensively O-glycosylated and O-glycans appear to account for most of its mass.
Fig. 5. Glycosylation of BoHV-4 gp180 and importance of BoHV-4 glycans in neutralization evasion. 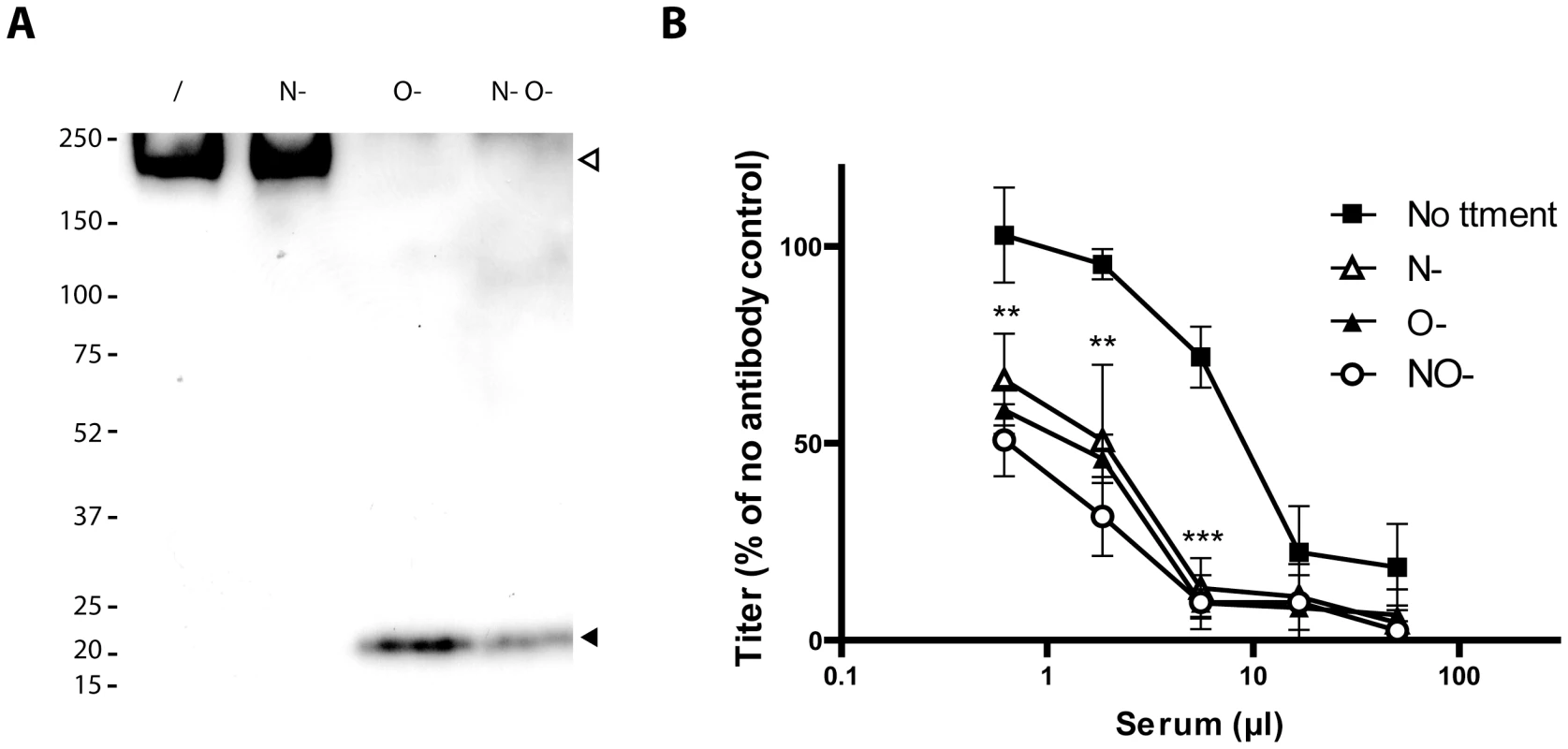
A. Purified BoHV-4 WT V.test strain virions were deglycosylated after denaturation. /, no enzyme; N-, protein N-glycanase; O-, sialidase A + β1-4 Galactosidase + O-glycanase; N O-, protein N-glycanase + sialidase A + β1-4 Galactosidase + O-glycanase. Each was then immunoblotted for gp180 with anti-Bo10-c15 serum as described in the Material and Methods. The position of a MM standard (in kDa) is shown on the left. B. Intact MDBK cell-derived BoHV-4 V.test strain WT virions were deglycosylated without denaturation. /, no enzyme, N-, protein N-glycanase, O-, sialidase A + O-glycanase, N-O- protein N-glycanase + sialidase A + O-glycanase. Each was then tested for neutralization by serum of rabbits immunized by the BoHV-4 V.test strain. After incubation (2 h, 37°C) the viruses were plaque assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. The data presented are the average ± SEMs for 4 measurements and were analyzed by 2way ANOVA and Bonferroni posttests, ** p<0.01, *** p<0.001. Statistical significance was only shown for O- treatment. Equivalent data were obtained in two further experiments. Glycans protect BoHV-4 against neutralization
To test whether O-glycans protect BoHV-4 against neutralization, we removed N - and/or O-glycans from intact virions before testing their susceptibility to neutralization. As removing glycans itself could affect viral titers (Figure S2), we expressed the results as a percentage of the number of plaques for each treatment without neutralization. As observed for other viral species [39]–[41], removing N-glycans increased virion susceptibility to neutralization by immune serum (Figure 5B). Removing O-glycans had a similar effect (Figure 5B). Therefore BoHV-4 uses both N - and O-linked glycans to limit its neutralization. Interestingly, gp180 bears most of the predicted BoHV-4 envelope O-glycans (122/155) [37]. These results suggest therefore that gp180 O-glycans provide part of a glycan shield for otherwise vulnerable viral epitopes.
Altered antigenicity of BoHV-4 lacking gp180
Our subsequent analysis focused on the identification of the neutralization epitopes hidden by gp180. The MuHV-4 gp150 seems to form a multiprotein entry complex with gB, gH and gL [42]. We therefore focused on antibodies raised against the BoHV-4 gB, gH and gL. Monoclonal antibodies (mAbs) were screened for gB, gH, gL or gH/gL specificity as described in the Material and Methods. Mabs 16, 29 and 33 recognize gL, gB and the heterodimer gH/gL, respectively (Figure S3). Mab 35 recognizes gB as previously stated [43].
As with MuHV-4 [32], infected cell surfaces provide a means of probing antigenic differences between BoHV-4 glycoprotein mutants. We compared cells infected by WT, Bo10 Del, Bo10 Rev, WT BAC, Bo10 STOP and Bo10 STOP Rev BoHV-4 viruses. MAbs 29 and 35 (recognizing gB), mAb 16 (recognizing gL) and mAb 33 (recognizing the gH/gL complex) all stained cells infected with the Bo10 Del and Bo10 STOP strains better than they stained those infected with wild-type or revertant viruses (Figure 6). This result was not due to differences in protein expression, as permeabilized cells gave similar staining with each virus (Figure 6).
Fig. 6. Antigenicity of infected cells. 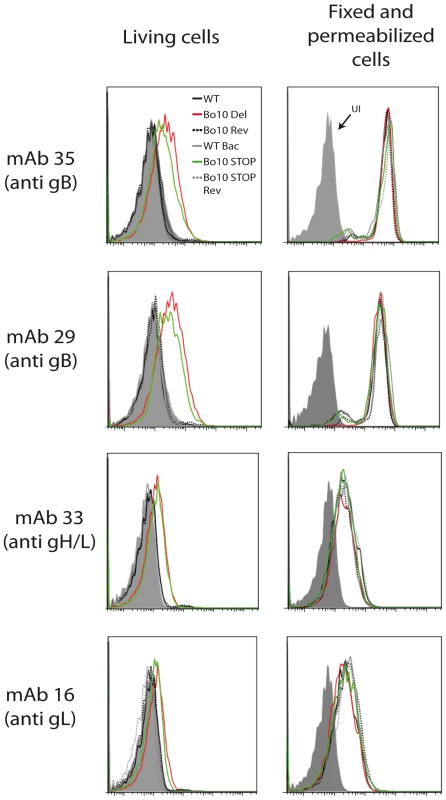
MDBK cells were infected (2 PFU/cell, 36 h) with WT (solid black lines), Bo10 Del (red lines), Bo10 Rev (dotted black lines), WT BAC (grey lines), Bo10 STOP (green lines) or Bo10 STOP Rev (dotted grey lines) of BoHV-4 V.test, and then analyzed by flow cytometry. The filled histogram shows uninfected cells. MAb 29 and 35 recognize gB, mAb 16 recognizes gL, and mAb 33 recognizes gH/L. Fixed (PFA 4%, 4°C for 30 min) and permeabilized (saponin 0.1%) cells were used as control of protein expression. We then analyzed WT and Bo10 Del virions by immunogold labeling with mAb 35 raised against gB. While binding of gold particles was observed with both strains (Figure 7A), there were statistically more particles on Bo10 Del virions than on WT virions (p<0.001). Representative particules are shown in Figures 7B and S4. This difference did not reflect a greater gB content of Bo10 del virions, since immunoblotting on the same viral preparations with the same antibody showed equivalent signals between the mutant and the WT (Figure 7C). The different stocks displayed also similar particle/PFU ratios as shown in Figure 7D. Finally, increased accessibility of some epitopes on Bo10 mutant virions was confirmed by immunofluorescence (Figure 7E) of virions bound to cell surfaces. The cells were scanned by confocal microscopy with settings unchanged between different viruses stained with the same antibody. Glow pseudo-color analysis established that the staining was stronger when Bo10 was deleted. The difference was particularly evident for mAbs 16 (anti-gL) and 33 (anti gH/L) (Figure 7E). Together these results established that gB, gL and gH/L epitopes were more accessible on Bo10 mutant virions than on WT or revertant viruses, consistent with gp180 hiding key epitopes from neutralization.
Fig. 7. Antigenicity of purified virions. 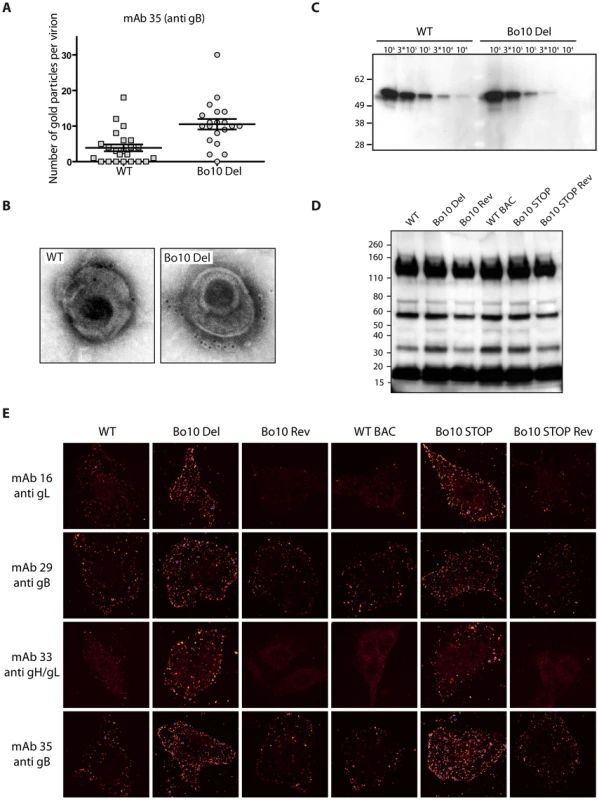
A. and B. Immuno-electron microscopy. Purified BoHV-4 WT V.test or Bo10 Del virions were processed for immune-electron microscopy as described in Material and Methods. These samples were stained for gB with mAb 35 followed by secondary goat anti-mouse IgG-10 nm gold labeled. Pictures of individual virions were then taken and gold particles were counted on each virion. The data presented are the average ± SEMs for 20 different virions and were analyzed by Student’s t-test, *** p<0.001 (A). Pictures of representative virions are showed (B). C. Purified BoHV-4 WT V.test and Bo10 Del virus stocks were compared for gB content per PFU by immunoblotting for gB (with mAb 35) as described in the Material and Methods. The position of a MM standard (in kDa) is shown on the left. D. Purified BoHV-4 WT V.test, Bo10 Del, Bo10 Rev, WT BAC, Bo10 STOP and Bo10 STOP Rev virions (105 PFU per lane) were compared for particles content per PFU by immunoblotting with anti BoHV-4 V.test polyserum as described in the Material and Methods. The position of a MM standard (in kDa) is shown on the left. E. Antigenicity of bound purified virions. MDBK cells were exposed to BoHV-4 V.test WT, Bo10 Del, Bo10 Rev, WT BAC, Bo10 STOP and Bo10 STOP Rev virions (20 PFU/cell, 2 h, 4°C), then directly fixed with acetone 95%/water 5%. The cells were then stained for gB with mAb 29 and 35, for gL with mAb 16 and for gH/gL with mAb 33. The pictures were taken with a confocal laser microscope. Images are shown with a pseudo-color glow scale (White, highest to black, lowest intensity, blue represents overexposed pixels). Increased susceptibility of Bo10 - BoHV-4 to gL directed neutralization
We next tested whether mAbs recognizing Bo10 mutants better (Figure 6 and 7) could also neutralize them better than WT or revertant virions. While mAbs 29, 35 (anti-gB) and 33 (anti-gH/L) did not neutralize any strain (data not shown), mAb 16 (anti-gL) neutralized the Bo10 mutants better in different cell types (Figure 8A, Figure S5). It was not possible to achieve complete neutralization as it had been with immune sera (Figure 8B). Therefore, gL is likely to be only one of several neutralization targets protected by gp180 or other protection mechanisms exist. However, it was clearly one such target, establishing that the reduction in gL accessibility by gp180 was functionally important.
Fig. 8. Sensitivity of Bo10- mutants to anti gL directed neutralization. 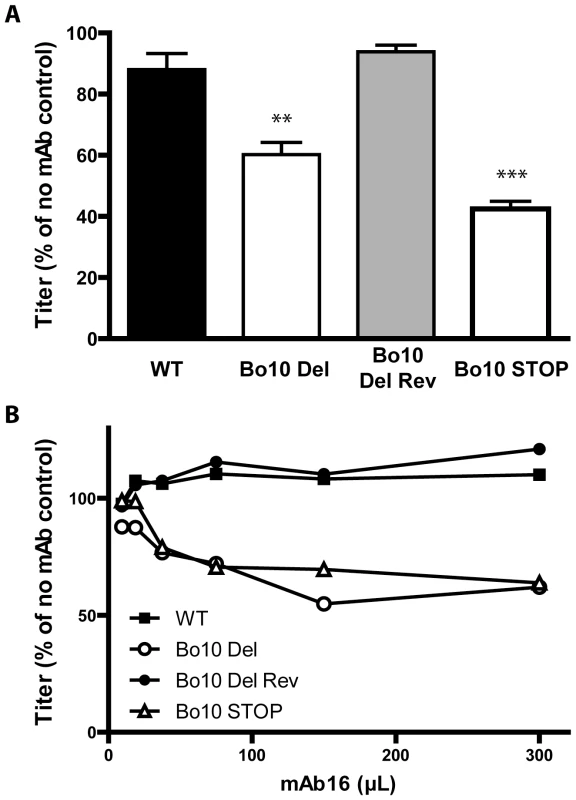
A. BoHV-4 WT V.test, Bo10 del, Bo10 Rev and Bo10 STOP virions were incubated with the gL-specific neutralizing mAb 16. After incubation (2 h, 37°C) the viruses were plaque assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. The data presented are the average ± SEMs for triplicate measurements and were analyzed by 1way ANOVA and Bonferroni posttests, ** p<0.01, *** p<0.001. B. BoHV-4 WT V.test, Bo10 del, Bo10 Rev and Bo10 STOP virions were incubated with increasing amounts of the gL-specific neutralizing mAb 16, and then assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. Equivalent data were obtained in two further experiments. Immunogenicity of BoHV-4 lacking gp180
The results obtained above showed that removal of gp180 results in the unmasking of several viral envelope epitopes among which some neutralization targets. To test whether gp180 might also affect BoHV-4 immunogenicity, we compared the humoral immune response induced in the rabbits by the Bo10 STOP strain to that observed with the wild type parental strain (Figure 9). Over the course of infection, no difference in total anti-BoHV-4 antibody response was observable between the groups of infected rabbits (Figure 9A). However, as the anti-herpesvirus antibody response is often dominated by capsid proteins, some subtle changes could be masked. We therefore investigated specific responses against gB, gH and gL. 293T cells expressing GPI-linked forms of gL, the gB extracellular domain or the gH extracellular domain, were stained with anti-BoHV-4 WT sera or with anti-BoHV-4 Bo10 STOP sera. The results obtained showed that sera of both groups of rabbits stained similarly gB and gH, whereas no detectable gL staining was observed (Figure 9B) although specific monoclonal antibodies confirmed cell surface expression of all proteins (data not shown). Finally, we compared the neutralization potential of these sera against WT, Bo10 Del, Bo10 STOP or Bo10 Rev virions. As observed previously for anti-BoHV-4 WT serum, anti-BoHV-4 Bo10 STOP serum neutralized Bo10 mutant viruses better. However, no significative difference in neutralization potential was observable between both groups of serum (Figure 9C). Our results suggest therefore that gp180 deficient virions display enhanced susceptibility to neutralizing antibodies but do not elicit markedly enhanced antibody response in infected rabbits. Thus, antigenicity does not predict immunogenicity.
Fig. 9. Immunogenicity of BoHV-4 lacking gp180. 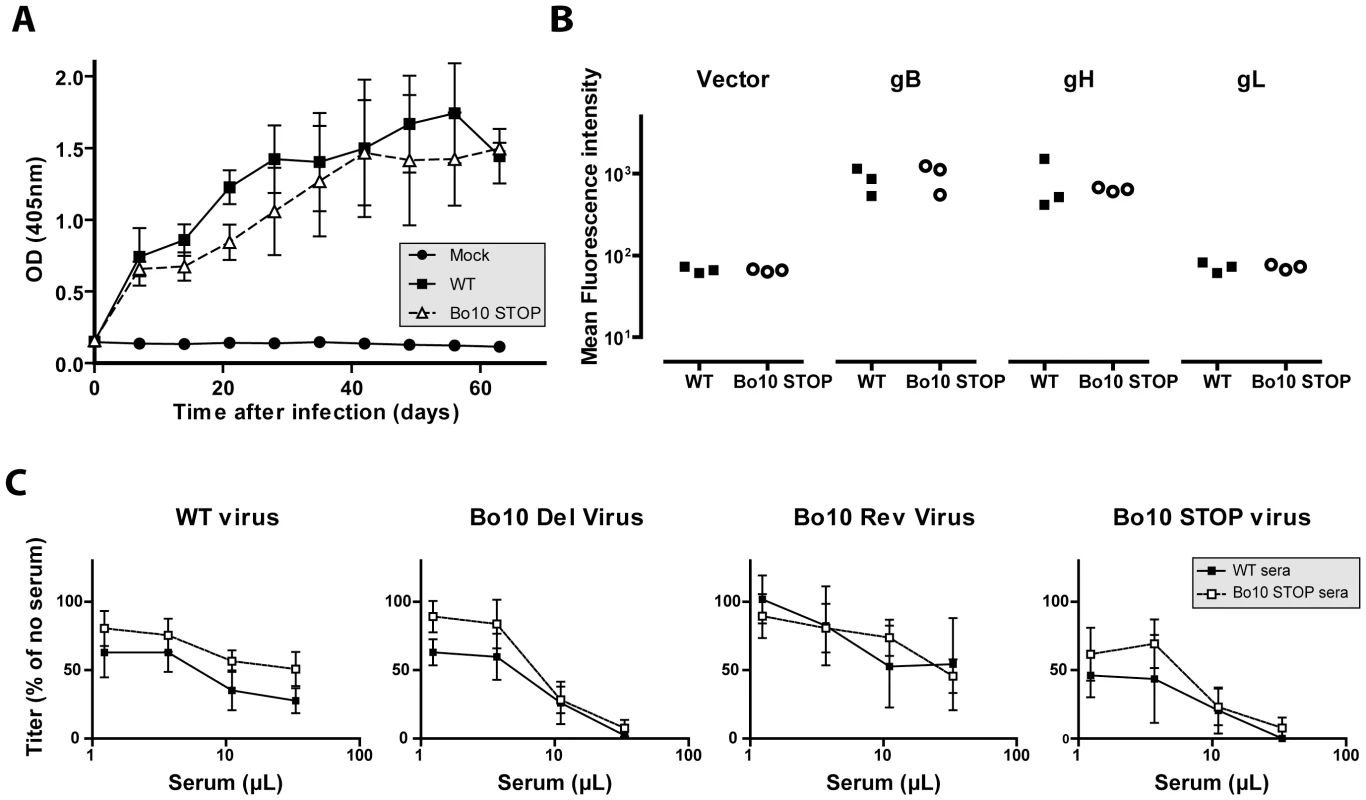
Rabbit anti-BoHV-4 V.test WT antibody response. Groups consisting of 3 rabbits were infected with 107 PFU of BoHV-4 WT V.test and Bo10 STOP excised strains. A. Sera were collected at different times post-infection and the titre of anti-BoHV-4 antibodies was estimated by ELISA as described in the Material and Methods. Each value represents the mean +/- SD of the data obtained for the three rabbits of each group. The serum of a mock infected rabbit was taken as control. The data were analyzed by 2way ANOVA and Bonferroni posttests. No significative difference was observed between groups. B. Specific anti gB, gH and gL antibody responses were investigated by staining unfixed 293T cells expressing GPI-linked forms of gL, the gB extracellular domain or the gH extracellular domain. These staining were performed with sera collected 63 days post-infection. The data were analyzed by Student t-test. No significative difference was observed between WT and Bo10 STOP sera. C. BoHV-4 WT V.test, Bo10 Del, Bo10 Rev and Bo10 STOP virions were incubated with sera of WT or Bo10 STOP infected rabbits collected 63 days post-infection (3 sera per group). After incubation (2 h, 37°C) the viruses were plaque assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. The data presented are the average ± SEMs for 3 measurements and were analyzed by 2way ANOVA and Bonferroni posttests. No significative difference was observed between WT and Bo10 STOP sera. Gp180 O-glycans can be a target for neutralization
While O-glycans help BoHV-4 to evade neutralizing antibodies, they can potentially be targeted by carbohydrate binding agents, as proposed for other viruses. Gp180 is not essential for BoHV-4 replication, but lectins could still compromise virus entry by steric hindrance. We therefore tested the capacity of jacalin, an O-glycan-specific lectin, to inhibit BoHV-4 infection (Figure 10A). Inhibition was evident for WT and Bo10 Rev virions, whereas Bo10 deleted virions were relatively resistant. Therefore O-glycan-directed neutralization was possible for BoHV-4 and appeared to target mainly gp180.
Fig. 10. Glycans on BoHV-4 gp180 allow virus neutralization. 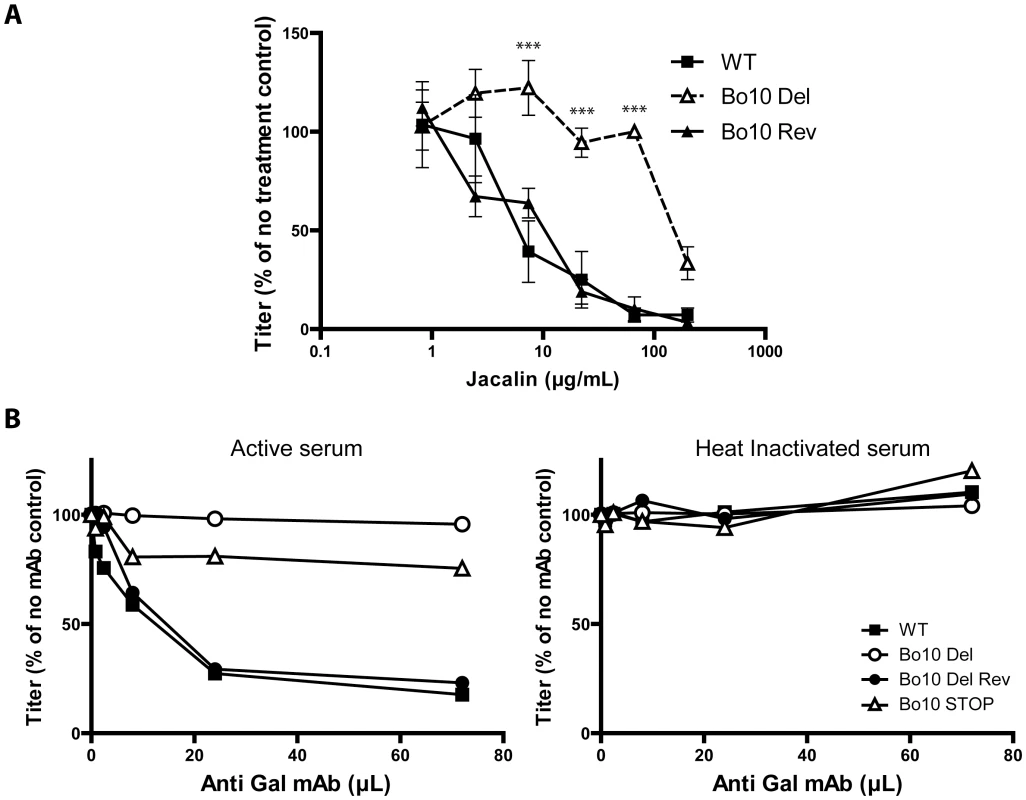
A. Sensitivity of WT, Bo10 Del and Bo10 Rev strains to Jacalin mediated neutralization. BoHV-4 WT V.test, Bo10 Del and Bo10 Rev virions were incubated with increasing amounts of O-glycan specific lectin jacalin (1 h, 37°C) and then assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without lectin. The data presented are the average ± SEMs for 3 measurements and were analyzed by 2way ANOVA and Bonferroni posttests, *** p<0.001. B. Sensitivity to anti-Gal antibodies induced complement dependent neutralization of BoHV-4 WT, Bo10 Del, Bo10 Rev and Bo10 STOP strains. Virions of the different strains derived from MDBK cells were assayed for their sensitivity to neutralization by horse serum supplemented with increasing amounts of mAb M86 raised against Galα1-3Gal. Horse sera (heat inactivated or not by incubation at 56°C for 30 min) were tested at the final concentration of 10% (vol/vol). After incubation (2 h, 37°C) the viruses were plaque assayed for infectivity on MDBK cells. BoHV-4 titers are expressed relative to virus without antibody. Another strategy would be to use specific antibodies, much as HIV can be neutralized by an antibody that binds to the high-mannose glycans of its gp120 “silent face” [44]. In animals apart from humans, apes and Old World monkeys, the α1-3-galactosyltransferase enzyme adds a terminal galactose onto glycoproteins and glycolipids in a specific α1-3 linkage to generate the Gal epitope [45]. We have previously shown that human sera consequently exhibit innate BoHV-4 neutralization through complement activation by anti-Gal antibodies [46]. We therefore compared the sensitivity BoHV-4 WT, Bo10 Del, Bo10 Rev and Bo10 STOP virions to anti-Gal dependent neutralization. While complement-containing horse serum supplemented with anti-Gal antibodies neutralized WT and Bo10 Rev virions in a dose-dependent manner, Bo10 Del and Bo10 STOP virions were only slightly affected (Figure 10B). Thus gammaherpesvirus glycan shields are potentially accessible to neutralization by carbohydrate-specific antibodies.
Discussion
Persistent viruses must evade multiple arms of the host immune response to maintain infectivity [47]–[48]. Gammaherpesviruses are archetypal persistent viruses, and their cytotoxic T cell evasion mechanisms are well-known [49]–[51]. Much less is known about how they evade neutralizing antibodies. Gammaherpesviruses all share a major glycoprotein homologous to EBV gp350. EBV remains infectious despite the presence of anti-gp350 antibodies in serum and saliva [52]–[55]. Moreover immunization with gp350 fails to reduce either infection rates or virus shedding [27]–[28]. Therefore, we still have much to learn about the interplay between gp350, gp350-specific antibodies and EBV host entry. For example, the inhibition of B cell infection by gp350-specific antibodies [54], [56] could have limited relevance to host entry, or even promote it by enhancing epithelial infection [26]. Similarly, antibodies to the MuHV-4 gp150 strongly enhance infection via IgG Fc receptors [57]. Here we showed that BoHV-4 gp180 is dispensable for establishment and maintenance of latency in vivo (Figure 2), but drastically reduced the susceptibility of BoHV-4 virions to neutralization by immune serum on various cell types (Figure 3, Figure S1). Gp180 seemed to hide at least partially several different epitopes on gB, gH and gL (Figures 6, 7 and S4), which included neutralization targets (Figures 8 and S5). Gp180 is extensively O-glycosylated and O-glycans account for most of its mass (Figures 4 and 5). These results suggest therefore that gp180 O-glycans provide part of a glycan shield for otherwise vulnerable viral epitopes. Since extensive O-glycosylation is a common feature of gammaherpesvirus gp350 homologs, this evasion mechanism may be widely shared. Another common feature of some of these proteins is strong immunogenicity [20], [23], [58]. The reason is not fully understood, however if gp180 homologs shield other virion glycoproteins, their location at the viral surface could favor development of an antibody response against them.
The substantial gp180 divergence between different BoHV-4 strains (Table S1, Figure 4A) remains to be explained. It is possible that much of the protein does not require a very specific amino acid sequence for its function. Thus, a key feature of the gp350 homologs of different gammaherpesviruses may simply be that they are type I transmembrane proteins with extensive O-glycosylation [33] (Figures 4 and 5A). An importance of glycans for immune evasion has also been hypothesized for gp350 [59]. Similarly, the HIV gp120 [60] uses glycans to provide a “silent face” protected against most antibodies [41]. Thus while neutralization is possible [61]–[62], this and other mechanisms ensure that it is difficult. The Ebola virus glycoprotein (EBOV GP) - again involved in virus binding [63] and a target for vaccine design - is also extensively glycosylated and in this way partially protected against antibody [64]. As with gp180, different filoviruses show huge glycoprotein diversity but retain the basic protein organization and extensive glycosylation [64]. This immune evasion mechanism appears therefore to be shared by several viral families.
While carbohydrates on SIV and HIV envelope proteins can shield these viruses from antibody recognition and neutralization [41], [65], it appears that these glycans could also limit the neutralizing antibody response in the context of SIV infection [66] or HIV immunization [67]. We did not observe an increased BoHV-4 immunogenicity in the absence of gp180 (Figure 9). While most of the epitopes hidden by the gp120 glycan shield are located on gp120 itself [68], BoHV-4 gp180 protects other virion glycoproteins in trans, rather than simply protecting itself in cis. Studies on different viruses [69]–[70] suggest that protection of a limited number of entry complexes from neutralization is probably sufficient to preserve virion infectivity. In contrast, influence on immunogenicity probably requires covering of all entry complexes in order to render them invisible to the immune system. Gp180 does not hide all the vulnerable epitopes at the viral surface. Indeed, even if gp180 hide most of the epitopes recognized by mAb 16, 29, 33 and 35, some remains accessible at the surface of WT or revertant virions (Figure 7). Moreover, infected cell debris provides also certainly a source of uncovered antigens. It therefore appears that gp180 influence virion antigenicity but not immunogenicity. Similarly, BoHV-4 gB N-term protects some vulnerable epitopes, but its deletion does not result in an enhanced ability to induce neutralizing antibody responses [71].
Another unusual feature of gp180 was the likely importance of O-linked glycans for viral antibody evasion. In other viruses, most protection against antibody seems to involve N-linked glycans [41], [72]. While BoHV-4 surface N-glycans are also involved in antibody evasion (Figure 5B), this study strengthens the role of O-glycans in neutralization evasion. O-linked glycans have also been shown to protect MuHV-4 gB N-terminal ectodomain against antibody [71]. Indeed, although MuHV-4 gB N-term confers protection to some neutralization epitopes on gH/L, gB N-term is itself a neutralization target [19], [71]. However, depending on the host cell, this part of gB can be largely protected against antibody by O-linked glycans [71]. These glycans could also possibly assist in protecting a neutralization epitope on gH/L. In BoHV-4, gB is the only other described envelope protein that could bear O-glycans. Indeed, gp180 and gB N-term contain respectively 122 and 33 predicted O-glycosylation sites [37]. These two proteins and their glycans could therefore cooperate to render BoHV-4 particularly resistant to neutralization [73]. Similar N-terminal O-glycans occur in the Herpes Simplex virus gC [74], but do not have a known function. Because N-linked glycans are relatively bulky, O-linked glycans may be better suited to protecting small or linear glycoprotein domains while still allowing protein/protein interactions. It seems with gp180 that protection by O-linked glycans can also be “scaled up” for more extensive protection. Because gp180 is likely to be part of a multi-protein complex, too many N-glycans might disrupt important protein/protein interactions. Another consideration is that glycans can on occasion be targeted by the immune response [75]. In this context, glycan diversity might be useful for a virus, and providing such diversity is a potential function of the BoHV-4 Bo17 gene, which encodes a mucin-type beta-1,6-N-acetylglucosaminyltransferase [76].
While glycans offer mainly protection in the natural setting, they can also be artificially targeted for neutralization by carbohydrate binding agents (CBAs) [77]. Evading CBAs would require a virus to compromise its glycan shield, thereby promoting neutralization by antibody [78]. As CBAs might be expected to elicit their own antibody response after repeated dosing, thereby attenuating their effect, anti-carbohydrate antibodies might be more useful in long-term settings. This also opens the possibility of vaccination against specific pathogen carbohydrates to target their glycan shields [79].
All together, our results suggest that BoHV-4 gp180 and, by extension, its homologs in other gammaherpesviruses shield the virus from immune recognition. This probably contributes to the ineffectiveness of the antibody response against these viruses.
Materials and Methods
Ethics statement
The experiments, maintenance and care of rabbits complied with the guidelines of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (CETS n° 123). The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Liège, Belgium (Permit Number: 1035). All efforts were made to minimize suffering.
Cells and virus
Madin-Darby bovine kidney (MDBK) (ATCC CCL-22), Bovine Turbinates (BT) (ATCC CRL-1390), Embryonic Bovine Trachea (EBTr) (ATCC CCL-44), Embryonic Bovine Lung (EBL) (DSMZ ACC-192), Bovine Macrophages (BOMAC) [80], bovine mammary epithelial (MacT) [81] and EBL-NLS-Cre [30] cells were cultured in Dulbecco’s modified Eagle Medium (Invitrogen) containing 10% fetal calf serum (FCS), 2% Penicillin/Streptomycin (Invitrogen) and 1% non Essential amino acids (Invitrogen). Bovine PBMC were prepared as described elsewhere [82] and cultured in RPMI Glutamax Medium containing 10% FCS, 2% Penicillin/Streptomycin (Invitrogen), 1% Essential amino acids (Invitrogen), 1 mM Sodium pyruvate, 25 mM HEPES and 50 µM 2-mercaptoethanol. The BoHV-4 V.test strain initially isolated from a case of orchitis [83], the BoHV-4 WTeGFP, Bo10 Del and Bo10 Rev strains [33] and the derived recombinant strain cloned as an Bacterial Artificial Chromosome (BAC) [30], were used throughout.
Plasmids
The coding sequence for BoHV-4 V.test gL amino acid residues 1-140 was amplified by PCR (Hi-Fidelity PCR kit, Roche Diagnostics Ltd) with 5' AvrII-restricted and 3' NotI-restricted primers. Similarly, the coding sequences for BoHV-4 V.test gB amino acid residues 1–725 and gH amino acid residues 1–678 were amplified by PCR with 5' XbaI-restricted and 3' NotI-restricted primers. These PCR products were cloned into the XbaI/NotI sites of pBRAD, thereby attaching a C-terminal glycosyl-phosphatidyl-inositol (GPI) membrane anchor [20], generating gL-GPI, gB-GPI and gH-GPI expression plasmids.
Antibodies and reagents
The O-glycan specific lectin, jacalin, was purchased from Vector Laboratories. For detection of gp180 on western blotting, we used a rabbit monospecific polyserum raised against the C-term end of the Bo10 encoded protein (anti-Bo10-c15) [33]. For the neutralization experiments, we used sera of 4 different rabbits infected intravenously with 108 PFU of the BoHV-4 V.test strain and collected 63 days post inoculation. The mouse mAb M86 raised against the Galα1-3Gal epitope was purchased from Alexis and used free of sodium azide as previously described [46]. Horse serum was collected as a source of complement. The serum was treated as described previously to preserve complement activity, aliquoted and stored at −80°C [46].
Four mouse mAbs raised against BoHV-4 were also used in the present study [84]. Their specificities were unraveled on 293T cells transfected with the vectors encoding gB-GPI, gH-GPI or gL-GPI. The epitopes depending on the gH-gL heterodimer were reconstituted by co-expressing gH-GPI and gL-GPI. Briefly, transfected 293T cells were fixed and permeabilized in Acetone 95% for 5 min and then stained with the different antibodies in PBS containing 10% FCS (v/v). These antibodies were detected with Alexa 488-coupled Goat anti-mouse IgG-specific antibodies (Invitrogen). Nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole). Fluorescence was visualized with a Nikon TE-2000 microscope and a Leica CCD camera.
Production of the BoHV-4 Bo10 STOP and Bo10 STOP Rev strains
We disrupted the BoHV-4 V.test Bo10 coding sequence (genomic coordinates 65,696 to 66,595, Genbank JN133502) by introducing stop codons into the coding sequence for the Bo10 signal peptide (Bo10 STOP). BoHV-4 recombinants were produced using BAC cloning and prokaryotic recombination technologies as described before [30]. The V.test BAC G plasmid was used as parental plasmid [30]. The BoHV-4 V.test Bo10 STOP was produced using a two step galactokinase (galK) positive/negative selection in bacteria [85]. The first recombination process (galK positive selection) consisted to introduce the galK gene into the Bo10 coding sequence (genomic coordinate 65,760) resulting in the V.test BAC G Bo10 galK plasmid. Recombination was achieved using the Bo10 galK cassette. It consisted of the galK gene flanked by 50-bp sequences corresponding to Bo10 regions (65,711-65,760 and 65,810-65,761 of the BoHV-4 V.test strain genome). This cassette was produced by PCR using pgalK vector [85] as template and Bo10-fwd-galK 5’agatctgtcatacattcaaattgcatgctttttatattcagcctcgcctgCCTGTTGACAATTAATCATCGGCA 3’ and Bo10-rev-galK 5’ atacggtggtggatgtgctggtgctgttgctggcagttaacccatatataTCAGCACTGTCCTGCTCCTT 3’ as forward and reverse primers, respectively (galK sequences are indicated in capital letters, Bo10 start codon is in bold). The second recombination process (galK negative selection) consisted to replace the galK sequence by a Bo10 STOP cassette to generate the BoHV-4 V.test Bo10 STOP plasmid. The Bo10 STOP cassette consisted of a synthetic double strand DNA corresponding to genomic coordinates 65,696 to 65,831 with the introduction (genomic coordinate 65,761) of 36 nucleotides coding for in-frame STOP codons and restriction sites (Figure 1A). These 36 nucleotides do not insert STOP codons in any of the 5 other frames of the genome. The BoHV-4 V.test Bo10 STOP Rev plasmid was produced similarly from BoHV-4 V.test Bo10 STOP plasmid. The first recombination process (galK positive selection) was identical to the one described above. The second recombination process (galK negative selection) consisted to restore Bo10 to generate a revertant plasmid. This cassette was produced by PCR using BoHV-4 V.test genome as template and Bo10-rec-sens (genomic coordinates 65,183 to 65,207) and Bo10-rec-rev (genomic coordinates 67,278 to 67,257) as forward and reverse primers, respectively. Reconstitution of infectious virus from BAC plasmids was obtained by transfection in MDBK cells to obtain Bo10 STOP BAC and Bo10 STOP BAC Rev strains. To excise the BAC cassette, reconstituted viruses were propagated in EBL-NLS-Cre cells expressing Cre recombinase to generate the corresponding excised strain.
Southern blot
Southern blot analysis [82] of viral DNA digested with EcoRI was performed with probe corresponding to genomic coordinates 65,696 to 66,595 of the BoHV-4 V.test genome.
Virus purification
BoHV-4 strains grown on MDBK cells were purified as follows. Virions were harvested from infected MDBK cell supernatants by ultracentrifugation (100,000× g, 2 h); infected-cell debris was then removed by low-speed centrifugation (1,000× g, 10 min). Virions were then centrifuged through a 20 to 50% (w/v) potassium tartrate gradient in PBS (100,000× g, 2 h). Virions were recovered from the gradient and finally washed and concentrated in PBS (100,000× g, 2 h).
Western blot
Virions were lysed and denatured by heating (95°C, 5 min) in SDS-PAGE sample buffer (31.25 mM Tris-HCl pH 6.8, 1% (w/v) SDS, 12.5% (w/v) glycerol, 0.005% (w/v) Bromophenol Blue, 2.5% (v/v) 2-mercaptoethanol). Proteins were resolved by electrophoresis on Mini-PROTEAN TGX (Tris-Glycine eXtended) precast 7.5% resolving gels (Bio-Rad) in SDS-PAGE running buffer (25 mM Tris-base, 192 mM glycine, 0.1% (w/v) SDS) and transferred to polyvinylidene difluoride membranes (Immobilon-P transfer membrane, 0.45 µM pore size, Millipore). The membranes were blocked with 3% non-fat milk in PBS/0.1% Tween-20, and then incubated with anti-Bo10-c15 rabbit antibodies, mAb 35 or rabbit anti-BoHV-4 polyserum in the same buffer. Bound antibodies were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG pAb or goat anti-mouse IgG pAb (Dako Corporation), followed by washing in PBS/0.1% Tween-20, development with ECL substrate (GEHealthcare) and exposure to X-ray film.
Animals
Specific-pathogen-free New-Zealand white rabbits were used throughout this study. Rabbits were inoculated intravenously with purified stocks of the different viral strains. In one experiment we infected rabbits with WT, Bo10 Del or Bo10 Rev strains (108 PFU). In a second experiment, rabbits received WT or Bo10 STOP strains (107 PFU). At the end of the experiment, rabbits were euthanized and a necropsy examination was performed during which the spleen was collected.
Isolation of peripheral blood mononuclear cells and preparation of spleen cell suspension
Blood samples were collected and PBMC were separated by Ficoll (Ficoll-Paque Plus, GE Healthcare) density gradient as described previously [86]. Immediately after euthanasia, spleen was removed and half-part of it was homogenized using a tissue grinder (VWR), passed through a stainless steel sieve and washed in FCS-free MEM before further analyses.
Viral genome detection by Real time-PCR
DNA was purified from the spleen and PBMC using the QIAamp DNA Mini kit (Qiagen). Real-time PCR was performed as described elsewhere [87]. A 103 bp fragment corresponding to BoHV-4 ORF8 was amplified with the forward primer 8startfw (5’ - CAAATAGTTCATTAGCTGCCTCTCC -3’) and the reverse primer 8middlerev (5’ - TCATCAGTAACAGTTGGAATAGTGG -3’) in the presence of the fluorescent probe 5’-FAM-AACACGTCAACA AGCAAGCCATCCACTG-TAMRA-3’. pGEM-T easy containing BoHV-4 gB ORF was used to establish standard curves. PCR amplifications and fluorescence reactions were carried out in a iCycler system (Bio-Rad) under the following conditions: initial activation of the Taq polymerase (Bio-Rad) at 94°C for 5 min followed by 50 cycles at 94°C for 1 min, 50 cycles at 51°C for 30 sec and 50 cycles at 72°C for 1 min.
Virus detection by infectious centre assay
Viral detection in spleen cell suspension was assayed by infectious centre assay (ICA) as follows. 5.105 MDBK cells grown in 6 well cluster dishes (Becton Dickinson) were co-cultured for 7 days at 37°C with spleen cells in MEM containing 10% FCS, 2% PS, 0.6% CMC and 5.10−5M of β-mercaptoethanol (Merck). Cells were then fixed and stained with mAb 35 for indirect immunofluorescent detection of intracellular viral antigen as described previously [33]. Fluorescence was then visualized with a TE2000-S Nikon and a Leica DC300F CCD camera system.
Oligosaccharide digestion
All reagents were obtained from New England BioLabs. For SDS-PAGE analysis, samples were denatured in Glycoprotein Denaturing Buffer (0.5% SDS, 40 mM DTT) for 10 min at 100°C and then digested for 3 h at 37°C with 250 NEB units PNGase F and/or 250 NEB units of neuraminidase, β1-4 Galactosidase, O-glycanase in G7 reaction buffer (50 mM sodium phosphate, pH 7.5) with 1% NP-40. Reactions were stopped by the addition of Laemmli sample buffer and proteins were analyzed by immunoblotting as described below. For neutralization assays, N - or O-linked glycans were removed with the same enzymes, but without reduction or denaturation. Thus, intact virions were incubated with the different enzymes (3 h, 37°C) in PBS/5% fetal calf serum buffered to pH 6.
Flow cytometry
For cell surface staining, cells infected by the different virus strains (2 PFU/cell, 36 h) were washed in PBS and analyzed directly for green channel fluorescence [71]. For intracellular staining, cells were fixed in 1% paraformaldehyde (30 min at room temperature) and then permeabilized with 0.1% saponin. Cells were incubated (1 h, 4°C) with the different mAbs specific for BoHV-4 glycoproteins followed by Alexa 633-conjugated goat anti-mouse pAb (Invitrogen). Cells were then washed and analyzed on a FACSAria cytometer (Becton Dickinson).
Immunogold labeling of virions
Copper grids of 400 mesh (Agar Scientific Ltd) were incubated for 10 min with 2% Alcian blue 8G solution (Gurr Microscopy Materials, BHD) to add positive charges. After washing, purified virions (108 PFU/ml) were adsorbed to the grids for 10 min. Non-specific interactions were blocked by incubation of the grids for 15 min in PBS containing 0.1% (w/v) cold water fish skin gelatin (CWFG, Aurion) and 5% (w/v) goat serum (Invitrogen). This solution was also used for further incubation and washes. Immunogold labeling was performed by incubation of the grids with mAb 35 as primary antibody for 60 min at room temperature. After washing with PBS and incubation in PBS 0.1% CWFG 5% goat serum for 5 min, the grids were incubated with Goat anti-mouse IgG-10 nm gold labeled conjugate (diluted 1∶50, BBInternational) as secondary antibody for 60 min at RT. A final incubation step was performed in 2% uranyl acetate solution for 10 sec (Agar Scientific). Immunogold stained virions were observed using a transmission electron microscope (FEI, TEcnai Biotwin). Micrographs of virion were acquired for at least 20 individual virions per strain.
Indirect immunofluorescent staining of bound virions
Infected cells (20 PFU/cell, 2 h, 4°C) were fixed in cold Acetone 95% for 5 min on ice. Immunofluorescent staining (incubation and washes) was performed in PBS containing 10% FCS (v/v). Samples were incubated at RT for 45 min with the different mAbs raised against BoHV-4 glycoproteins. After three washes, samples were incubated at RT for 45 min with Alexa Fluor 488 or Alexa Fluor 568 goat anti-mouse IgG (2 µg/ml; Invitrogen). Images were acquired on a Leica TCS SP confocal laser scanning microscope with settings specific for Alexa Fluor 488 or Alexa Fluor 568. Acquisition settings (PMT voltage and offset) were kept identical between slides stained with the same antibodies.
Quantification of anti-BoHV-4 antibodies by ELISA
Nunc Maxisorp ELISA plates (Nalgene Nunc) were coated for 18 h at 37°C with 0.1% Tween 20-disrupted BoHV-4 virions (2.106 PFU/well), blocked in PBS/0.1% Tween-20/3% BSA, and incubated with rabbit sera (diluted 1/300 in PBS/0.1% Tween-20/3% BSA). Bound antibodies were detected with Alkaline Phosphatase conjugated goat anti-rabbit Ig polyclonal antibody (Sigma). Washing were performed with PBS/0.1% Tween-20/3% BSA. p-Nitrophenylphosphate (Sigma) was used as substrate and absorbance was read at 405 nm using a Benchmark ELISA plate reader (Thermo).
Nucleotide sequence accession numbers
Sequence data reported here have been deposited in the GenBank database under the following accession numbers: BoHV-4 V.test Long Unique region (JN133502).
Supporting Information
Zdroje
1. Thorley-LawsonDAGrossA 2004 Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 350 1328 1337
2. VermaSCRobertsonES 2003 Molecular biology and pathogenesis of Kaposi sarcoma-associated herpesvirus. FEMS Microbiol Lett 222 155 163
3. SalvettiMGiovannoniGAloisiF 2009 Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol 22 201 206
4. SerafiniBRosicarelliBFranciottaDMagliozziRReynoldsR 2007 Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med 204 2899 2912
5. HadinotoVShapiroMSunCCThorley-LawsonDA 2009 The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog 5 e1000496
6. NiedermanJCMillerGPearsonHAPaganoJSDowalibyJM 1976 Infectious mononucleosis. Epstein-Barr-virus shedding in saliva and the oropharynx. N Engl J Med 294 1355 1359
7. YaoQYRickinsonABEpsteinMA 1985 A re-examination of the Epstein-Barr virus carrier state in healthy seropositive individuals. Int J Cancer 35 35 42
8. DesgrangesCde-TheG 1978 Presence of Epstein-Barr virus specific IgA in saliva of nasopharyngeal carcinoma patients: their activity, origin and possible clinical value. IARC Sci Publ 459 469
9. SaridOAnsonOYaariAMargalithM 2001 Epstein-Barr virus specific salivary antibodies as related to stress caused by examinations. J Med Virol 64 149 156
10. BuismanAMAbbinkFScheppRMSonsmaJAHerremansT 2008 Preexisting poliovirus-specific IgA in the circulation correlates with protection against virus excretion in the elderly. J Infect Dis 197 698 706
11. OgraPLKarzonDTRighthandFMacGillivrayM 1968 Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N Engl J Med 279 893 900
12. BurtonDR 2002 Antibodies, viruses and vaccines. Nat Rev Immunol 2 706 713
13. HeldweinEEKrummenacherC 2008 Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 65 1653 1668
14. ChandranB 2010 Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J Virol 84 2188 2199
15. Hutt-FletcherLM 2007 Epstein-Barr virus entry. J Virol 81 7825 7832
16. PertelPE 2002 Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J Virol 76 4390 4400
17. MillerNHutt-FletcherLM 1988 A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol 62 2366 2372
18. GillMBGilletLColacoSMayJSde LimaBD 2006 Murine gammaherpesvirus-68 glycoprotein H-glycoprotein L complex is a major target for neutralizing monoclonal antibodies. J Gen Virol 87 1465 1475
19. GilletLGillMBColacoSSmithCMStevensonPG 2006 Murine gammaherpesvirus-68 glycoprotein B presents a difficult neutralization target to monoclonal antibodies derived from infected mice. J Gen Virol 87 3515 3527
20. GilletLMayJSColacoSStevensonPG 2007 The murine gammaherpesvirus-68 gp150 acts as an immunogenic decoy to limit virion neutralization. PLoS One 2 e705
21. NemerowGRMoldCSchwendVKTollefsonVCooperNR 1987 Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J Virol 61 1416 1420
22. TannerJWeisJFearonDWhangYKieffE 1987 Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell 50 203 213
23. Thorley-LawsonDAPoodryCA 1982 Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol 43 730 736
24. JanzAOezelMKurzederCMautnerJPichD 2000 Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J Virol 74 10142 10152
25. Shannon-LoweCDNeuhierlBBaldwinGRickinsonABDelecluseHJ 2006 Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci U S A 103 7065 7070
26. TurkSMJiangRChesnokovaLSHutt-FletcherLM 2006 Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. J Virol 80 9628 9633
27. SokalEMHoppenbrouwersKVandermeulenCMoutschenMLeonardP 2007 Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis 196 1749 1753
28. CoxCNaylorBAMackettMArrandJRGriffinBE 1998 Immunization of common marmosets with Epstein-Barr virus (EBV) envelope glycoprotein gp340: effect on viral shedding following EBV challenge. J Med Virol 55 255 261
29. NashAADutiaBMStewartJPDavisonAJ 2001 Natural history of murine gamma-herpesvirus infection. Philos Trans R Soc Lond B Biol Sci 356 569 579
30. GilletLDaixVDonofrioGWagnerMKoszinowskiUH 2005 Development of bovine herpesvirus 4 as an expression vector using bacterial artificial chromosome cloning. J Gen Virol 86 907 917
31. ZimmermannWBrollHEhlersBBuhkHJRosenthalA 2001 Genome sequence of bovine herpesvirus 4, a bovine Rhadinovirus, and identification of an origin of DNA replication. J Virol 75 1186 1194
32. de LimaBDMayJSStevensonPG 2004 Murine gammaherpesvirus 68 lacking gp150 shows defective virion release but establishes normal latency in vivo. J Virol 78 5103 5112
33. MachielsBLeteCde FaysKMastJDewalsB 2010 Bovine Herpesvirus-4 Bo10 gene encodes a non-essential viral envelope protein that regulates viral tropism through both positive and negative effects. J Virol 85 1011 1024
34. DewalsBThirionMMarkine-GoriaynoffNGilletLde FaysK 2006 Evolution of Bovine herpesvirus 4: recombination and transmission between African buffalo and cattle. J Gen Virol 87 1509 1519
35. ShinYCJonesLRManriqueJLauerWCarvilleA 2010 Glycoprotein gene sequence variation in rhesus monkey rhadinovirus. Virology 400 175 186
36. DunowskaMLetchworthGJCollinsJKDeMartiniJC 2001 Ovine herpesvirus-2 glycoprotein B sequences from tissues of ruminant malignant catarrhal fever cases and healthy sheep are highly conserved. J Gen Virol 82 2785 2790
37. JuleniusKMolgaardAGuptaRBrunakS 2005 Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15 153 164
38. MaleyFTrimbleRBTarentinoALPlummerTHJr 1989 Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180 195 204
39. SkehelJJStevensDJDanielsRSDouglasARKnossowM 1984 A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A 81 1779 1783
40. AguilarHCMatreyekKAFiloneCMHashimiSTLevroneyEL 2006 N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol 80 4878 4889
41. WeiXDeckerJMWangSHuiHKappesJC 2003 Antibody neutralization and escape by HIV-1. Nature 422 307 312
42. GilletLStevensonPG 2007 Evidence for a multiprotein gamma-2 herpesvirus entry complex. J Virol 81 13082 13091
43. LomontePFileePLyakuJRBublotMPastoretPP 1997 Glycoprotein B of bovine herpesvirus 4 is a major component of the virion, unlike that of two other gammaherpesviruses, Epstein-Barr virus and murine gammaherpesvirus 68. J Virol 71 3332 3335
44. ScanlanCNPantophletRWormaldMROllmann SaphireEStanfieldR 2002 The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1—>2 mannose residues on the outer face of gp120. J Virol 76 7306 7321
45. GaliliUClarkMRShohetSBBuehlerJMacherBA 1987 Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci U S A 84 1369 1373
46. MachielsBGilletLNascimento BritoSDDrionPDelforgeC 2007 Natural antibody—complement dependent neutralization of bovine herpesvirus 4 by human serum. Microbes Infect 9 1530 1537
47. LavineJSPossMGrenfellBT 2008 Directly transmitted viral diseases: modeling the dynamics of transmission. Trends Microbiol 16 165 172
48. VillarrealLPDefilippisVRGottliebKA 2000 Acute and persistent viral life strategies and their relationship to emerging diseases. Virology 272 1 6
49. TortorellaDGewurzBEFurmanMHSchustDJPloeghHL 2000 Viral subversion of the immune system. Annu Rev Immunol 18 861 926
50. YewdellJWHillAB 2002 Viral interference with antigen presentation. Nat Immunol 3 1019 1025
51. StevensonPGSimasJPEfstathiouS 2009 Immune control of mammalian gamma-herpesviruses: lessons from murid herpesvirus-4. J Gen Virol 90 2317 2330
52. HoffmanGJLazarowitzSGHaywardSD 1980 Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A 77 2979 2983
53. MillerGHestonLHoffmanG 1982 Neutralization of lymphocyte immortalization by different strains of Epstein-Barr virus with a murine monoclonal antibody. Infect Immun 37 1028 1031
54. SashiharaJBurbeloPDSavoldoBPiersonTCCohenJI 2009 Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology 391 249 256
55. MoutschenMLeonardPSokalEMSmetsFHaumontM 2007 Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 25 4697 4705
56. MillerGNiedermanJCStittDA 1972 Infectious mononucleosis: appearance of neutralizing antibody to Epstein-Barr virus measured by inhibition of formation of lymphoblastoid cell lines. J Infect Dis 125 403 406
57. RosaGTGilletLSmithCMde LimaBDStevensonPG 2007 IgG fc receptors provide an alternative infection route for murine gamma-herpesvirus-68. PLoS One 2 e560
58. ChandranBSmithMSKoelleDMCoreyLHorvatR 1998 Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology 243 208 217
59. SzakonyiGKleinMGHannanJPYoungKAMaRZ 2006 Structure of the Epstein-Barr virus major envelope glycoprotein. Nat Struct Mol Biol 13 996 1001
60. JohnsonWEDesrosiersRC 2002 Viral persistence: HIV's strategies of immune system evasion. Annu Rev Med 53 499 518
61. WuXYangZYLiYHogerkorpCMSchiefWR 2010 Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329 856 861
62. ZhouTGeorgievIWuXYangZYDaiK 2010 Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329 811 817
63. LeeJESaphireEO 2009 Neutralizing ebolavirus: structural insights into the envelope glycoprotein and antibodies targeted against it. Curr Opin Struct Biol 19 408 417
64. LeeJEFuscoMLHessellAJOswaldWBBurtonDR 2008 Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454 177 182
65. BackNKSmitLDe JongJJKeulenWSchuttenM 1994 An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199 431 438
66. ReitterJNMeansREDesrosiersRC 1998 A role for carbohydrates in immune evasion in AIDS. Nat Med 4 679 684
67. LiYClevelandBKlotsITravisBRichardsonBA 2008 Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol 82 638 651
68. PantophletRBurtonDR 2006 GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24 739 769
69. YangXKurtevaSLeeSSodroskiJ 2005 Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J Virol 79 3500 3508
70. PiersonTCXuQNelsonSOliphantTNybakkenGE 2007 The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1 135 145
71. GilletLStevensonPG 2007 Antibody evasion by the N terminus of murid herpesvirus-4 glycoprotein B. EMBO J 26 5131 5142
72. HelleFVieyresGElkriefLPopescuCIWychowskiC 2010 Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol 84 11905 11915
73. DubuissonJGuillaumeJBoulangerDThiryEBublotM 1990 Neutralization of bovine herpesvirus type 4 by pairs of monoclonal antibodies raised against two glycoproteins and identification of antigenic determinants involved in neutralization. J Gen Virol 71 Pt 3 647 653
74. BillerMMardbergKHassanHClausenHBolmstedtA 2000 Early steps in O-linked glycosylation and clustered O-linked glycans of herpes simplex virus type 1 glycoprotein C: effects on glycoprotein properties. Glycobiology 10 1259 1269
75. DooresKJFultonZHongVPatelMKScanlanCN 2010 A nonself sugar mimic of the HIV glycan shield shows enhanced antigenicity. Proc Natl Acad Sci U S A 107 17107 17112
76. VanderplasschenAMarkine-GoriaynoffNLomontePSuzukiMHiraokaN 2000 A multipotential beta -1,6-N-acetylglucosaminyl-transferase is encoded by bovine herpesvirus type 4. Proc Natl Acad Sci U S A 97 5756 5761
77. BalzariniJ 2007 Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol 5 583 597
78. BalzariniJ 2005 Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis 5 726 731
79. AstronomoRDBurtonDR 2010 Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov 9 308 324
80. DonofrioGvan SantenVL 2001 A bovine macrophage cell line supports bovine herpesvirus-4 persistent infection. J Gen Virol 82 1181 1185
81. HuynhHTRobitailleGTurnerJD 1991 Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp Cell Res 197 191 199
82. GilletLSchroederHMastJThirionMRenauldJC 2009 Anchoring tick salivary anti-complement proteins IRAC I and IRAC II to membrane increases their immunogenicity. Vet Res 40 51
83. ThiryEPastoretPPDessy-DoizéCHanzenCCalberg-BacqCM 1981 Herpesvirus in infertile bull's testicle. Vet rec 108 426
84. DubuissonJThiryEBublotMSneyersMBoulangerD 1989 Production and characterization of monoclonal antibodies to bovid herpesvirus-4. Vet Microbiol 19 305 315
85. WarmingSCostantinoNCourtDLJenkinsNACopelandNG 2005 Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33 e36
86. DewalsBBoudryCFarnirFDrionPVVanderplasschenA 2008 Malignant catarrhal fever induced by alcelaphine herpesvirus 1 is associated with proliferation of CD8+ T cells supporting a latent infection. PLoS ONE 3 e1627
87. BoudryCMarkine-GoriaynoffNDelforgeCSpringaelJYde LevalL 2007 The A5 gene of alcelaphine herpesvirus 1 encodes a constitutively active G-protein-coupled receptor that is non-essential for the induction of malignant catarrhal fever in rabbits. J Gen Virol 88 3224 3233
88. ThompsonJDGibsonTJPlewniakFJeanmouginFHigginsDG 1997 The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 4876 4882
89. BendtsenJDNielsenHvon HeijneGBrunakS 2004 Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340 783 795
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



