-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
Plants have evolved pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI) to protect themselves from infection by diverse pathogens. Avirulence (Avr) effectors that trigger plant ETI as a result of recognition by plant resistance (R) gene products have been identified in many plant pathogenic oomycetes and fungi. However, the virulence functions of oomycete and fungal Avr effectors remain largely unknown. Here, we combined bioinformatics and genetics to identify Avr3b, a new Avr gene from Phytophthora sojae, an oomycete pathogen that causes soybean root rot. Avr3b encodes a secreted protein with the RXLR host-targeting motif and C-terminal W and Nudix hydrolase motifs. Some isolates of P. sojae evade perception by the soybean R gene Rps3b through sequence mutation in Avr3b and lowered transcript accumulation. Transient expression of Avr3b in Nicotiana benthamiana increased susceptibility to P. capsici and P. parasitica, with significantly reduced accumulation of reactive oxygen species (ROS) around invasion sites. Biochemical assays confirmed that Avr3b is an ADP-ribose/NADH pyrophosphorylase, as predicted from the Nudix motif. Deletion of the Nudix motif of Avr3b abolished enzyme activity. Mutation of key residues in Nudix motif significantly impaired Avr3b virulence function but not the avirulence activity. Some Nudix hydrolases act as negative regulators of plant immunity, and thus Avr3b might be delivered into host cells as a Nudix hydrolase to impair host immunity. Avr3b homologues are present in several sequenced Phytophthora genomes, suggesting that Phytophthora pathogens might share similar strategies to suppress plant immunity.
Published in the journal: Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity. PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002353
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002353Summary
Plants have evolved pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI) to protect themselves from infection by diverse pathogens. Avirulence (Avr) effectors that trigger plant ETI as a result of recognition by plant resistance (R) gene products have been identified in many plant pathogenic oomycetes and fungi. However, the virulence functions of oomycete and fungal Avr effectors remain largely unknown. Here, we combined bioinformatics and genetics to identify Avr3b, a new Avr gene from Phytophthora sojae, an oomycete pathogen that causes soybean root rot. Avr3b encodes a secreted protein with the RXLR host-targeting motif and C-terminal W and Nudix hydrolase motifs. Some isolates of P. sojae evade perception by the soybean R gene Rps3b through sequence mutation in Avr3b and lowered transcript accumulation. Transient expression of Avr3b in Nicotiana benthamiana increased susceptibility to P. capsici and P. parasitica, with significantly reduced accumulation of reactive oxygen species (ROS) around invasion sites. Biochemical assays confirmed that Avr3b is an ADP-ribose/NADH pyrophosphorylase, as predicted from the Nudix motif. Deletion of the Nudix motif of Avr3b abolished enzyme activity. Mutation of key residues in Nudix motif significantly impaired Avr3b virulence function but not the avirulence activity. Some Nudix hydrolases act as negative regulators of plant immunity, and thus Avr3b might be delivered into host cells as a Nudix hydrolase to impair host immunity. Avr3b homologues are present in several sequenced Phytophthora genomes, suggesting that Phytophthora pathogens might share similar strategies to suppress plant immunity.
Introduction
Plant innate immunity, which has been continuously refined by challenges from a diversity of pathogens during evolution, employs at least two defense systems in response to pathogen attacks [1]. One is basal defense through host receptor recognition of conserved pathogen-associated molecular patterns (PAMPs), termed PAMP-triggered immunity (PTI). In most cases, the basal defense response can successfully prevent infections from becoming established. However, successful pathogens deliver secreted proteins (effectors) to suppress basal defense. Therefore, plants have developed a second immunity system that relies on plant resistance (R) protein perception of specific pathogen effectors and is called effector-triggered immunity (ETI). Pathogen effectors recognized by plant R proteins have historically been termed avirulence (Avr) effectors [1], [2]. ETI usually results in faster and stronger plant resistance responses, including programmed cell death (PCD), reactive oxygen species (ROS) accumulation, and induction of plant hormone-mediated signal pathways [1]. Thus, the interaction between host R proteins and pathogen Avr effectors can determine the outcome of an infection.
Many plant R genes encode polymorphic proteins characterized by nucleotide binding (NB) and leucine-rich repeat (LRR) domains. These R proteins are located inside the cell, indicating that Avr effectors are usually delivered into plant cells by pathogens [2], [3]. A motif, RXLR (Arg-any-Leu-Arg), was identified in the N-terminus of Phytophthora effectors such as P. sojae Avr1b and P. infestans Avr3a [3], [4], [5], [6] and was experimentally shown to be a host-targeting motif that could translocate effectors into host cells [7], [8], [9]. Recent evidence has shown that Phytophthora RXLR effectors could enter host cells without pathogen machinery by binding to the host external lipid phosphatidylinositol-3-phosphate (PI3P), suggesting that the RXLR-PI3P-mediated cell-entering mechanism is used by pathogens to deliver effectors into host cells [10]. Interestingly, nearly all of the Avr effectors identified from oomycetes so far carry the RXLR motif. These Avr effectors include Avr1b, Avr1a, Avr3a/5, Avr3c, and Avr4/6 from P. sojae; Avr-blb1, Avr-blb2, Avr2, Avr3a, and Avr4 from P. infestans; and ATR1 and ATR13 from the downy mildew pathogen Hyaloperonospora arabidopsidis [3], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. Although a recently cloned H. arabidopsidis avirulence effector, ATR5, does not employ a strict RXLR motif [21], RXLR motifs are still considered to be characteristic signatures of oomycete Avr effectors. Based on the genome sequencing of several oomycete plant pathogens, a large number of RXLR effector candidates were predicted. Altogether, more than 1200 RXLR effector candidates were predicted in the genomes of P. sojae, P. infestans, P. ramorum, and H. arabidopsidis [5], [6], [22], [23]. The RXLR motif and the large collection of predicted RXLR effector candidates provide a good resource for Avr effector identification.
Avr effectors are assumed to be important virulence factors that function by manipulating host immunity. However, only a few fungal and oomycete effectors have been functionally characterized [1], [2]. In the fungal pathogen Cladosporium fulvum, the Avr effector Avr2 is a cysteine protease inhibitor, and Avr4 acts as a chitin-binding protein that protects C. fulvum against host chitinases [24], [25]. The Avr-Pita effector is a zinc-dependent metalloprotease that is required by the fungus Magnaporthe oryzae for full virulence on rice [26], [27]. The polyketide synthase activity of M. oryzae Ace1 is required for avirulence [27]. In the flax rust Melampsora lini, AvrP123 effectors contain a Kazal protease inhibitor signature, but the activity has not been verified [28]. Recently, Fusarium oxysporum f. sp. lycopersici Avr1 was found to suppress Avr2 - and Avr3-triggered ETI in tomato [29]. Avr1b and many other effectors from P. sojae could abolish cell death triggered in plants by BAX (a mammalian pro-apoptotic factor), by the PAMP infestin1 (INF1) and by several effectors [30], [31]. Downy mildew H. arabidopsidis Avr effectors ATR1 and ATR13 could enhance bacterial virulence on susceptible hosts, while some ATR13 alleles could suppress bacterial PAMP-triggered callose deposition or reduce PAMP-triggered ROS production [32]. Phytophthora infestans Avr3a could interact with and stabilize host U-box E3 ligase CMPG1, preventing it from being degraded by the proteasome system and subsequently blocking triggered cell death triggered by INF1 [33]. However, the virulence mechanism of most oomycete and fungal Avr effectors remains to be further explored.
Here, we identify a new oomycete Avr effector, Avr3b, from P. sojae that is recognized by soybean plants containing the R gene Rps3b. Biochemical assays validate the bioinformatic prediction that Avr3b has ADP-ribose/NADH pyrophosphorylase activity. Avr3b contributes to virulence through suppression of plant immunity and that suppression is dependent on enzyme activity. However the enzyme activity of Avr3b is not required for recognition by Rps3b-containing plants.
Results
RXLR effector candidates for Avr3b
Briefly summarized, previous studies have described the following features of Avr effectors [12], [13], [14]. (1) The transcripts of Avr effectors are detectable in avirulent strains; (2) all of the Phytophthora Avr effectors cloned so far possess the RXLR motif; and (3) many Avr proteins are polymorphic. Previous genetic studies also indicated that Avr3b segregates as a single dominant gene [34]. Thus, we hypothesized that Avr3b was likely encoded by an infection-expressed RXLR effector that is polymorphic among P. sojae strains. To identify expressed RXLR effector candidates in avirulent strains, we examined transcription of potential RXLR effector genes in P. sojae using digital gene expression (DGE), Affymetrix arrays, and expressed sequence tags (ESTs). The DGE method resulted in the identification of 81 expressed RXLR effector genes [35]. A BLAST search in the P. sojae EST database identified 16 expressed RXLR effector genes. After the addition of 59 previously identified expressed RXLR effector genes from microarray analysis [12], [31], a total of 119 RXLR effectors were detected as expressed in the P. sojae Avr3b avirulent strain P6497 (Table S1).
Genomic and mitochondrial DNA RFLP results delineated P. sojae strains into four major genetic lineages [36]. Phytophthora sojae isolates P6497, P7064, P7074, and P7076 are representative isolates from each lineage. In addition to the published genome sequence of P6497, the re-sequencing of three P. sojae lineages (P7064, P7074, and P7076) has recently been completed [31]. Investigation of the 131 expressed effector sequences among these lineages revealed that six effectors (Avh20, Avh113, Avh238, Avh258, Avh288, and Avh307) showed a sequence polymorphism pattern consistent with the Avr3b phenotype; namely, identical in P6497 and P7074 (Avr3b avirulent strains) but variant in P7074 and P7076 (virulent strains) (Table 1). Furthermore, Avh6 showed a presence/absence polymorphism between avirulent and virulent strains (Table 1). Thus, these seven effectors were selected as Avr3b candidates for further investigation.
Tab. 1. Seven expressed RXLR effectors showed Avr3b polymorphism. 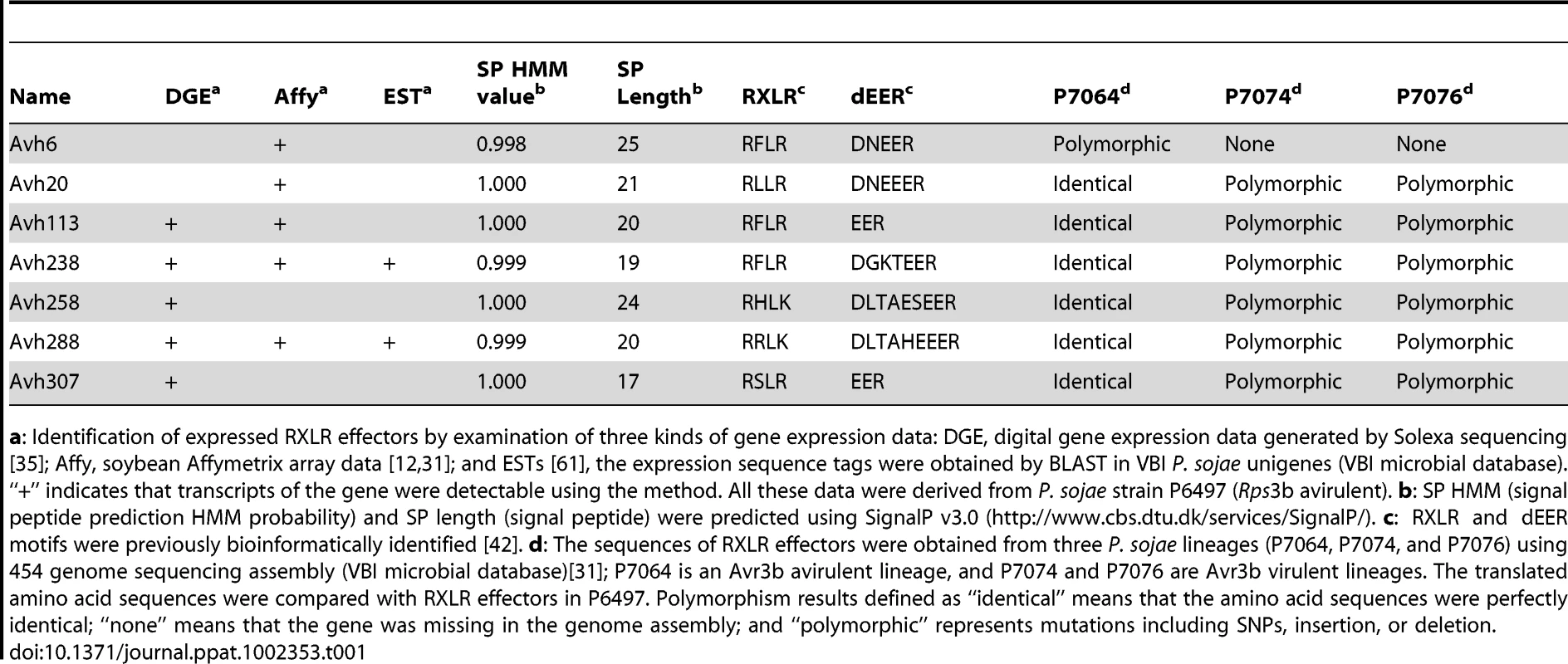
a: Identification of expressed RXLR effectors by examination of three kinds of gene expression data: DGE, digital gene expression data generated by Solexa sequencing [35]; Affy, soybean Affymetrix array data [12], [31]; and ESTs [61], the expression sequence tags were obtained by BLAST in VBI P. sojae unigenes (VBI microbial database). “+” indicates that transcripts of the gene were detectable using the method. All these data were derived from P. sojae strain P6497 (Rps3b avirulent). b: SP HMM (signal peptide prediction HMM probability) and SP length (signal peptide) were predicted using SignalP v3.0 (http://www.cbs.dtu.dk/services/SignalP/). c: RXLR and dEER motifs were previously bioinformatically identified [42]. d: The sequences of RXLR effectors were obtained from three P. sojae lineages (P7064, P7074, and P7076) using 454 genome sequencing assembly (VBI microbial database)[31]; P7064 is an Avr3b avirulent lineage, and P7074 and P7076 are Avr3b virulent lineages. The translated amino acid sequences were compared with RXLR effectors in P6497. Polymorphism results defined as “identical” means that the amino acid sequences were perfectly identical; “none” means that the gene was missing in the genome assembly; and “polymorphic” represents mutations including SNPs, insertion, or deletion. Genetic co-segregation of the Avh307 genotype with the Avr3b phenotype
To determine whether candidate effectors might match Avr3b, we conducted genetic mapping to identify effectors that co-segregated with the Avr3b avirulence phenotype. First, we crossed P6497 and P7076, and identified 3 F1 individuals. All the 3 F1 individuals showed avirulence on Rps3b soybeans. Then, one F1 individual was selected for self-crossing, resulting in a total of 71 F2 individuals (P. sojae is diploid and homothallic, and wild-type strains show low levels of heterozygosity, hence segregation for most loci occurs in the F2 generation). Avirulence assays of these progeny on soybean plants carrying resistance gene Rps3b distinguished 51 avirulent and 20 virulent F2 individuals. The observed F2 population segregation ratio for Avr3b fit a 3∶1 ratio (χ2 = 0.38, p >0.05), suggesting that the Avr3b avirulence phenotype is conferred by a single dominant allele, consistent with a previous report [34]. Cleaved amplified polymorphic (CAP) DNA markers were designed based on the sequence polymorphisms of the candidate effector genes (Table S2). The Avh307 genotype was found to perfectly match (0% recombination) the Avr3b avirulence phenotype among the 71 F2 progeny (Figure 1A). In contrast, Avh238 (8.3% recombination), Avh288 (29%), Avh20 (33%), Avh6 (40%), Avh113 (35%), and Avh258 (31%) did not match.
Fig. 1. Avh307 corresponds to Avr3b based on genetic mapping, sequence polymorphisms, and bioassays. 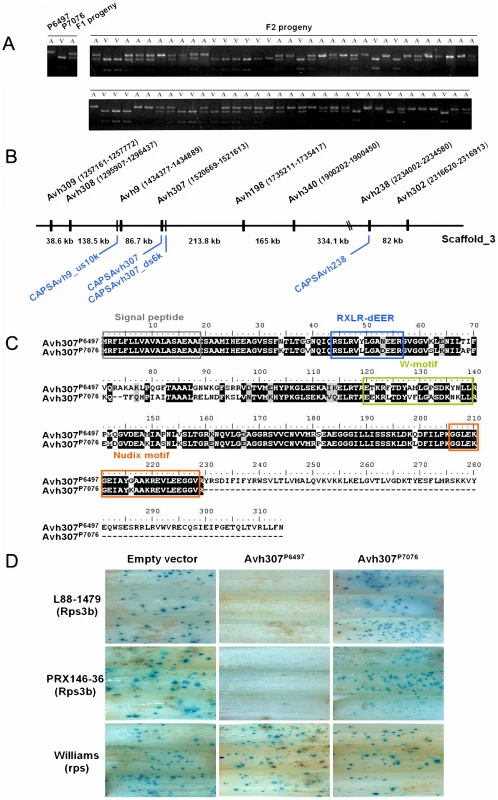
(A) Co-segregation of Avh307 with the Avr3b virulence phenotype in F2 progeny from a cross between P. sojae isolates P6497 and P7076. A virulence assay was performed with cultures scored as virulent (V) or avirulent (A) on Rps3b soybean plants. Cleaved amplified polymorphic (CAP) markers, co-dominant and specific for Avh307, were scored using genomic DNA from the avirulent (A) and virulent (V) parents and F1 and F2 progeny. Shown is a photograph of an ethidium bromide-stained agarose gel of the CAP marker results for Avh307. (B) Physical map of the Avh307 region. Predicted RXLR genes and CAP DNA markers for genetic mapping are shown. Genes (black color) and CAP markers (blue color) are shown on P. sojae P6497 genome sequence scaffold_3 (assembly version 5.0), not to scale. (C) Predicted amino acid sequences of Avh307P6497 (from P6497 and other Rps3b-avirulent strains) and Avh307P7076 (from P7076 and other Rps3b-virulent strains). Predicted signal peptide, RXLR-dEER motif, W-motif, and a Nudix hydrolase motif are shown in grey, blue, green and orange frames. (D) Transient expression assays of Avh307 alleles in Rps3b plant tissue. Soybean hypocotyls of cultivars Williams (rps), L88-1479 (Rps3b), and PRX146-36 (Rps3b) were transformed by co-bombardment with a plasmid mixture consisting of a glucuronidase reporter gene (GUS) expression vector and a test construct. Expression of the GUS reporter and Avh307 alleles were controlled by the 35S promoter. The test constructs used in combination with the GUS reporter are shown on the top of the panel. Avh238 and Avh307 are genetically linked and are located on the same genome sequence scaffold, 713 kb apart (Avh238, scaffold_3 : 2234002–2234580; Avh307, scaffold_3 : 1520669–1521613) (Figure 1B). Examination of 1.06 Mb of DNA sequence surrounding Avh307 revealed another six RXLR effector genes (Avh309, Avh308, Avh9, Avh198, Avh340, and Avh302) in this region (Figure 1B; Table S3). Analysis by RT-PCR of all of these effector candidates revealed that only Avh309 and Avh340 were expressed in P6497 during infection (Table S3). However, neither Avh309 nor Avh340 displayed sequence polymorphisms. The predicted effector gene Avh308 displayed polymorphisms but was not expressed during infection. To generate a more detailed genetic map of the Avr3b locus, we identified another two markers (CAPSAvh307_ds6k and CAPSAvh9_us10k) in the vicinity of Avh307. The marker CAPSAvh307_ds6k (scaffold_3 : 1515512–1516432) is located 6 kb downstream of Avh307; this marker also co-segregated with the Avr3b avirulence phenotype without recombination in our 71 F2 progeny. The marker CAPSAvh9_us10 k (scaffold_3 : 1424151–1424781) is located 96 kb upstream of Avh307; this marker showed 4.4% recombination with the Avr3b avirulence phenotype (Figure 1B). Thus, of the eight RXLR genes examined in this region, Avh307 emerged as the best candidate for Avr3b (Table S3).
Oomycete Avr effectors often show significant sequence polymorphisms among field populations [12], [13], [14]. We examined Avh307 polymorphisms among 20 P. sojae field isolates from China. As shown in Figure 1C, Avh307 from each of the 16 avirulent strains encoded a 315 amino acid protein identical in sequence to Avh307P6497. However, the four virulent isolates encoded a protein identical to Avh307P7076 which is truncated to 230 amino acids by a premature stop codon (Figure 1C). Outside of the truncated region, an additional 46 amino acid substitutions and two deletions were present in Avh307P7076 compared to Avh307P6497.
In addition to a secretory signal peptide and RXLR and dEER motifs, the sequence of the Avh307P6497 allele contains a W motif [30], and a nuclear diphosphate hydrolase (Nudix) motif, G5XE7XREUXEEXGU (Conserved Domain Database ID: cd04666, E-value = 1.52e-07) (Figure 1C). The Avh307P7076 allele retained all of these motifs but with polymorphic sites in the dEER-, Nudix-, and W-motifs, as illustrated in Figure 1C.
Expression of Avh307 from avirulent strains triggers Rps3b-mediated cell death
The proteins encoded by P. sojae Avr genes trigger cell death when they are expressed in or enter into soybean cells containing their matching soybean Rps proteins. To test whether Avh307 alleles could trigger cell death in the presence of Rps3b, we conducted a soybean transient expression experiment by co-bombardment of soybean hypocotyls to measure cell death [37]. Plasmid constructs carrying Avh307 alleles encoding mature proteins without native signal peptides were delivered by particle bombardment into soybean hypocotyls, along with a beta-glucuronidase (GUS) reporter gene to measure cell survival. The Avh307P6497 allele triggered cell death in the presence of Rps3b, but not in the absence of Rps3b, as evidenced by a strong reduction in GUS staining. This experiment was performed on two different soybean lines carrying the Rps3b gene and on control plants without Rps3b (Figure 1D). In contrast, expression of Avh307P7076 did not reduce GUS staining in the presence of the Rps3b gene, nor in its absence (Figure 1D). To further confirm our results, we performed a double-barreled bombardment which could compare cell death in a more direct fashion [30]. These data confirmed that expression of the Avh307P6497 allele strongly triggered cell death on Rps3b soybeans, but no reaction on near-isogenic soybean lines that do not contain Rps3b (Figure S1). Avh307P7076, the virulence allele, did not induce significant cell death on Rps3b plants. Thus, both kinds of co-bombardment assays provided functional evidence that Avh307 is Avr3b, and that Avh307P6497 and Avh307P7076 are avirulence and virulence alleles, respectively, of Avr3b.
The Avr3b gene is expressed during infection and shows transcriptional polymorphisms between virulent and avirulent strains
To explore how Avr3b may be involved in P. sojae-soybean interactions, we examined the Avr3b transcriptional profile at different stages of infection and among different isolates. To define the transcriptional pattern of Avr3b, we isolated total RNA from mycelia, zoospores, cysts, germinating cysts, and from infected susceptible soybean leaves at 6 hours post-infection (hpi), 12 hpi, and 24 hpi. Real-time RT-PCR data indicated that Avr3b transcript levels are significantly elevated during infection (Figure 2A). Compared with in vitro grown mycelia, the Avr3b transcript level was elevated 20-fold, 150-fold, and 50-fold in the cyst, germinating cyst, and 24-hpi stages, respectively. These data indicate that Avr3b is strongly induced in pre-infection structures and during infection.
Fig. 2. Avr3b is induced during infection and shows transcriptional polymorphisms. 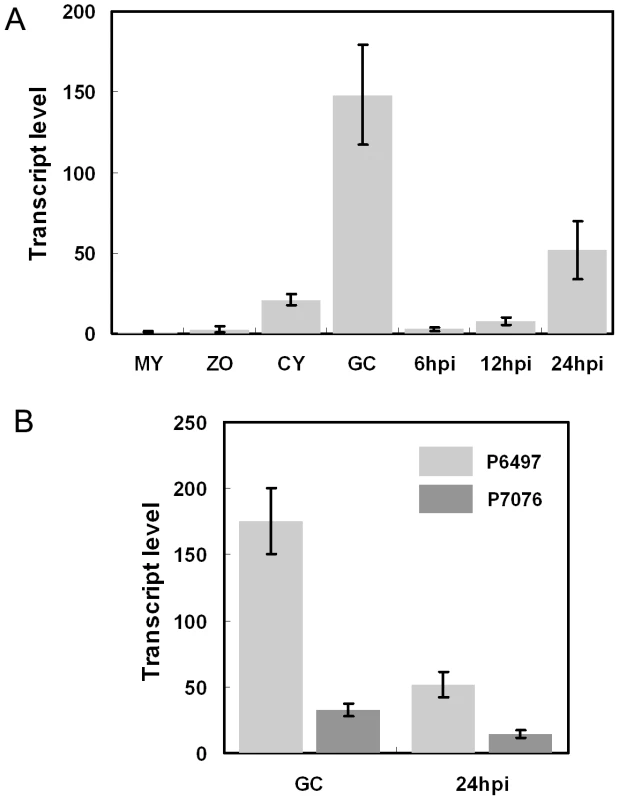
(A) Avr3b transcript levels in different developmental and infection stages measured by real-time RT-PCR. Total RNA was extracted from mycelia (MY), zoospores (ZO), cysts (CY), germinating cysts (GC), and infected soybean leaves at 6 hours post-infection (hpi), 12 hpi, and 24 hpi. All inoculations for RNA isolation were performed on susceptible soybean cultivar Williams. Real-time RT-PCR analysis employed primers specific for Avr3b and the actin gene. Transcrip level represent the P. sojae Avr3b mRNA levels compared with actin mRNA levels. Bars represent standard errors from 2 independent replicates each. (B) Avr3b transcriptional polymorphism between virulent (P7076) and avirulent (P6497) strains. Real-time RT-PCR analysis was conducted to compare Avr3b transcript levels at two infection-related stages, germinating cysts (GC) and infected leaves (24 hpi). Primers used in this assay amplified identical regions from Avr3bP6497 and Avr3bP7076. Bars represent standard errors from 2 independent replicates each. P. sojae Avr effector genes Avr1b, Avr1a, and Avr3a all showed transcriptional polymorphisms among P. sojae strains [12], [13], [14]. To test for transcriptional polymorphisms of Avr3b, we compared Avr3b transcript levels in the germinating cyst and 24-hpi stages between P. sojae isolates P6497 (avirulent) and P7076 (virulent) using real-time RT-PCR. In P6497 compared to P7076, Avr3b transcript levels were five times higher at the germination cyst stage and three times higher at the 24-hpi stage (Figure 2B), indicating that Avr3b exhibits significant transcriptional polymorphism. However, despite the relatively lower expression level, transcripts of the Avr3b virulence allele remain detectable, indicating that it might be required during infection.
Avr3b enhances Phytophthora virulence on N. benthamiana and decreases ROS accumulation
Several oomycete avirulence effectors can suppress aspects of plant immunity [8], [32], [38]. To determine whether Avr3b interferes with plant immunity, we transiently expressed recombinant Avr3bP6497 (without a signal peptide) fused with a FLAG tag in N. benthamiana using agroinflitration with a potato virus X (PVX) vector. Total protein samples were isolated from agroinfiltrated plant leaves and subjected to western blot analysis, employing an anti-FLAG monoclonal antibody as a probe. The results show expression of a protein of the expected size (34-kDa) in the tissue infiltrated with the Agrobacterium cells carrying the FLAG:Avr3bP6497 construct (Figure 3A), consistent with expression of FLAG:Avr3bP6497 in N. benthamiana.
Fig. 3. Expression of Avr3bP6497 in N. benthamiana increased Phytophthora susceptibility and suppressed ROS accumulation. 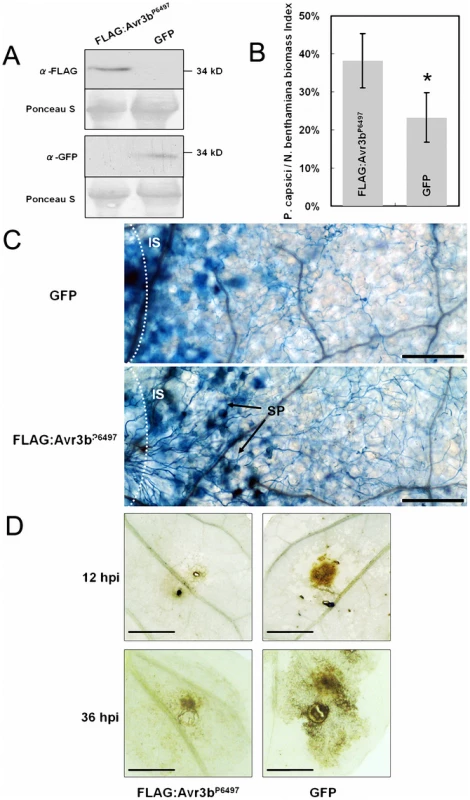
(A) Western blot assay of Avr3bP6497 and GFP protein levels in N. benthamiana leaves following Agrobacterium-mediated transient expression. Anti-FLAG monoclonal antibody was used to detect levels of FLAG-tagged Avr3bP6497 protein. Anti-GFP monoclonal antibody was used to detect GFP protein levels. (B) Real-time PCR measurement of pathogen and plant DNA ratios was used to determine P. capsici biomass in infected plant tissues following Agrobacterium-mediated transient expression of FLAG-Avr3b or GFP. Nicotiana benthamiana leaves infiltrated with Agrobacterium carrying different constructs were grown in a greenhouse for 48 hours then inoculated with agar plugs containing P. capsici mycelium. DNA from P. capsici infected regions was isolated at 36 hpi. Real-time PCR employed primers specific for the N. benthamiana and P. capsici actin genes. Statistical analysis was performed by the Wilcoxon rank sum test (asterisk indicates p<0.01). Bars represent standard errors from 6 replicates (two technical replicates each from three biological replicates). (C) Photomicrographs from P. capsici infected regions. Leaf regions transiently expressing Avr3bP6497 or GFP, and inoculated with P. capsici for 16 hours, were stained with trypan blue. Bar = 2.5 µm. IS: inoculation sites, SP: sporangia. (D) ROS generation during P. capsici infection following Avr3bP6497 or GFP transient expression in N. benthamiana leaves at 12 hpi (upper panel) and 36 hpi (lower panel). Leaves were stained with DAB. Bar = 5 mm. Typical results are shown from 4 replicates. Two Phytophthora pathogens of N. benthamiana, P. capsici and P. parasitica, were used to the challenge the infiltrated tissue. After 48 hours following Agrobacterium infiltration, we inoculated the infiltrated regions with an agar plug (5×5 mm) containing freshly grown P. capsici and P. parasitica mycelia, then evaluated disease development. On leaves infiltrated with strains carrying a control gene (GFP), the diameter of the disease lesions was approximately 0.7 to 0.8 cm at 36 hpi; however, on leaves infiltrated with strains carrying Avr3bP6497 the lesion diameter expanded to 1.3 to 1.4 cm, as shown in Figure S2. To more precisely measure P. capsici infection, we measured the ratio of P. capsici DNA to N. benthamiana DNA using real-time PCR to determine the Phytophthora biomass in the infected plant tissues (Figure 3B). The data show that the P. capsici/N. benthamiana biomass ratio was significantly higher in Avr3bP6497-infiltrated leaves (38%) than in GFP leaves (24%) (P value < 0.01). Both the lesion size data and the biomass data suggest that Avr3b expression in planta enhanced the susceptibility of N. benthamiana to Phytophthora.
To explore possible mechanisms behind the increased Phytophthora susceptibility, trypan blue and diaminobenzidine (DAB) staining were performed to examine infected plant tissues for the distribution of hyphae and for ROS production, respectively. At 16 hpi, a higher density of infected mycelium was observed in Avr3bP6497-transformed tissues (Figure 3C), consistent with the real-time PCR data showing greater P. capsici biomass in Avr3bP6497-expressing tissues. Furthermore sporangia had formed within the infected regions of Avr3bP6497-expressing leaves, whereas none were found within the infected regions of GFP-expressing leaves (Figure 3C). In contrast, there was more extensive trypan blue staining of plant cells in infected tissues expressing the GFP control than in those expressing Avr3bP6497, suggesting that more defense-related cell death may have occurred in those tissues. Substantially less DAB staining of 16-hpi plant tissues was observed in infected regions of Avr3bP6497-expressing leaves compared to the GFP control, suggesting that ROS accumulation in response to infection was significantly reduced in the Avr3bP6497-expressing tissue (Figure 3D). Overall, transient expression of Avr3bP6497 in N. benthamiana leaf tissue resulted in a greater degree of Phytophthora infection with less ROS accumulation and less cell death, compared to the GFP control.
Avr3b-like RXLR-Nudix proteins are conserved in Phytophthora species, but not in other oomycetes
To examine the distribution of Avr3b homologs in other oomycete species, we performed a BLAST similarity search in several oomycete genome databases. A search of the P. sojae genome identified another 12 predicted genes that had the Nudix motif. A total of 15 Nudix hydrolases were found in the P. infestans genome; four of these hydrolases (PITG05846, PITG06308, PITG15679, and PITG15732) had a signal peptide and RXLR motif. The P. ramorum genome encoded a total of 14 Nudix hydrolases; three (PrAvh165, PrAvh268, and PrAvh281) were RXLR effectors. Only one RXLR Nudix hydrolase (ID: 102433) was defined in the JGI P. capsici genome. We also searched other plant and animal oomycete databases, including H. arabidopsidis, Pythium ultimum, and Saprolegnia parasitica without finding any predicted RXLR Nudix hydrolase effectors, suggesting that this gene family might be present only in the Phytophthora genus. Thus, an Avr3b family composed of nine members from four Phytophthora species was identified (Table S4).
Sequence alignment of Avr3b family members plus two characterized Nudix hydrolases (AtNUDT7 from Arabidopsis and ScYSA1 from Saccharomyces cerevisiae) revealed that all of these sequences contain the conserved residues of the Nudix hydrolase motif (GX5EX7REUXEEXGU) (Figure 4A). The Nudix motif is located at the C-terminus of each of the oomycete effectors. The motif is located at the extreme C-terminus in many family members including P. capsici Pc102433, P. infestans PITG05846, PITG06308, PITG15679, and the three P. ramorum Nudix hydrolase effectors, a pattern similar to that of Avr3bP7076 (Figure S3). However, in Avr3bP6497 and PITG15732 the motif is closer to the middle of the protein, which is more similar to AtNUDT7 and ScYSA1.
Fig. 4. Avr3b is an ADP-ribose/NADH pyrophosphorylase. 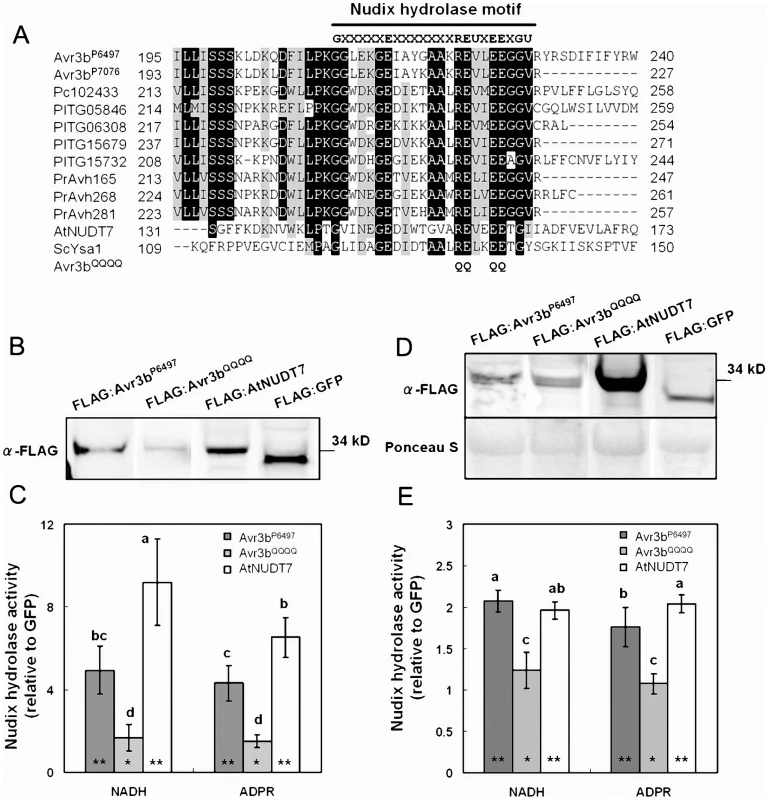
(A) Sequence alignment of the Nudix motif region from Avr3b homologs and characterized nudix hydrolases. Pc102433 is a predicted P. capsici RXLR effector. PITG05846, PITG06308, PITG15679, and PITG15732 are predicted P. infestans RXLR effectors. PrAvh165, PrAvh268, and PrAvh281 are P. ramorum RXLR effectors. AtNUDT7 is an ADP-ribose/NADH pyrophosphorylase from Arabidopsis. ScYSA1 is an ADP-ribose pyrophosphorylase from Saccharomyces cerevisiae. The black line refers to the Nudix hydrolase motif “G5XE7XREUXEEXGU.” The threshold for amino acid shading in this alignment is 70%. Avr3bQQQQ is an Avr3bP6497 non-functional mutant; the glutamine (Q) substitutions in the nudix hydrolase motif are marked. (B) Western blot of purified Avr3b and control proteins. Immuno-precipitated recombinant proteins FLAG:AtNUDT7, FLAG:GFP, FLAG:Avr3bP6497 and predicted non-functional Avr3b mutant FLAG:Avr3bQQQQ were detected with anti-FLAG antibody. (C) Enzyme assays of purified Avr3b and control proteins. The hydrolase activities of FLAG:AtNUDT7, FLAG:Avr3bP6497 and FLAG:Avr3bQQQQ were measured separately with FLAG:GFP as a control for each assay. The relative hydrolase activity was calculated as a ratio of Avr3b/AtNUDT7 over GFP. ADPR: adenosine diphosphate ribose, NADH: nicotinamide adenine dinucleotide (reduced form). Bars represent standard errors from 6 independent replicates each. Student's t-test was used to compare whether testing sample activities are significantly different from the GFP control. The double asterisk and single asterisk indicates statistical significance p <0.01 and p <0.05, respectively. The letters represent statistical significance (P <0.01) as measured by Duncan's multiple range test. (D) Western blot of the total plant extract from the Agrobacterium infiltrated region. The supernatants from FLAG:AtNUDT7, FLAG:GFP, FLAG:Avr3bP6497 and FLAG:Avr3bQQQQ transgenic N. benthamiana leaves were electrophoresed and detected with anti-FLAG antibody. (E) Enzyme assays of the total protein extracts from the Agrobacterium infiltrated region. The relative hydrolase activities were measured as a ratio to extracts from a GFP-expression control. Bars represent standard errors from 4 independent replicates each. Student's t-test was used to compare whether testing sample activities are significantly different from the GFP control. The double asterisk and single asterisk indicates statistical significance p <0.01 and p <0.05, respectively. The letters represent statistical significance (P <0.01) as measured by Duncan's multiple range test. Avr3b has ADP-ribose/NADH pyrophosphorylase activity
To examine whether Avr3b has Nudix hydrolase activity, and to identify possible substrates for it, we expressed FLAG:Avr3bP6497 (with the FLAG tag fused to the Avr3b N-terminus) in N. benthamiana (attempts to produce active protein in E. coli were unsuccessful). Western blot data showed a clear 34∼35 kDa band which suggested the recombinant protein was expressed. As a positive control, we fused AtNUDT7 from Arabidopsis with a FLAG tag. We then conducted immuno-precipitation using anti-FLAG M2 affinity gel. After immuno-precipitation, western blotting of the elution samples showed clear signals, indicating that, Avr3b protein had been recovered (Figure 4B). To test the hydrolase activity of the precipitated FLAG:Avr3bP6497 protein and its possible substrates, relative hydrolase activity was measured as a ratio of FLAG:Avr3bP6497 samples over immuno-precipitated GFP, using a series of nucleotide derivatives as substrates. The results showed that FLAG:AtNUDT7 and FLAG:Avr3bP6497 immunoprecipitates could both hydrolyze ADPR and NADH, as shown in Figure 4C and Table S5. The AtNUDT7 showed higher activity than Avr3bP6497. To confirm Avr3bP6497 that is a nudix hydrolase, and to validate that the hydrolase activity in the immunoprecipitates was due to Avr3b, we mutated Avr3bP6497 at four conserved residues (R220Q, E221Q, E225Q, E226Q simultaneously, Figure 4A) to generate a non-functional Nudix mutant [39], called Avr3bQQQQ. The hydrolase activity of immunoprecipitated Avr3bQQQQ was significantly weaker than Avr3bP6497, on either ADPR or NADH, and not significantly greater than the GFP control (Figure 4C). Besides ADPR and NADH, other nucleotide derivatives including NADPH, NAD, Ap4A, FAD and Coenzyme A were also examined as possible substrates for Avr3bP6497. The Avr3bP6497 immunoprecipitate caused weak but significant hydrolysis of all these derivatives except NAD, whereas there was no significant hydrolysis by the Avr3bQQQQ immunoprecipitate, except possibly of NADPH (Table S5). In our assay, the hydrolase data from AtNUDT7 (Figure 4C), a positive control, is consistent with previous reports [40]. Together, these data are consistent with the hypothesis that Avr3bP6497 contains ADP-ribose and NADH pyrophosphorylase activity.
To measure the elevation of total nudix hydrolase activity in the plant tissue resulting from Avr3bP6497 expression, we examined the hydrolase activity of total plant extracts. Avr3bP6497, Avr3bQQQQ , GFP and AtNUDT7 were transiently expressed in N. benthamiana by using Agrobacterium infiltration. Western blotting of total plant extracts confirmed expression of the proteins (Figure 4D). Consistent with the results from the immunoprecipitated protein, the extracts from Avr3bP6497-expressing plant tissue showed significantly elevated hydrolase activity with NADH and ADPR than the control whereas expression of Avr3bQQQQ did not significantly elevate the hydrolase activity (Figure 4E). No significant elevation of hydrolase activity against NADPH, NAD, Ap4A, FAD or Coenzyme A could be detected in total extracts from Avr3bP6497 - and Avr3bQQQQ-expressing tissue (Table S5). Thus, in planta expression of Avr3bP6497 elevated the ADP-ribose/NADH pyrophosphorylase activity in the plant tissue by approximately two-fold.
Avr3b virulence functions depend on its nudix motif, but its avirulence activity does not
To examine the relationship between Avr3b's Nudix motif and its virulence and avirulence activities, we fused a FLAG tag to the N-terminus of Avr3bP6497, Avr3bQQQQ, Avr3bP7076 (virulence allele), Avr3b16-174 (N-terminus of Avr3bP6497), and Avr3b170-315 (C-terminal of Avr3bP6497) (Figure 5A). These proteins were then transiently expressed in N. benthamiana leaves using agroinfiltration. Western blots confirmed that all of the proteins were expressed in planta with the expected sizes (Figure 5B).
Fig. 5. Functional assays of Avr3b alleles and mutants. 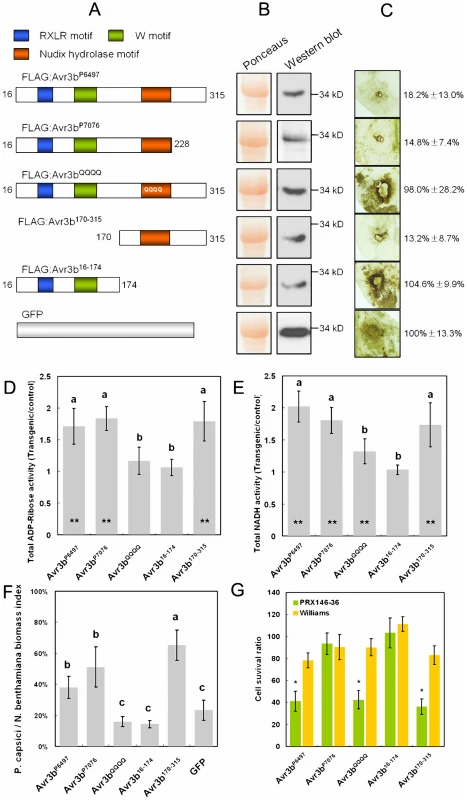
(A) Schematic view of pGR107-FLAG:Avr3b constructs. Avr3b16-174, Avr3b170-315 and Avr3bQQQQ are derived from the Avr3bP6497 avirulence allele. (B) Western blot confirmation of in planta expression of Avr3b alleles and mutants (as in Figure 4A). (C) ROS accumulation assay. DAB staining of inoculation sites around P. capsici infection sites on Agrobacterium-infiltrated N. benthamiana leaf tissue expressing Avr3b alleles and mutants at 36 hpi, as in Figure 3D. The quantification of the DAB staining based on three replicates is shown on the right. The quantification data was shown in a pattern of mean ± standard deviation. (D, E) ADP-ribose (D) and NADH (E) hydrolase activity of total protein extracts from Agrobacterium-infiltrated N. benthamiana tissue transiently expressing Avr3b alleles and mutants, as in Figure 4C. The letters represent statistical significance (P <0.01) determined by Duncan's multiple range test. Bars represent standard errors from 5 independent replicates each. Student's t-test was used to compare whether testing sample activities are significantly different from the GFP control. The double asterisk indicates statistical significance p <0.01 and p <0.05, respectively. (F) Virulence assay. The P. capsici biomass index in N. benthamiana infected tissues was determined by PCR of plant and pathogen DNA as in Figure 3B. The letters represent statistical significance (P <0.01). (G) Avirulence assay. Avr3b alleles and mutants were transiently expressed in leaves of soybean cultivar Williams (rps) and PRX146–36 (Rps3b) by double-barreled bombardment, as described in the Methods. Statistical analysis was performed using Wilcoxon rank sum test for each pair of Williams and PRX146–36 measurements. The asterisks indicate a statistically significant difference between Williams and PRX146–36 (P <0.01). A significantly reduced cell survival ratio indicates avirulence activity. Next, N. benthamiana leaves expressing each protein were challenged with P. capsici and then stained with DAB to test for suppression of ROS production. Expression of Avr3bP6497, Avr3bP7076, and Avr3b170-315 all noticeably reduced ROS generation, whereas, expression of Avr3b16-174 and Avr3bQQQQ failed to do so, similar to expression of the GFP control (Figure 5C).
To measure the elevation in hydrolase activity caused by expression of the Avr3b proteins, total proteins from leaves expressing each construct were assayed with ADP-ribose and NADH as substrates. Protein extracts from leaf tissue expressing Avr3bP6497, Avr3bP7076, and Avr3b170-315 exhibited significantly elevated ADP-ribose and NADH pyrophosphorylase activity whereas leaves expressing Avr3b16-174 did not (Figure 5D, 5E). Avr3bQQQQ expression resulted in no significant elevation in ADP-ribose pyrophosphorylase activity, and a weak but not statistically significant elevation in NADH pyrophosphorylase activity (Figure 5D, 5E).
To determine the effect of each mutant on susceptibility to P. capsici infection, real-time PCR was used to quantify the pathogen biomass in infected plant tissues expressing each construct. The pathogen biomass was significantly higher in tissues expressing Avr3bP6497, Avr3bP7076, and Avr3b170-315 (p <0.01) than in tissues expressing the GFP control, but was not significantly higher in tissues expressing Avr3b16-174 or Avr3bQQQQ (p >0.05) (Figure 5F).
To quantitate the avirulence activity of each Avr3b allele and mutant, the double-barreled version of the co-bombardment assay was used. This assay allows precise quantitation of cell death triggered by a construct by comparing the number of blue spots on one side of a leaf with the number produced by a parallel empty vector bombardment on the other side of the leaf [29]. Consistent with the qualitative assay results shown in Figures 1D and S1, Avr3bP6497, but not Avr3bP7076, significantly reduced the cell survival ratio to around 40% on leaves containing Rps3b, whereas no significant effector-triggered cell death was observed in control leaves lacking Rps3b (Figure 5G). Transient expression of Avr3bQQQQ and Avr3b170-315 also reduced the cell survival ratio to around 40% in leaves with Rps3b but not in those without Rps3b. On the other hand, Avr3b16-174 did not trigger significant Rps3b-dependent cell death (Figure 5G). Therefore the avirulence activity of Avr3b (i.e. triggering of Rps3b-dependent cell death) required the C-terminal half of the protein, containing the Nudix motif, but did not require the Nudix motif to be intact.
Transient silencing of Avr3bP6497 impaired P. sojae virulence on susceptible soybean
To more directly test the contribution of Avr3b, to P. sojae virulence, transient silencing of Avr3b expression in P. sojae was carried out. In vitro synthesized Avr3bP6497 dsRNA was introduced into P. sojae P6497, and 16 lines were recovered. Avr3b transcript levels were determined in germinating cysts from two biological replicates of each line using Real-Time PCR. In five lines (T5, T7, T9, T10, T13) levels of the Avr3bP6497 transcript were significantly reduced (14%∼29%) compared to P6497 (Figure 6A). In five non-silenced lines (T1, T2, T3, T12, T16) levels of the Avr3bP6497 transcript were consistently similar to P6497. In six lines, Avr3b transcript levels were not consistent between the two biological replicates; these lines were not examined further.
Fig. 6. Transient silencing of Avr3b impaired P. sojae virulence on soybean. 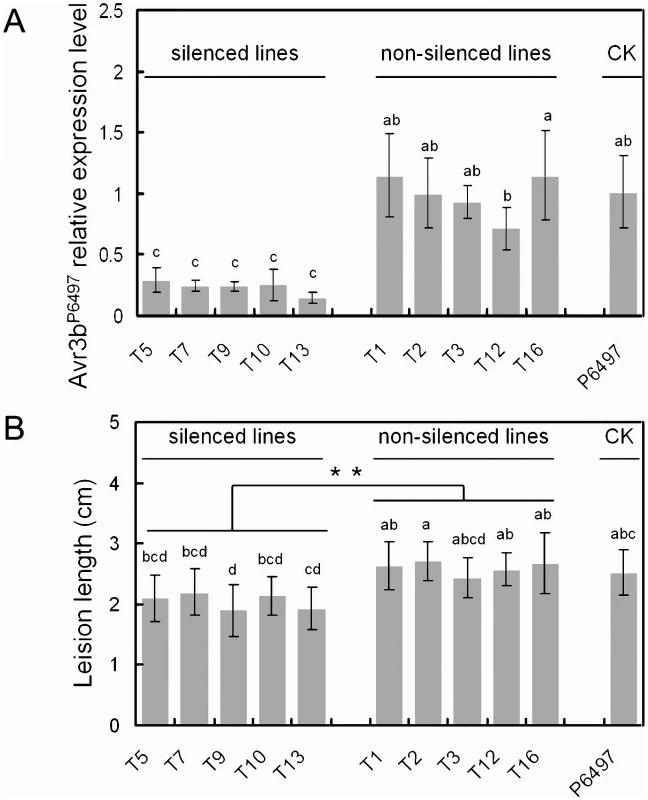
In vitro synthesized Avr3b dsRNA was introduced into P. sojae P6497, generating 16 lines. Avr3b transient transformants and recipient strain P6497 were examined for Avr3b transcript levels and virulence. RNA samples were extracted at the germinating cyst stage. (A) Avr3b transcript levels were determined by Real-time PCR using P. sojae actin transcripts as a reference and then normalized to the wildtype (P6497). Bars represent standard errors from 2 independent RNA isolation and Real-time PCR replicates. Letters indicated above each column represent statistical significance based on Duncan's multiple range test (p <0.01). (B) The disease lesion lengths in etiolated soybean hypocotyls (susceptible cultivar Williams) infected with zoospores of Avr3b transient transformants and P6497 at 36 hpi. Means and standard errors from at least 10 measurements are shown. Two statistical tests was used. The letters indicated above each column represent statistical significance based on Duncan's multiple range test (p <0.01). The asterisk indicates statistical significance based on student's t-test (p <0.01). Zoospore inoculation of etiolated soybean hypocotyls was performed on susceptible soybean cultivar Williams. The disease lesion length was measured to quantify the virulence of Avr3b-silenced lines at 36 hpi, as shown in Figure 6B. The lesion lengths generated by the Avr3b-silenced lines were significantly smaller than those of the non-silenced lines and of P6497 (P <0.01). Thus, the silencing of Avr3b expression in P6497 significantly impaired virulence.
The nudix motif is required for Avr3bP6497 to suppress ETI
To further elucidate the virulence mechanism of Avr3b, we tested if Avr3b could suppress ETI in soybean using a double-barreled bombardment protocol [31]. This experiment involves measuring ETI-associated cell death triggered by P. sojae effector Avr1b in the presence of soybean R gene Rps1b, in the presence or absence of Avr3b. The side-by-side comparison of cell death triggered by co-bombardment of Avr1b alone compared to Avr1b+Avr3bP6497 on Rps1b soybean indicated that Avr1b-triggered cell death was strongly suppressed in the present of Avr3bP6497 as shown in Figure 7A.
Fig. 7. Avr3b suppresses ETI triggered by P. sojae effector Avr1b. 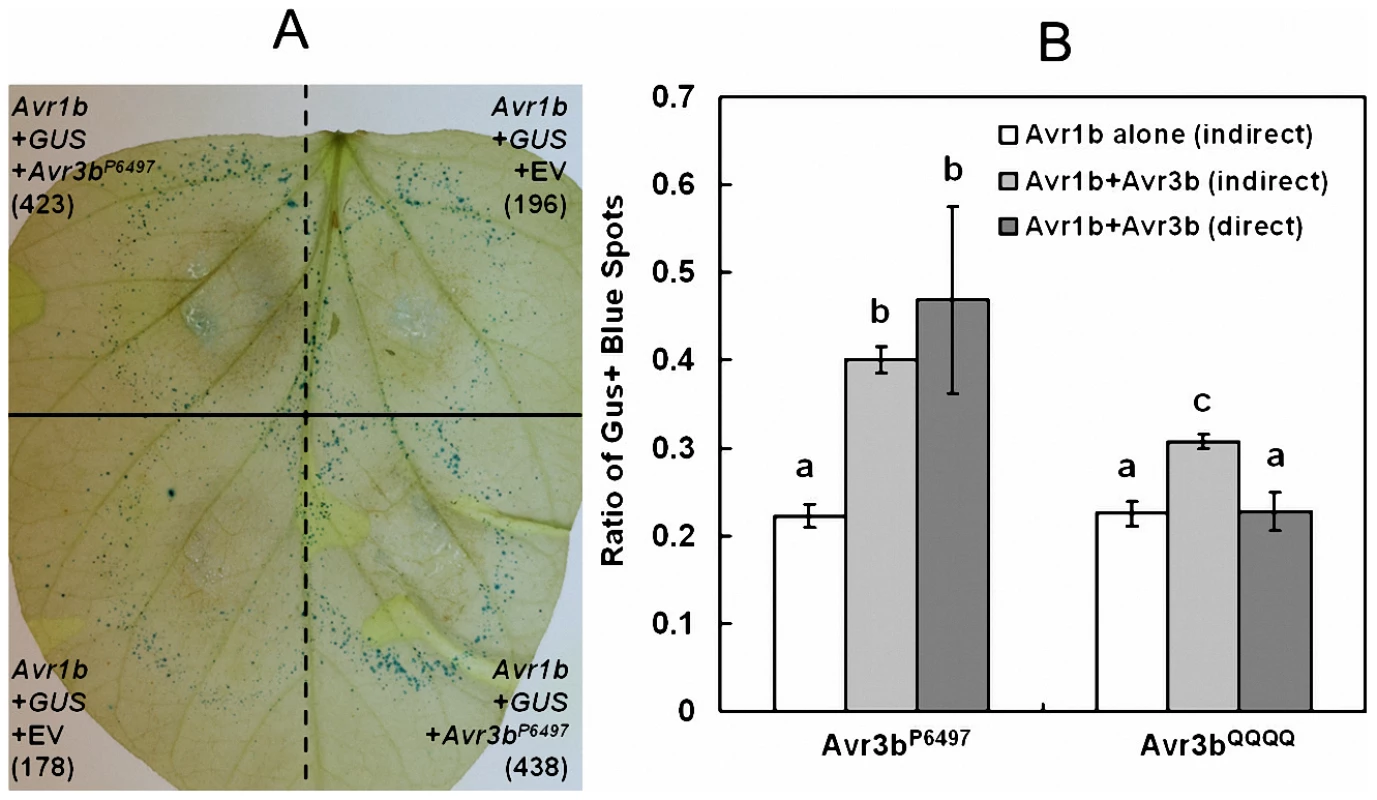
Suppression of Avr1b-triggered PCD in soybean cultivar L77–1863 (Rps1b) by Avr3bP6497 and Avr3bQQQQ measured by indirect and direct double barrel particle bombardment assays. (A) Side-by-side comparison of cell death triggered by co-bombardment of Avr1b alone compared to Avr1b+Avr3bP6497 on Rps1b soybean. Numbers in parenthesis indicate total GUS positive spots counted for each treatment on the leaf shown. Co-bombardments occur horizontally and are separated by dashed lines. Replicate bombardments are separated by the solid black line. (B) Quantitation of suppression by indirect and direct co-bomardment assays. For the indirect assay the number of GUS-expressing spots surviving in the presence of Avr1b on Rps1b soybean, was measured relative to a parallel GUS-only control in the presence (light grey bars) or absence (white bars) of Avr3bP6497 or Avr3bQQQQ. For the direct assay (dark gray bars) one barrel delivered DNA encoding Avr1b + Avr3bP6497 or Avr3bQQQQ and the other barrel delivered Avr1b DNA only. The direct ratio was then multiplied by the cell survival in the presence of Avr1b alone on Rps1b Soybean (white bars) to enable comparison to the results of the indirect assays. Based on the Wilcoxon signed ranks test (direct assays) or the Wilcoxon rank sum test (indirect assays and comparisons of two direct assays), outcomes that were significantly different p <0.001 are marked with different letters. To quantify suppression, and to determine whether the Nudix motif is required for suppression, an indirect and a direct assay were carried out (Figure 7B). For the indirect assay, the number of GUS-expressing spots surviving in the presence of Avr1b on Rps1b soybean was measured relative to a parallel GUS-only control in the presence or absence of Avr3bP6497 or Avr3bQQQQ. For the direct assay one barrel delivered DNA encoding Avr1b + Avr3b (Avr3bP6497 or Avr3bQQQQ) and the other barrel delivered DNA encoding Avr1b alone. The results of both the direct and the indirect assays showed that the survival of GUS-spots from Avr1b + Avr3bP6497 was significantly higher than from Avr1b alone, confirming that Avr3b could suppress Avr1b-triggered cell death. The survival of GUS-spots from Avr1b + Avr3bQQQQ was significantly less than Avr1b + Avr3bP6497 and not significantly higher than from Avr1b alone indicating that Avr3bP6497 suppression of Avr1b mediated cell death is dependent on the nudix motif activity.
Discussion
Oomycete and fungal avirulence effectors form a highly diverse class of proteins, with few if any common sequence signatures. Therefore, the cloning of most oomycete and fungal Avr effectors has relied heavily on genetic mapping and/or genomic subtraction techniques. The discovery of the RXLR host-targeting signal motif in oomycetes has provided a rapid way to identify new Avr effector candidates in conjunction with mapping or screening strategies. For instance, RXLR effector prediction and high-throughput functional screening aided in the discovery of the P. infestans genes Avr3a, Avr-blb1, and Avr-blb2 [11], [16]. By combining RXLR effector prediction with transcriptional patterns, two RXLR effectors encoded by the P. sojae avirulence genes Avr3a and Avr3c were identified [12], [14]. Here, in addition to RXLR effector prediction and transcriptional data, sequence polymorphism analysis [41] was also used for the discovery of the P. sojae Avr effector Avr3b. This powerful combination of methods could significantly accelerate the identification of additional oomycete and fungi avirulence effectors.
The avirulence allele of Avr3b, Avr3bP6497, encodes a protein with an intact RXLR motif, a W-motif [5], [30], a Nudix hydrolase motif and two cysteine residues. In the virulence allele, Avr3bP7076, a premature stop codon creates a 90-amino-acid C-terminal deletion that removes one cysteine and places the Nudix motif at the extreme C-terminus of the protein. In the non-deleted region, sequence variation results in 46 amino acid substitutions and two deletions, making Avr3b the most polymorphic Avr effector among all reported P. sojae Avr genes [12], [13], [14], [15]. Transcripts from Avr3bP7076 accumulate at a significantly lower level than from Avr3bP6497. However, it is unlikely that Avr3bP7076 is a pseudogene because transcripts for this gene are detectable and in planta transient expression of Avr3bP7076 resulted in pyrophosphorylase enzyme activity and suppression of plant immunity.
Most RXLR effectors in Phytophthora have diverged in sequence to such a degree that the identification of orthologous effectors across species is often difficult or impossible. This is likely a result of the rapid diversification of RXLR effectors caused by the evolutionary host-pathogen arms race [3], [6], [18], [23], [42]. However, P. sojae Avr3b has one ortholog in P. capsici, three in P. ramorum, and four in P. infestans, forming an Avr3b-like Nudix RXLR effector family. Sequence alignment of the Avr3b family members shows that most of the sequence similarity occurs in the C-terminal region of the Nudix hydrolase motif (Figure S3). The key residues for Nudix hydrolase activity are conserved, consistent with enzyme activity being required for normal function. Avr3b from P. sojae has two cysteine residues, a feature that is uncommon in intracellular RXLR effectors but is not unusual for apoplastic effectors from oomycetes or fungi. However the positions of the cysteine residues are not conserved in other Avr3b-like effectors. Whether these cysteine residues are required for avirulence or virulence activities needs further investigation. With regard to expression of the Avr3b orthologs, microarray data shows that two P. infestans Avr3b-like effectors PITG06308, PITG15679 are up-regulated by 2.5 and 2.2 fold at 2 dpi, compared to mycelium stages, respectively [23], suggesting Avr3b-like effectors might also function during P. infestans infection. The orthologs' conservation among Phytophthora species suggests that they that provide a common virulence mechanism. However, this remains to be tested.
Recently evidence has shown that P. sojae, P. infestans, P. capsici, M. oryzae, M. lini and other pathogen effectors can enter inside plant cells, presumably to promote virulence [7], [8], [9], [10], [43], [44]. Discrete targeting motifs have been identified in these effectors that are required for their translocation into host cells [10], [43], [45], [46]. Like previously reported oomycete avirulence effectors [3], [11], [12], [14], [15], [16], [17], [18], [20], Avr3b has a signal peptide leader and an RXLR motif at its N-terminus. In our assays, expression of Avr3b in soybean or N. benthamiana cells without its signal peptide resulted in avirulence and virulence activities, suggesting that Avr3b normally acts inside plant cells; thus it should be able to enter into plant cells during infection. The RXLR motif was also conserved in the Avr3b-like Nudix RXLR effector family. Except for PITG15732 from P. infestans (RFLR) and PrAvh268 from P. ramorum (RSLH), all the other Avr3b-like Nudix RXLR effectors have the sequence “RSLR” (Figure S3). The dEER motif that is associated with the RXLR motif was not as highly conserved as the RXLR motif in the Avr3b family.
We failed to identify any Avr3b-like Nudix RXLR effectors in the genome of H. arabidopsidis, which has a reduced set of RXLR effectors [22] or in the genome of P. ultimum, which has no other RXLR effector genes [47]. Sequencing of additional oomycete pathogen genomes will be required to confirm whether this family is only present in Phytophthora species.
Nudix hydrolases are a family of pyrophosphatases containing the highly conserved Nudix motif GX5EX7REUXEEXGU. The family is widely distributed in many organisms, including viruses, bacteria, Archaea, and eukaryotes [39]. Nudix hydrolases catalyze the hydrolysis of a variety of nucleoside diphosphate derivatives linked to a second moiety with varying degrees of specificity [48]. The substrates of Nudix hydrolases identified so far include di - and triphosphates and their oxidized forms, dinucleoside polyphosphates, nucleotide sugars, NADH, coenzyme A, and the mRNA cap [39], [48]. In the genome of the model plant Arabidopsis, 29 putative Nudix hydrolases have been identified, and the substrates of a few Arabidopsis Nudix hydrolases have been characterized [49]. Like Avr3b, the Arabidopsis Nudix hydrolases AtNUDT2, AtNUDT7, and AtNUDT6 use both ADP-ribose and NADH as substrates [40], [50]. Interestingly, among these hydrolases, AtNUDT7 was reported to be a pathogen-responsive gene whose induction depends on plant defense regulatory genes EDS1 and PAD4 [51]. Furthermore, AtNUDT7 knockout mutants showed enhanced resistance against Pseudomonas syringae and H. arabidopsidis, suggesting that ADP-ribose/NADH pyrophosphatases may act as negative regulators of plant immunity to pathogenic bacteria and oomycetes [51]. Therefore, it is reasonable to suppose that pathogen effectors like Avr3b might mimic negative regulators of plant immunity such as AtNUDT 7 to repress plant defense.
Recently, a few putative type-three secretion system (TTSS) effectors with the Nudix motif were identified from the plant pathogenic bacterium Ralstonia solanacearum [52], suggesting that this pathogen might also translocate Nudix proteins into plant cells as virulence factors. However, those TTSS effectors were not tested for enzyme activity nor was their contribution to virulence measured. Recombinant Avr3b expressed in planta elevated NADH and ADP-ribose pyrophosphatase activities in plant extracts, converting NADH to a reduced form of nicotinamide mononucleotide (NMNH) plus AMP, and ADP-ribose to AMP plus ribose 5-P. The NADH and ADP-ribose pyrophosphatase activity co-purified with Avr3b protein in immunoprecipitation experiments, and the activity was not present when the nudix motif mutant Avr3bQQQQ was expressed. Therefore, it is very likely that Avr3b protein has NADH and ADP-ribose pyrophosphatase activity. However, because Avr3b was not purified to homogeneity, we cannot absolutely rule out an indirect stimulation and co-purification of a plant Nudix hydrolase with wildtype Avr3b in these experiments. NADH/NAD+ turnover plays an important role in maintaining the ROS balance. Recent reports have shown that AtNUDT7 and AtNUDT2 modulate redox homeostasis in response to biotic or abiotic stress by nucleotide recycling from free ADP-ribose molecules [40], [53], [54], [55]. Thus, we hypothesize that inside plant cells Avr3b can suppress pathogen-triggered ROS accumulation by reducing NADH content and recycling nucleotides from ADP-ribose. However, these mechanisms remain to be further investigated.
Rps3b recognizes the presence of Avr3b through the Avr3b C-terminal region (170 to 315 aa) but not the N-terminal region (16 to 174 aa) (Figure 5G). This is consistent with previous reports indicating that the C-terminal sequences of most P. sojae, P. infestans and H. arabidopsidis avirulence effectors show signs of positive selection [11], [30], [31], [56]. In contrast to other avirulence effectors, the Avr3bP6497 C-terminal region contains an identifiable enzyme domain, a Nudix hydrolase domain. In this study, we generated a mutant version of the protein, Avr3bQQQQ, that apparently lacks Nudix enzyme activity but nonetheless could still trigger a defense response in the presence of Rps3b. This result suggests that Rps3b recognition might not depend on Nudix hydrolase activity. However, Avr3bQQQQ retains residual hydrolase activity. Thus we can not rule out the possibility that Avr3b avirulence activity also required Nudix activity because the threshold for Avr3b-mediated avirulence activity is likely to be much lower than for its virulence activity. Due to the numerous polymorphic sites present within the Avr3b170-315 domain, the particular residues responsible for Avr3b-Rps3b recognition cannot readily be discerned. Only Avr3b mutants retaining Avr3b ADP-ribose and NADH pyrophosphorylase activity could suppress ETI and increase Phytophthora biomass in infected plant tissue, suggesting that the enzymatic activity is required to promote Phytophthora virulence.
Phytophthora genomes encoded hundreds of RXLR effector genes, suggesting many of these effectors might be redundant in function [57]. For examine, either overexpression or silencing of P. sojae Avr gene Avr3a/5 does not significantly effect P. sojae virulence on susceptible soybean [19]. Overexpression of Avr1b in P. sojae makes transformants more aggressive on soybean [30], but the effector is naturally silenced in some isolates [13]. On the other hand silencing of Avr3a impaired P. infestans pathogenicity on N. benthamiana [33], and silencing of Avh172 and Avh238 in P. sojae also impaired virulence [31], suggesting that these effectors are essential virulence factors. In this paper, transient silencing of Avr3bP6497 compromised the virulence of transformant recipient strains on susceptible soybean cultivar, identifying Avr3b also as an essential virulence factor. This is consistent with our finding that both Avr3b virulence and avirulence alleles are transcribed and both proteins retain Nudix enzyme activity. In conclusion, Avr3b plays dual roles (avirulence and virulence) in the P. sojae - soybean interaction. The virulence allele Avr3bP7076 may be considered to be a compromise between host recognition pressures and pathogen fitness.
Materials and Methods
Plant and microbial culture, and virulence scoring
Detailed information about the P. sojae strains used in this study is listed in Table S6. The P. capsici (Pc263) and P. parasitica (P24-3) strains were obtained from the Phytophthora species collection at Nanjing Agricultural University. All of these isolates and the P6497× P7076 F1 progeny and F2 progeny used in this paper were routinely maintained on 2.5% vegetable (V8) juice medium at 25°C in the dark [58]. Phytophthora sojae mycelia, zoospores, and cysts were prepared as previously described [59]. Cysts germinating at 25°C for 6 hours were used for this study. For RNA samples, infection assays with P. sojae were performed by sandwiching P. sojae mycelium between pairs of soybean leaves [60]. The mycelium was removed at 6 hpi, and the infected soybean leaves were collected at 12 hpi and 24 hpi. All of the collected samples were immediately frozen in liquid nitrogen and stored at −80°C until used for RNA isolation.
Soybean (Glycine max) cultivar Williams (rps), Williams isoline L88–1479 (Rps3b), and PRX146–36 (Rps3b) from the collections at Nanjing Agricultural University and Northeast Agricultural University (Harbin, China) were used to score the virulence of P. sojae cultures. Williams isoline L77–1863 (Rps1b) was from the collection at the Virginia Bioinformatics Institute. Etiolated soybean seedlings were grown in vermiculite soaked with water at 25°C without light for 4 days before harvest for co-bombardment. For light-grown soybeans, ten soybean seeds were sown in 10 cm pots (a minimum of three pots per isolate) containing a soil (70%) and vermiculite (30%) mix soaked with water. Soybeans were grown in a greenhouse with a 16-hour photoperiod at 25°C. Soybeans were grown for 7 days for virulence assays, or for 13 to 14 days for use in co-bombardment. Phytophthora sojae cultures were grown on 0.9% (v/v) V8 agar plates 5 to 7 days prior to light-grown plant inoculations. The virulence of P. sojae cultures was scored in exactly the same manner as that previously described [14]. A minimum of three independent replicates of the disease assay were performed for each P. sojae culture tested. N. benthamiana plants were grown at 25°C with a 16 hr photoperiod in a greenhouse in styrofoam cups containing disinfected soil. Plants of 5 to 6 weeks old were used for agroinfiltration.
Bioinformatics
In total, 395 predicted RXLR effectors in P. sojae P6497 were previously predicted [42]. For DGE expression data, RNA samples were collected at Nanjing Agricultural University, and sequencing and analysis were performed at Beijing Genomics Institute. Affymetrix microarray data were previously reported [12], [31]. All RXLR sequences were used as queries for BLAST searches of the P. sojae EST unigene database (http://vmd.vbi.vt.edu) [61] , with an E-value cutoff at e−20. We identified a total of 131 RXLR effectors that are expressed in P. sojae P6497. P. sojae 454 genome sequencing data (P7064, P7074, and P7076) [31] were accessed from Virginia Microbial Database (http://vmd.vbi.vt.edu). Nudix homologs were identified in two ways: genome annotation searching and BLAST searching. The P. sojae, P. capsici and P. ramorum homolog searches were performed at the Joint Genome Institute database (http://genome.jgi-psf.org), the H. arabidopsidis search was performed at Virginia Microbial Database, and the P. infestans search was conducted at the Broad Institute P. infestans database (http://www.broadinstitute.org). For Pythium ultimum genome searching, the genome sequence was downloaded from the Pythium genome database available at http://pythium.plantbiology.msu.edu and a local BLAST search was conducted. SignalPv3.0 (http://www.cbs.dtu.dk/services/SignalP) was used for secretion signal peptide prediction. Protein domain and motif analyses were conducted using the NCBI conserved domain database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) and Motif Scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan). Sequence alignment was performed using BioEdit2005 (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The Avr3b-like family member sequences have been deposited into NCBI GenBank with accession numbers (Table S7).
Generation of F2 mapping population and scoring of genotypes
F1 hybrids were derived from P6497 (Avr3b) × P7064 (avr3b) crosses. Oospores from F1 hybrids were produced as described [14]. Single germinating oospores were harvested for DNA isolation. DNA isolation was performed using a fast DNA isolation kit (Axygen Biotech, China). Of 79 germinating oospores, three F1 hybrids were confirmed using two different CAP DNA markers corresponding to polymorphisms within the predicted genes Avh20 and Avh238). One of the F1 hybrids was cultured on 2.5% (v/v) V8-medium plates for F2 progeny oospore generation. A total of 71 F2 progeny were produced to establish an expanded mapping population to test Avr3b candidate effectors. For each F2 individual, virulence was scored on Williams (rps) and L88–1479 (Rps3b) plants, and DNA samples from mycelia were also isolated for genotyping. CAP markers for seven Avh genes (Avh238, Avh307, Avh113, Avh258, Avh20, Avh288, and Avh6) were designed based on polymorphisms between P6497 and P7076; details are provided in Table S2. The genotype of the candidate gene and the virulence type of the F2 progeny were compared. For Avr3b allele sequencing in different strains, the specific primers Avh307 MF, Avh307 MR, Avh307 MFa, and Avh307 MFb were used for PCR amplification and sequencing. Primer sequences are presented in Table S2.
Soybean transient expression assays
The Avr3bP6497 and Avr3bP7076 genes, excluding regions encoding the predicted signal peptide sequences, were amplified using specific primers and cloned into a 35S promoter-derived plant expression vector pFF19 using BamH I and Sph I restriction sites (primers sequences are shown in Table S8). Similarly, constructs for bombardment of Avr3bP6497-derived mutants (Avr3b16-174, Avr3b170-315 and Avr3bQQQQ) were also generated. Co-bombardment and transient expression assays for Avr3bP6497 and Avr3bP7076 on etiolated soybean hypocotyls were performed as described [37]. Leaves were photographed using a digital camera system (V12, Zeiss Germany). For Avr3b-derived mutant avirulence assay and Avr3b suppression of Avr1b ETI assay, double-barreled particle bombardment assays on soybean leaves were performed as previously described [30]. Both hypocotyl co-bombardment and double-barreled particle bombardment were conducted using a Bio-Rad (USA) He/1000 particle delivery system. For each paired shot, the logarithm of the ratio of the spot numbers of the test construct to that of the control was calculated. The log ratios obtained from the Rps3b and non-Rps3b leaves were then compared using the Wilcoxon rank-sum test.
RNA isolation and real-time RT-PCR
To monitor Avr3b transcript profiling in P. sojae P6497 by real-time RT-PCR, total RNA samples from mycelia, zoospores, cysts, germinating cysts, and infected plant tissue samples were extracted using a PureLink RNA Mini Kit (Invitrogen USA). For Avr3b transformants, germinating cysts was prepared at 9 and 11 days after transformation. The RNA samples were isolated from these germinating cysts (two biological replicates for each transformants). The first-strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) following the manufacturer's directions. For Avr3b transcript profiling analysis, SYBR green real-time RT-PCR assays were carried out. Primer pairs (Table S8) were designed at the identical region between Avr3bP6497 and Avr3bP7076. Two P. sojae housekeeping genes were selected as endogenous controls, namely actin (JGI Gene ID: 109046) and the molecular chaperone HSP70 superfamily gene (JGI Gene ID: 144810). PCR reactions (20 µL) included 20 ng cDNA, 0.2 µM of each primer, and 10 µL SYBR Premix ExTaq (TaKaRa Inc., Dalian, China). Reactions were performed on an ABI PRISM 7300 fast real-time PCR system (Applied Biosystems, USA) under the following conditions: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 31 s; followed by 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s to obtain melt curves. The expression of each gene relative to average Ct values of the two housekeeping genes (Ct = Ctgene - CtHKaverage) was determined and analyzed using ABI 7300 System Sequence Detection Software Version 1.4 [62].
Construction of recombinant Agrobacterium binary PVX vectors
Sequences corresponding to Avr3bP6497 and Avr3bP7076 with secretion signal peptides replaced by a FLAG tag sequence were amplified from P6497 and P7076 genomic DNA using high-fidelity DNA polymerase (TaKaRa, Inc.) with primers described in Table S8. The PVX vector pGR107 for N. benthamiana transient expression assay was isolated using a plasmid spin column small isolation kit (Axygen Biotech. China.). For the Avr3bP6497 mutant, a method similar to that used for vector construction was used. The Avr3bQQQQ mutant was generated with additional primers (Table S8) by overlapping PCR. High-fidelity PCR products were sub-cloned into pGR107 pre-digested by the restriction enzyme Sma I. Recombinant binary vectors were maintained and propagated in the Escherichia coli strain JM109, grown in the presence of 50 µg/mL kanamycin. The recombinant binary vectors were transformed into the Agrobacterium tumefaciens strain GV3101 by electroporation. After growing at 28°C on LB agar plates supplemented with 50 µg/mL kanamycin as selective agents for 2 days, individual Agrobacterium colonies were verified with PCR using vector primers. Agrobacterium tumefaciens was grown in LB broth cultures supplemented with 25 µg/mL kanamycin for 2 day at 28°C with constant shaking. The cultures were centrifuged at 4,000 rpm for 4 min in a tabletop centrifuge. The pellet was resuspended in 1 mL of induction medium (10 mM MES, 10 mM MgCl2, and 150 mM acetosyringone, pH = 5.6). The final concentration of Agrobacterium cells was adjusted to OD600 = 0.4. Leaves of N. benthamiana were infiltrated with Agrobacterium cultures using a blunt syringe. The infiltrated plants were then kept in a greenhouse for 72 hours prior to Western blot, immuno-precipitation, or virulence assays.
Virulence assay of Phytophthora on N. benthamiana
Agrobacterium-infiltrated N. benthamiana plants were grown in a greenhouse for 48 hours, and transformed leaves were then detached and maintained on half-strength MS medium in a petri dish. Next, 2.5% V8 juice agar plugs (0.5×0.5 cm) infested with fresh P. capsici or P. parasitica mycelia were inoculated onto the infiltrated regions. The diameter of the disease lesion was photographed and measured at 36 hpi. Total DNA isolated from P. capsici-infected regions (2×2 cm) was isolated at 36 hpi. Real-time PCR was used to quantify the ratio of host to pathogen ratio DNA sequences, employing primers specific for the N. benthamiana and P. capsici housekeeping actin genes (Table S8). Three independent biological replicates were conducted. Diseased plant tissues at 16 hpi were stained by trypan blue and DAB according as described [63]. Quantification DAB was performed by analyzing DAB staining image by a combination of Photoshop and Quantity One. GFP was considered to be 100% standard.
Western blot and immuno-precipitation assays
Total plant protein was isolated as described [63] for Western blot and immuno-precipitation assays. The protein samples were quantified using a BioPhotometer (Eppendorf, Germany). A standard sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) protocol was used for protein separation. The amount of total protein loaded per gel lane varied from 80 µg to 120 µg depending on each experiment. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes using a semi-wet apparatus (Bio-Rad) according to the product instructions. Western blotting was performed as a standard protocol. Anti-FLAG monoclonal antibody (Sigma-Aldrich) and anti-mouse IgG-peroxidase conjugate (Beyotime Biotech, China) were used as the primary and secondary antibodies. The membrane was treated with Chemiluminescent Peroxidase Substrate-1 (Thermo Scientific Pierce, No. 34080, USA) for 5 min. The membrane was briefly drained and exposed to BioMax (Kodak, USA) light film several times (depending on results) for exposure signal development.
For immuno-precipitation, anti-FLAG M2 affinity gel (Sigma-Aldrich) was used. The gel suspension (40 µL) was transferred into a fresh 1.5 mL test tube and centrifuged in a pre-cooled rotor at 6000 g for 30 s. The resin was washed twice with 1 mL TBS (50 mM Tris HCL, 150 mM NaCl, pH = 7.4), and all washing buffer was removed. Total protein lysate was clarified by centrifugation (10000 g, 2 min) then 1000 µL of the supernatant was added to the washed resin, and the mixture was incubated using a roller shaker for 4 hours at 4°C. The suspension was centrifuged at 6000 g and the supernatant was removed. The remaining resin was washed three times with 1 mL of TBS. A total of 100 µL elution buffer (0.5 M Tris-HCl buffer, 150 ng/µL 3xFLAG, pH = 7.2) was then added to the resin, and the samples were incubated using a roller shaker for 10 min at room temperature. The resin was centrifuged for 30 s at 6000 g, and the supernatants were transferred to fresh tubes previously loaded with 10 µL of 0.5 M Tris HCl and 1.5 M NaCl, pH 7.4. The purified protein samples were used for gel electrophoresis and enzyme activity assay.
Nudix hydrolase activity assays
A general method for the Nudix hydrolase activity assay was performed to test for Avr3b enzymatic activity [40], [53]; a 50-µL reaction mixture contained 50 mM Tris-HCl, pH 8.5, 5 mM MgCl2, 1 mM dithiothreitol, 2 units of calf alkaline phosphatase, 2 mM substrate (ADP-ribose, NADH, FAD, or Ap4A; Sigma-Aldrich), and certain amount of protein samples. Equal amount of protein (5 µg for purified protein, 2 mg for total plant protein extract) was loaded for hydrolase activity assay in each reaction. After incubation for 1 hr at 37°C, the reaction was stopped by adding 150 µL of 1 N H2SO4. For color development, 100 µL of water and 700 µL of a freshly made mixture containing 600 µL of 3.4 mM ammonium molybdate and 100 µL of 570 mM ascorbic acid was used. The reaction tubes were incubated in a 45°C dry bath for 10 min for color development and then cooled on ice. The solutions were measured using the Du-640 spectrophotometer (Beckman, USA) at A820 The reaction without protein was used as a blank control for each substrate. For direct assay of purified proteins, immuno-precipitation eluate was directly added into enzymatic assay reaction mixture.
For hydrolase activity assay of total plant protein extract, two regions per leaf transiently expressing FLAG:GFP and FLAG:Avr3bP6497 were created in N. benthamiana leaves using Agrobacterium infiltration. A total of 0.1 g of GFP - or Avr3b-infiltrated leaf tissue was collected from each leaf. For both kinds of protein preparations, the relative hydrolase enzyme activity was calculated as a ratio of Avr3b extract (A820 reading) over GFP extract (A820 reading). For NADH and ADPR, 17 and 21 biological replicates were performed, respectively. For FAD, Ap4A, NAD and NADPH, 4 biological replicates were conducted.
In vitro dsRNA synthesis and P. sojae transformation
Avr3b in vitro dsRNA synthesis was carried out as described [64]. Primers AVH307-T7F/R with T7 promoter sequence added to the 5′ end of both primers (see Table S8) were used to amplify a C-terminal specific Avr3b DNA fragment from the Avr3bP6497 allele. The PCR product was cloned into pMD19 vector (TaKaRa) and sequenced. AVH307-T7F/R primers were also used to generate Avr3b in vitro dsRNA by using the Megascript RNAi kit (Ambion AM1626). A total of around 200 µg dsRNA was obtained as measured by spectrophotometry. P. sojae transient transformation was performed as described [30], [59] with a few modifications [32]. About 100 µg of Avr3b dsRNA was added into 1 mL MMg solution (0.4 M mannitol, 15 mM MgCl2, 4 mM MES, pH 5.7) containing 5000–10000 P. sojae P6497 protoplasts. After transformation, the regenerated protoplasts were suspended in liquid pea agar (40 °C ) containing 0.5 M mannitol. The visible colonies could be observed after 36 h incubation at 25 °C. A total of 16 single colonies were randomly selected and propagated on V8 agar plates for RNA extraction and virulence assays.
Supporting Information
Zdroje
1. JonesJDDanglJL 2006 The plant immune system. Nature 444 323 329
2. De WitPJMehrabiRVan den BurgHAStergiopoulosI 2009 Fungal effector proteins: past, present and future. Mol Plant Pathol 10 735 747
3. RehmanyAPGordonARoseLEAllenRLArmstrongMR 2005 Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell 17 1839 1850
4. BirchPRRehmanyAPPritchardLKamounSBeynonJL 2006 Trafficking arms: oomycete effectors enter host plant cells. Trends Microbiol 14 8 11
5. JiangRHYTripathySGoversFTylerBM 2008 RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving super-family with more than 700 members. Proc Natl Acad Sci U S A 105 4874 4879
6. TylerBMTripathySZhangXDehalPJiangRH 2006 Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313 1261 1266
7. WhissonSCBoevinkPCMolelekiLAvrovaAOMoralesJG 2007 A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450 115 118
8. DouDKaleSDWangX JiangRHBruceNA 2008 RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell 20 1930 1947
9. SchornackSvan DammeMBozkurtTOCanoLMSmokerM 2010 Ancient class of translocated oomycete effectors targets the host nucleus. Proc Natl Acad Sci U S A 107 17421 17426
10. KaleSDGuBCapellutoDGDouDFeldmanE 2010 External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell 142 284 295
11. OhSKYoungCLeeMOlivaRBozkurtTO 2009 In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 21 2928 2947
12. DongSQutobDTedman-JonesJKufluKWangY 2009 The Phytophthora sojae avirulence locus Avr3c encodes a multi-copy RXLR effector with sequence polymorphisms among pathogen strains. PLoS One 4 e5556
13. ShanWCaoMLeungDTylerBM 2004 The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol Plant Microbe Interact 17 394 403
14. QutobDTedman-JonesJDongSKufluKPhamH 2009 Copy number variation and transcriptional polymorphisms of Phytophthora sojae RXLR effector genes Avr1a and Avr3a. PLoS One 4 e5066
15. DouDKaleSDLiuTTangQWangX 2010 Different domains of Phytophthora sojae effector Avr4/6 are recognized by soybean resistance genes Rps4 and Rps6. Mol Plant Microbe Interact 23 425 435
16. ArmstrongMRWhissonSCPritchardLBosJIVenterE 2005 An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc Natl Acad Sci U S A 102 7766 7771
17. van PoppelPMGuoJvan de VondervoortPJJungMWBirchPR 2008 The Phytophthora infestans avirulence gene Avr4 encodes an RXLR-dEER effector. Mol Plant Microbe Interact 21 1460 1470
18. AllenRLBittner-EddyPDGrenville-BriggsLJMeitzJCRehmanyAP 2004 Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306 1957 1960
19. DongSYuDCuiLQutobDTedman-JonesJ 2011 Sequence Variants of the Phytophthora sojae RXLR Effector Avr3a/5 Are Differentially Recognized by Rps3a and Rps5 in Soybean. PLoS One 6 e20172
20. GilroyEMBreenSWhissonSCSquiresJHeinI 2011 Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2-like in Phytophthora infestans determine virulence on R2 plants. New Phytol 191 763 776
21. BaileyKCevikVHoltonNByrne-RichardsonJSohnKH 2011 Molecular cloning of ATR5(Emoy2) from Hyaloperonospora arabidopsidis, an avirulence determinant that triggers RPP5-mediated defense in Arabidopsis. Mol Plant Microbe Interact 24 827 838
22. BaxterLTripathySIshaqueNBootNCabralA 2010 Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330 1549 1551
23. HaasBJKamounSZodyMC JiangRHHandsakerRE 2009 Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461 393 398
24. van EsseHPVan't KloosterJWBoltonMDYadetaKAvan BaarlenP 2008 The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 20 1948 1963
25. van EsseHPBoltonMDStergiopoulosIde WitPJThommaBP 2007 The chitin-binding Cladosporium fulvum effector protein Avr4 is a virulence factor. Mol Plant Microbe Interact 20 1092 1101
26. JiaYMcAdamsSABryanGTHersheyHPValentB 2000 Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19 4004 4014
27. BohnertHUFudalIDiohWTharreauDNotteghemJL 2004 A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell 16 2499 2513
28. CatanzaritiAMDoddsPNLawrenceGJAyliffeMAEllisJG 2006 Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell 18 243 256
29. HoutermanPMCornelissenBJRepM 2008 Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog 4 e1000061
30. DouDKaleSDWangXChenYWangQ 2008 Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell 20 1118 1133
31. WangQHanCFerreiraAOYuXYeW 2011 Transcriptional Programming and Functional Interactions within the Phytophthora sojae RXLR Effector Repertoire. Plant Cell 23 2064 2086
32. SohnKHLeiRNemriA JonesJD 2007 The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell 19 4077 4090
33. BosJIArmstrongMRGilroyEMBoevinkPCHeinI 2010 Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci U S A 107 9909 9914
34. MayKJWhissonSCZwartRSSearleIRIrwinJA 2002 Inheritance and mapping of 11 avirulence genes in Phytophthora sojae. Fungal Genet Biol 37 1 12
35. YeWWangXTaoKLuYZhaoW 2011 Digital gene expression profiling of the Phytophthora sojae transcriptome. Mol Plant Microbe Interact
36. ForsterHTylerBMCoffeyMD 1994 Phytophthora sojae races have arisen by clonal evolution and by rare outcrosses. Mol Plant Microbe Interact 7 780 791
37. QutobDKamounSGijzenM 2002 Expression of a Phytophthora sojae necrosis-inducing protein occurs during transition from biotrophy to necrotrophy. Plant J 32 361 373
38. BosJIKannegantiTDYoungCCakirCHuitemaE 2006 The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana. Plant J 48 165 176
39. MildvanASXiaZAzurmendiHFSaraswatVLeglerPM 2005 Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys 433 129 143
40. GeXLiGJWangSBZhuHZhuT 2007 AtNUDT7, a negative regulator of basal immunity in Arabidopsis, modulates two distinct defense response pathways and is involved in maintaining redox homeostasis. Plant Physiol 145 204 215
41. BosJIBArmstrongMWhissonSCTortoTOchwoM 2003 Intraspecific comparative genomics to identify avirulence genes from Phytophthora. New Phytologist 159 63 72
42. JiangRHTripathySGoversFTylerBM 2008 RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc Natl Acad Sci U S A 105 4874 4879
43. RafiqiMGanPHRavensdaleMLawrenceGJEllisJG 2010 Internalization of flax rust avirulence proteins into flax and tobacco cells can occur in the absence of the pathogen. Plant Cell 22 2017 2032
44. KhangCHBerruyerRGiraldoMCKankanalaPParkSY 2010 Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22 1388 1403
45. TianMWinJSavoryEBurkhardtAHeldM 2011 454 Genome Sequencing of Pseudoperonospora cubensis Reveals Effector Proteins with a QXLR Translocation Motif. Mol Plant Microbe Interact 24 543 553
46. OlivaRWinJRaffaeleSBoutemyLBozkurtTO 2010 Recent developments in effector biology of filamentous plant pathogens. Cell Microbiol 12 1015
47. LevesqueCABrouwerHCanoLHamiltonJPHoltC 2010 Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol 11 R73
48. BessmanMJFrickDNO'HandleySF 1996 The MutT proteins or "Nudix" hydrolases, a family of versatile, widely distributed, "housecleaning" enzymes. J Biol Chem 271 25059 25062
49. KraszewskaE 2008 The plant Nudix hydrolase family. Acta Biochim Pol 55 663 671
50. OgawaTUedaYYoshimuraKShigeokaS 2005 Comprehensive analysis of cytosolic Nudix hydrolases in Arabidopsis thaliana. J Biol Chem 280 25277 25283
51. BartschMGobbatoEBednarekPDebeySSchultzeJL 2006 Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18 1038 1051
52. TamuraNMurataYMukaiharaT 2005 Isolation of Ralstonia solanacearum hrpB constitutive mutants and secretion analysis of hrpB-regulated gene products that share homology with known type III effectors and enzymes. Microbiology 151 2873 2884
53. IshikawaKOgawaTHirosueENakayamaYHaradaK 2009 Modulation of the poly(ADP-ribosyl)ation reaction via the Arabidopsis ADP-ribose/NADH pyrophosphohydrolase, AtNUDX7, is involved in the response to oxidative stress. Plant Physiol 151 741 754
54. JambunathanNPenagantiATangYMahalingamR 2010 Modulation of redox homeostasis under suboptimal conditions by Arabidopsis nudix hydrolase 7. BMC Plant Biol 10 173
55. OgawaTIshikawaKHaradaKFukusakiEYoshimuraK 2009 Overexpression of an ADP-ribose pyrophosphatase, AtNUDX2, confers enhanced tolerance to oxidative stress in Arabidopsis plants. Plant J 57 289 301
56. WinJKamounS 2008 Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Signal Behav 3 251 253
57. BirchPRBoevinkPCGilroyEMHeinIPritchardL 2008 Oomycete RXLR effectors: delivery, functional redundancy and durable disease resistance. Curr Opin Plant Biol 11 373 379
58. QutobDHraberPTSobralBWGijzenM 2000 Comparative analysis of expressed sequences in Phytophthora sojae. Plant Physiol 123 243 254
59. HuaCWangYZhengXDouDZhangZ 2008 A Phytophthora sojae G-protein alpha subunit is involved in chemotaxis to soybean isoflavones. Eukaryot Cell 7 2133 2140
60. ChenXShenGWangYZhengX 2007 Identification of Phytophthora sojae genes upregulated during the early stage of soybean infection. FEMS Microbiol Lett 269 280 288
61. Torto-AlaliboTATripathySSmithBMArredondoFDZhouL 2007 Expressed sequence tags from Phytophthora sojae reveal genes specific to development and infection. Mol Plant Microbe Interact 20 781 793
62. SchuhmacherTLemuthKHardimanTVacunGReussM 2010 Quantifying cytosolic messenger RNA concentrations in Escherichia coli using real-time polymerase chain reaction for a systems biology approach. Anal Biochem 398 212 217
63. DongSZhangZZhengXWangY 2008 Mammalian pro-apoptotic bax gene enhances tobacco resistance to pathogens. Plant Cell Rep 27 1559 1569
64. WhissonSCAvrovaAOPVANWJonesJT 2005 A method for double-stranded RNA-mediated transient gene silencing in Phytophthora infestans. Mol Plant Pathol 6 153 163
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



