-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
Antimicrobial peptides (AMPs) contribute to host innate immune defense and are a critical component to control bacterial infection. Nontypeable Haemophilus influenzae (NTHI) is a commensal inhabitant of the human nasopharyngeal mucosa, yet is commonly associated with opportunistic infections of the upper and lower respiratory tracts. An important aspect of NTHI virulence is the ability to avert bactericidal effects of host-derived antimicrobial peptides (AMPs). The Sap (sensitivity to antimicrobial peptides) ABC transporter equips NTHI to resist AMPs, although the mechanism of this resistance has remained undefined. We previously determined that the periplasmic binding protein SapA bound AMPs and was required for NTHI virulence in vivo. We now demonstrate, by antibody-mediated neutralization of AMP in vivo, that SapA functions to directly counter AMP lethality during NTHI infection. We hypothesized that SapA would deliver AMPs to the Sap inner membrane complex for transport into the bacterial cytoplasm. We observed that AMPs localize to the bacterial cytoplasm of the parental NTHI strain and were susceptible to cytoplasmic peptidase activity. In striking contrast, AMPs accumulated in the periplasm of bacteria lacking a functional Sap permease complex. These data support a mechanism of Sap mediated import of AMPs, a novel strategy to reduce periplasmic and inner membrane accumulation of these host defense peptides.
Published in the journal: Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in. PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002360
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002360Summary
Antimicrobial peptides (AMPs) contribute to host innate immune defense and are a critical component to control bacterial infection. Nontypeable Haemophilus influenzae (NTHI) is a commensal inhabitant of the human nasopharyngeal mucosa, yet is commonly associated with opportunistic infections of the upper and lower respiratory tracts. An important aspect of NTHI virulence is the ability to avert bactericidal effects of host-derived antimicrobial peptides (AMPs). The Sap (sensitivity to antimicrobial peptides) ABC transporter equips NTHI to resist AMPs, although the mechanism of this resistance has remained undefined. We previously determined that the periplasmic binding protein SapA bound AMPs and was required for NTHI virulence in vivo. We now demonstrate, by antibody-mediated neutralization of AMP in vivo, that SapA functions to directly counter AMP lethality during NTHI infection. We hypothesized that SapA would deliver AMPs to the Sap inner membrane complex for transport into the bacterial cytoplasm. We observed that AMPs localize to the bacterial cytoplasm of the parental NTHI strain and were susceptible to cytoplasmic peptidase activity. In striking contrast, AMPs accumulated in the periplasm of bacteria lacking a functional Sap permease complex. These data support a mechanism of Sap mediated import of AMPs, a novel strategy to reduce periplasmic and inner membrane accumulation of these host defense peptides.
Introduction
Host-derived antimicrobial peptides (AMPs) are typically amphipathic, cationic innate immune defense molecules that target bacterial membranes, disrupt transmembrane potential and trigger cytoplasmic leakage resulting in bacterial cell death [1], [2]. Defensins (α - and β-) and cathelicidin (hCAP-18/LL37) molecules are primarily abundant in neutrophils (α-defensins and cathelicidin), respiratory epithelium (β-defensins 1–3 and cathelicidin), and are secreted by lung and trachea epithelia (cathelicidin) [3]–[6]. As a first line of innate defense, AMPs serve to limit bacterial colonization of mucosal surfaces [7]–[11]. Bacteria therefore adapt to resist AMP lethality through a series of countermeasures: remodeling the bacterial outer membrane surface to dampen charge and alter hydrophobicity [1], [12]–[14], export of AMPs via multiple transferable resistance (MTR)-mediated efflux pumps [15], secretion of exoproteases for AMP degradation [16], secretion of bacterial molecules to suppress host innate defense [17], [18], and release of proteins that function to adsorb extracellular AMPs [19].
Nontypeable Haemophilus influenzae (NTHI) is a commensal of the human nasopharnyx, yet causes opportunistic diseases such as conjunctivitis, sinusitis, exacerbations of chronic obstructive pulmonary disease, complications of cystic fibrosis and chronic and acute otitis media [20]–[25]. During the transition from a commensal to pathogen, NTHI must acquire nutrients and defend against host innate immune defense strategies including increased production of AMPs in response to infection. NTHI outer membrane remodeling provides a first line of defense against cationic AMPs. Lysenko and colleagues demonstrated that the presence of phosphorylcholine, a phase variable modification of NTHI lipooligosaccharide, alters outer membrane hydrophobicity that confers resistance to the cathelicidin LL-37 [26]. Additionally, HtrB is required for hexaacylation of NTHI lipid A, thus mutants lacking htrB are unable to fully acylate their lipid A rendering NTHI susceptible to AMP mediated killing [27].
NTHI lack the other described resistance mechanisms such as AMP efflux or exoprotease activity. The sap (sensitivity to antimicrobial peptides) operon encodes an inner membrane ABC-transporter, previously shown to play a crucial role in defense against AMPs [28]–[36]. Previously, we demonstrated that NTHI SapA, the periplasmic substrate binding protein of the Sap transporter, binds AMPs [37]. NTHI strains deficient in either SapA or the SapD ATPase are susceptible to killing by recombinant chinchilla β-defensin-1, an orthologue of human β-defensin-3, sharing 77% amino acid identity [37], [38]. Moreover, SapA and SapD are required for virulence in a mammalian host [37], [38]. Expression of the sap operon is up-regulated in vivo during NTHI-induced otitis media and in response to AMP exposure in vitro [37], [38]. The mechanism by which the Sap transporter complex confers AMP resistance remains unknown.
Here, we demonstrated that NTHI lacking the ligand binding protein SapA were directly susceptible to AMP exposure in the mammalian host, as neutralization of cBD-1 in vivo reversed attenuation and clearance of the sapA mutant strain. Further, we describe a novel mechanism of AMP import into the bacterial cytoplasm, which is dependent upon Sap transporter function. The Sap-permease was required for cytoplasmic localization and in a Sap-permease deletion strain, AMPs accumulated in the bacterial periplasm. Since AMPs were susceptible to intracytoplasmic peptidase activity, we hypothesize that AMP import, coupled with cytoplasmic degradation, decreases AMP accumulation in the bacterial periplasm and protects the bacterium from subsequent perturbation of the bacterial cytoplasmic membrane, a counter strategy to evade innate immune defense and ultimately benefit the bacterium nutritionally.
Results
SapA is required for NTHI to directly counter host defensin microbicidal activity in vivo
Previously, we demonstrated that the SapA periplasmic binding protein is required for NTHI to persist and cause disease in the middle ear of a mammalian model of otitis media [38]. In this co-infection model of middle ear disease (equal amounts of the wild type NTHI strain 86-028NP and the isogenic sapA mutant strain were introduced directly into the middle ear) we observed significant attenuation of the sapA mutant strain, which was unable to compete for survival and cleared from the middle ear. We subsequently demonstrated that recombinant SapA binds the AMP molecule, chinchilla β-defensin 1 (cBD-1) in vitro [37], and further, binds numerous AMPs, including the human β-defensin 3 (hBD-3) and cathelicidin (LL37) peptides [39]. These data support a mechanism whereby SapA confers protection from AMP lethality in vivo during disease progression. To directly assess this hypothesis, we sought to neutralize activity of cBD-1 in vivo and monitor the consequence of this treatment on survival of the SapA-deficient strain during a co-infection model of otitis media. We predicted that SapA was required by NTHI to counter the defensin bactericidal activity in vivo, and that neutralization of cBD-1 would reverse attenuation and clearance of the SapA-deficient strain in this environment.
Altered expression of AMPs can impact the ability of bacteria to colonize a host [11], [40]. We previously demonstrated that β-defensin expression controls NTHI bacterial colonization of the nasopharyngeal mucosal surface in a chinchilla model of NTHI-mediated disease [41]. Neutralization of available native cBD-1 via passive inhalation of affinity-purified antibody assessed the direct contribution of AMPs in mediating NTHI colonization levels. Here, we further developed and utilized this methodology to assess the direct contribution of AMPs in mediating attenuation of sapA mutant infection in the middle ear. We delivered affinity-purified anti-recombinant cBD-1 [(r)cBD-1] polyclonal antibody (or pre-immune serum as a negative control) to the chinchilla middle ear cavity (n = 5 per cohort) via the superior bullae, to inhibit the activity of native cBD-1. Twenty minutes after pre-treatment, we challenged chinchillas transbullarly with NTHI in a co-infection model, then measured the relative concentration of wild type and sapA mutant present in middle ear effusions over a 14 day period. A second dose of neutralizing antibody (or pre-immune serum) was delivered 24 hours after bacterial challenge.
We demonstrated that animals receiving anti-(r)cBD-1 neutralizing antibody remained colonized with the sapA mutant strain (Figure 1, panel B), which was sufficiently able to compete (in many cases out compete) with the wild type strain as shown in competitive index calculations (Figure 1, panel C). In contrast, the SapA-deficient strain is attenuated in animals receiving pre-immune serum (Figure 1, panel A). Consistent with our previous findings, we demonstrated that the sapA mutant was unable to persist over the course of infection (Figure 1, Panel A), and was significantly impaired (p<0.01) in its ability to infect the middle ear compared to that of the wild type strain 14 days after infection. Additionally, as previously described, anti-(r)cBD-1 antibody or the control pre-immune serum was not bactericidal against NTHI at concentrations used for AMP neutralization [41]. These data demonstrate that SapA functions to counter AMP lethality during NTHI infection in vivo. Since SapA binds and delivers periplasmic substrate to the inner membrane Sap transporter complex, we were interested to determine whether AMP molecules are transported to the cytoplasm of intact cells and whether this transport was dependent upon Sap permease activity.
Fig. 1. SapA is required for NTHI to directly counter host defensin lethality in vivo. 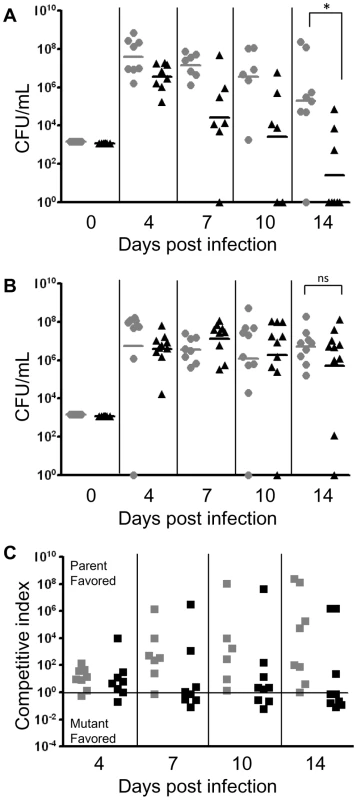
Chinchilla middle ears were pre-treated with (r)cBD-1 antiserum (B) or pre-immune serum (A) then co-infected with a mixture of wild type NTHI strain 86-028NP and the isogenic sapA mutant to determine the consequence of neutralization of native cBD-1 on persistence of the sapA mutant in vivo. A second dose of serum was delivered one day after bacterial challenge. (A) The sapA mutant strain (black triangles) was unable to persist in the middle ear, and showed a significant decrease in colonization relative to that of the wild type strain (gray circles) 14 days after infection (p<0.01). (B) Neutralization of cBD-1 restored the ability of the sapA mutant strain to persist in the middle ear, at levels equal to or greater than that of the wild type strain. (C) Competitive index ratios (wild type to sapA mutant strain) were determined for each cohort receiving either the pre-immune (gray squares) or (r)cBD-1 (black squares) neutralizing antiserum. The black line indicates a competitive index of 1 (equal wild type to sapA mutant ratio). Black and gray horizontal lines (panels A and B) represent the geometric mean value of each cohort (n = 5 animals per cohort). Statistical significance was determined by non-parametric Mann-Whitney U test of geometric means, significance at p≤0.05. Co-fractionation of AMPs in cytoplasmic extracts of NTHI
We predicted that AMP substrates, bound by the SapA periplasmic binding protein, would be transported into the bacterial cytoplasm. Thus, we sought to obtain cytoplasm-enriched fractions of NTHI by bacterial fractionation and monitor the presence of AMPs in these fractions following exposure to sub-lethal concentrations of hBD3 or LL37. Traditional methods previously used to fractionate Escherichia coli were not successful with the prototypic NTHI clinical isolate strain, 86-028NP. Therefore, we utilized membrane destabilization and differential centrifugation (see Materials and Methods) and obtained both periplasm and cytoplasm-enriched fractions from lysed NTHI cells. The protein profiles of these two fractions were unique when visualized by two dimensional gel electrophoresis (Figure 2A) and SDS-PAGE analysis (Figure 2B). We confirmed fractionation by immunodetection of the periplasmic enzyme β-lactamase and the cytoplasmic protein SapD (Figure 2C). β-lactamase was localized to periplasm-enriched fractions and the cytoplasmic protein SapD was only detected in the cytoplasm-enriched fraction (not present in the periplasm). Importantly, we demonstrated that hBD-3 and LL37 co-fractionated with cytoplasm-enriched fractions of NTHI, demonstrating that AMPs, even at sublethal concentrations, gained access to the bacterial cytoplasm of intact NTHI (Figure 2D).
Fig. 2. AMPs localize to cytoplasm-enriched fractions of NTHI. 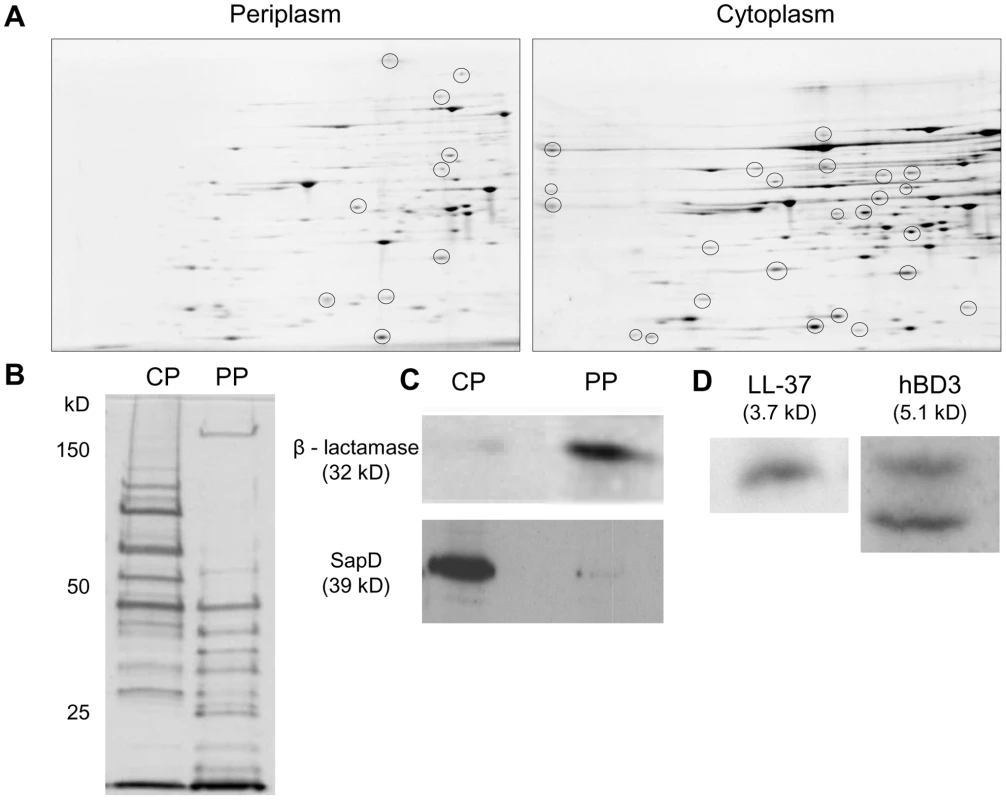
NTHI was fractionated to obtain cytoplasm and periplasm-enriched fractions. (A) Two dimensional gel electrophoresis of enriched fractions confirmed the presence of unique proteins in the periplasm and cytoplasm-enriched fractions. (B) Enriched fractions were separated by SDS-PAGE (12%) and silver stained to determine unique proteomic profiles. (C) Confirmation of bacterial fractionation by immunodetection of the periplasmic enzyme, β – lactamase and the cytoplasmic ATPase protein, SapD. (D) NTHI strain 86-028NP was exposed to LL-37 or hBD3 at sublethal levels for 30 minutes, fractionated and cytoplasm-enriched fractions were separated on a 16.5% Tris-Tricine gel and AMPs were detected by Western immunoblot analysis. No AMPs were detected in cytoplasm-enriched fractions prepared from cells alone. AMP structural classes display different kinetics of transport into the bacterial cytoplasm
We hypothesized that AMP substrates bound by SapA are delivered to the inner membrane Sap transporter complex for energy dependent transport into the bacterial cytoplasm. Indeed, we demonstrated that AMPs localized to the bacterial cytoplasm of intact cells within 30 minutes of exposure to sublethal AMP concentrations. Thus, we sought to characterize the kinetics of localization to the cytoplasm. NTHI were incubated with AMPs for 30 minutes (pulse period), cells were washed to removed unbound AMPs and then incubated in buffer alone (without AMPs) for 0, 2, or 4 hours (chase period). Incubation of AMP-exposed NTHI in buffer, during this chase period, did not affect NTHI viability during the time course (data not shown). At each time point, cytoplasm-enriched fractions were monitored for the presence of AMP molecules by immunoblot (Figure 3). We observed maximal import of hBD3 at the earliest time point tested (Figure 3A and 3C). Interestingly we observed a decrease in the detection of hBD3 over the remainder of the chase period (from 0 to 4 hours, Figure 3A and 3C). Import of hBD-3 was dependent upon SapA production as hBD-3 did not localize to the cytoplasm in a SapA-deficient strain (data not shown). Although similarly detected in the bacterial cytoplasm following the 30 minute pulse period (0 hr), LL-37 peptide appeared to accumulate during the first 2 hours of the chase period and decrease only slightly when incubated an additional 2 hours (4 hr, Figure 3A and 3C). These data suggest differential kinetics of AMP accumulation within NTHI, likely related to AMP structure and charge. We were unable to follow the fate of LL37 beyond the 4 hour chase period due to loss of NTHI viability.
Fig. 3. In vivo degradation of AMPs in NTHI cytoplasm. 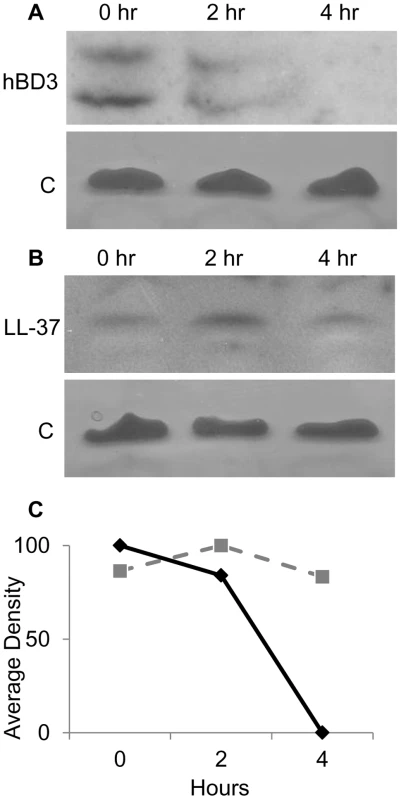
The parental NTHI strain was exposed to AMPs for 30 minutes, washed, resuspended in buffer without AMPs, and incubated for a chase period of 0, 2, or 4 hours followed by cell fractionation. Enriched cytoplasmic fractions were separated by SDS-PAGE on a 16.5% Tris-Tricine gel and hBD-3 (A) or LL-37 (B) was detected by immunoblot analysis. Samples were matched for viability, normalized for protein amount, and confirmed by silver stain of lipopeptide [C] contained in enriched cytoplasmic samples. Average density of bands is represented graphically (bottom panel C). There was no signal for control samples from cells incubated in the absence of AMPs. Data are representative of three independent assays. AMPs are degraded by cytoplasmic peptidase activity
The absence of known MTR in NTHI [15], combined with our observation of decreasing AMP levels in the cytoplasm, led us to hypothesize that the AMPs are degraded in the cytoplasm. To determine susceptibility of AMPs to peptidase activity, we incubated cytoplasm-enriched fractions of NTHI with hBD-3 or LL37 for 0, 2, 5, or 13 hours in the presence or absence of a protease inhibitor cocktail mix (−/+ inhibitor , Figure 4). At each time point, samples were collected and monitored for AMP by immunoblot (Figure 4). Similar to our observations in intact cells, we observed nearly complete loss of hBD-3 detection within 5 hours whereas LL37 loss was not observed to the same extent. After overnight incubation (T13) however, LL-37 detection was dramatically reduced. In both cases, AMPs were susceptible to cytoplasmic fraction peptidase activity that was blocked in the presence of protease inhibitors (Figure 4, + inhibitor). Collectively, these data suggest that AMP molecules are susceptible to cytoplasmic protease activity, and are likely beneficial to NTHI for metabolic purposes.
Fig. 4. Degradation of AMPs by cytoplasmic peptidase activity. 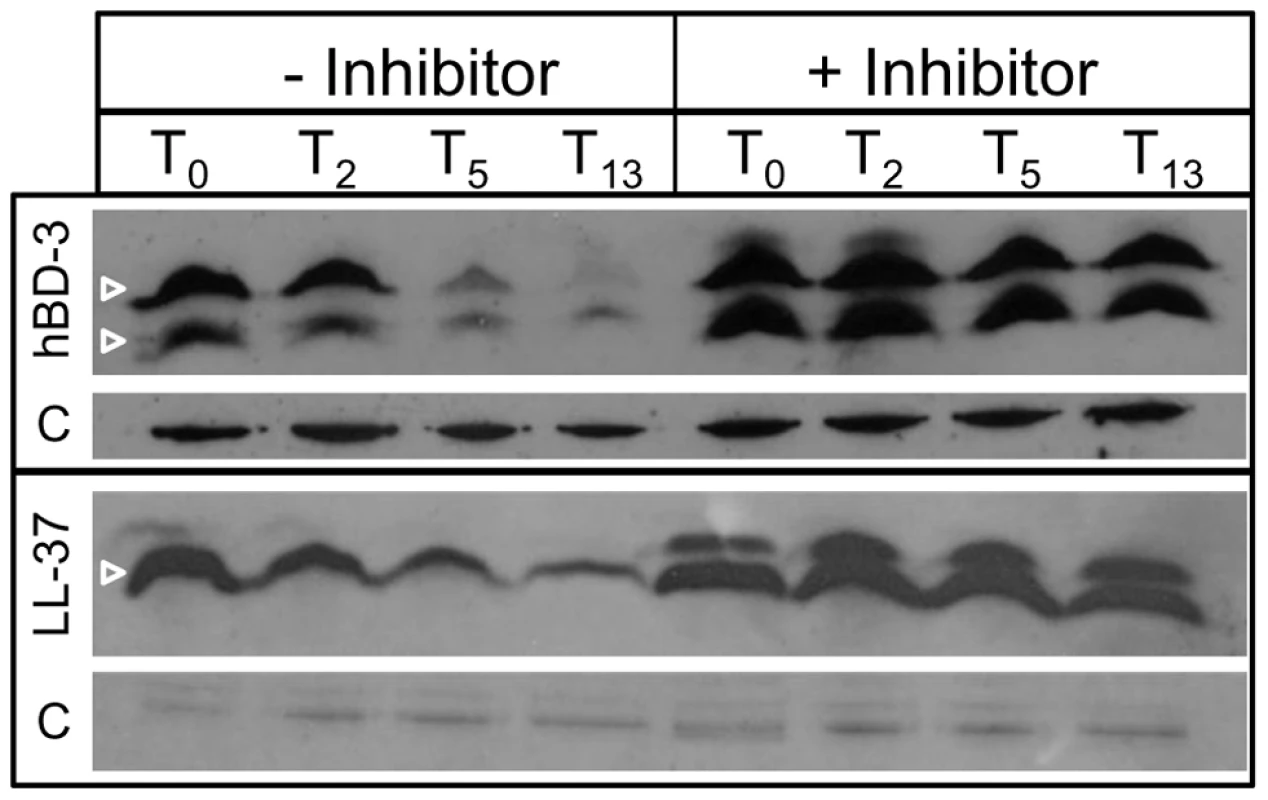
The parental NTHI strain was fractionated to obtain cytoplasm-enriched fractions. This fraction was combined with hBD-3 or LL37 in the presence or absence of a protease inhibitor cocktail (−/+ Inhibitor) and incubated for 0, 2, 5 or 13 hours. Samples were separated by SDS-PAGE on a 16.5% Tris-Tricine gel, and hBD3 or LL-37 was detected by immunoblot analysis. Samples were matched for viability and normalized for protein amount, as shown [C] for both hBD3 and LL-37. Transport of antimicrobial peptides to the cytoplasm of NTHI requires the Sap permease
The Sap transporter belongs to a family of ABC transporters that recognize and transport substrates that are typically small and cationic in nature [29]. We previously demonstrated that AMPs can displace additional substrates previously bound by SapA, and that mutations in Sap transporter proteins alter NTHI susceptibility to AMP exposure [37]–[39]. We hypothesized that the Sap transporter may mediate the transfer of AMPs across the cytoplasmic membrane, as a mechanism of AMP transport into the cell. We therefore generated a deletion mutant lacking the permease components of the Sap transporter (SapB and SapC) and determined the subcellular localization of LL-37 and hBD3. NTHI (parent and sapBC permease mutant) were exposed to sub-lethal concentrations of either LL-37 or hBD3 for 30 minutes and processed to assess AMP localization by transmission electron microscopy, to visualize AMP localization in intact cells. We observed that both LL-37 (Figure 5, top panels) and hBD3 (Figure 5, bottom panels) localized to the periplasm and the cytoplasm of the parental cells. Accumulation of AMPs was not observed in either the bacterial outer or inner membranes under these conditions. In contrast, neither LL-37 nor hBD3 were observed in the cytoplasm of the sapBC permease-deficient cells, yet a striking accumulation of AMPs was observed in the periplasm and cytoplasmic membrane. These data suggest that subcellular localization of AMP molecules to the bacterial cytoplasm is dependent upon Sap-mediated transport. Consistent with this observation, sapBC permease-deficient cells are more sensitive to AMP exposure (data not shown). This targeted transport of AMPs, to the cytoplasm for degradation, may serve to reduce periplasmic accumulation of AMPs, and thus protect the cytoplasmic membrane from AMP association and lethality. Thus, Sap-mediated transport of AMP to the cytoplasm for degradation serves as a newly described subversion mechanism that would provide a nutritional benefit to the bacterium thus coupling innate immune resistance to metabolic activity.
Fig. 5. The Sap transporter inner membrane permease is required for AMP transport to the bacterial cytoplasm. 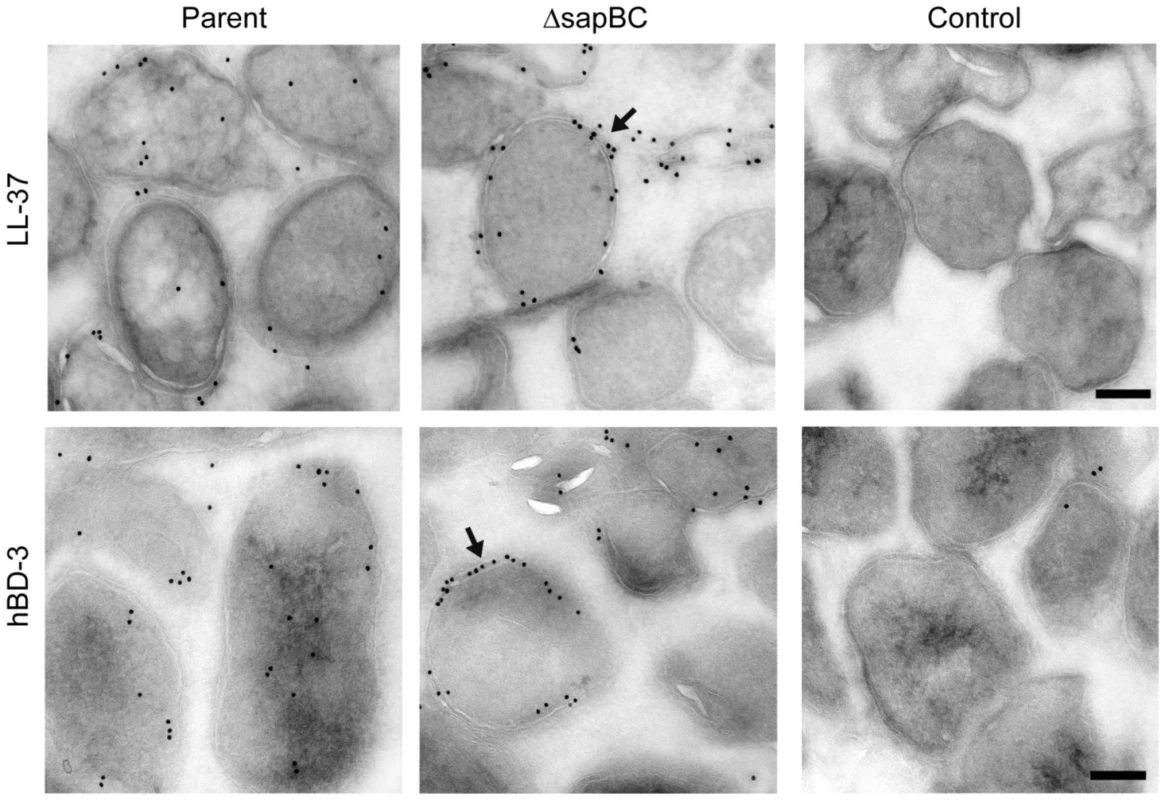
Parent and permease-deficient NTHI were incubated with sublethal concentrations of LL-37 or hBD3 for 30 minutes, washed, fixed, sectioned (50 nm) and immunogold labeled for AMP as visualized by transmission electron microscopy (TEM) analysis. Parallel samples not exposed to AMPs yet processed in parallel served as a labeling control. Scale bar = 200nm. Discussion
The ability of NTHI to persist in both the commensal and pathogenic lifestyles requires mechanisms to avert the host innate immune response. The epithelium in the upper airway produces AMPs that assist in the control of NTHI [41]–[43]. This led us to investigate the mechanisms used by NTHI to evade killing by AMPs. Modifications of the outer membrane primarily serve as a first line of defense against AMP lethality including addition of phosphorylcholine to lipooligosaccharide to repel positively charged AMPs [26] and lipid A acylation to increase NTHI hydrophobicity, thus altering membrane permeability [27]. Although effective as a first line defense strategy, AMP accumulation and disruption of the outer membrane, periplasmic localization and permeabilization of the cytoplasmic membrane is microbicidal. Many bacteria possess additional mechanisms to detect and resist AMP insult shown to be regulated through two-component regulatory signaling [44], [45], release of extracellular peptidases [16], secreted proteins to bind AMPs [19], and AMP efflux pumps [15], [46], [47]. NTHI however, lacks two-component regulatory systems that sense AMP exposure, and additional mechanisms of AMP resistance have not been identified.
We previously demonstrated that the Sap transporter conferred NTHI resistance to AMPs. The periplasmic binding protein, SapA, binds AMPs [37], contributes to AMP resistance [38] and is also upregulated in response to AMP exposure during otitis media. This, taken with our data presented here, suggests that resistance to AMP lethality, conferred via Sap mediated transport of AMPs, is important for NTHI pathogenesis. Since we observed marked attenuation of Sap transporter mutants in vivo [37], [38], we predicted that neutralization of AMPs would rescue this attenuated phenotype, supporting a critical role for Sap-dependent AMP import in NTHI pathogenesis. In support of this, in vivo neutralization of the hBD3 orthologue, chinchilla β-defensin-1, which is highly expressed in the chinchilla upper respiratory tract [48], results in increased colonization by NTHI [41]. These data suggest that AMPs play an important role in limiting infection in a mammalian host. Indeed, alterations in AMP production can affect the ability of other microorganisms to colonize a host [11], [40], [49]. The ability of NTHI to exploit mechanisms to resist AMP lethality may equip transition to that of opportunistic pathogen and ability to cause disease. We demonstrated here that neutralization of β-defensin activity in the middle ear of a mammalian model of otitis media rescues the attenuated phenotype of the SapA-deficient strain, such that the mutant strain is no longer cleared from this environment (Figure 1). This is the first direct evidence that SapA contributes to bacterial virulence by conferring protection from AMP lethality in vivo. Having established this, we monitored the consequence of SapA-AMP interaction in intact bacterial cells. We describe a novel mechanism of inner membrane transport of AMP molecules, via the Sap ABC transporter, to the bacterial cytoplasm resulting in AMP degradation (Figure 6). This direct mechanism of AMP influx serves to decrease both periplasmic and inner membrane accumulation of AMPs and protect NTHI from AMP bactericidal effects. Current models predict that AMP-induced microbicidal activity results from transmembrane pore formation subsequent to AMP accumulation at the cell surface [1]. However, we observed by TEM analysis, that AMPs localized to the bacterial cytoplasm, even when NTHI were exposed to sublethal concentrations that do not appear to accumulate in the bacterial membranes, suggesting a mechanism whereby bacteria can directly import AMP molecules. Since accumulation of AMPs in the bacterial cytoplasm would likely be detrimental, we hypothesized that AMPs are subsequently targeted for cytoplasmic degradation. Indeed, we observed that hBD3, localized to the bacterial cytoplasm, was susceptible to cytoplasmic degradation, as the ability to detect hBD3 decreased over time (Figure 3A, C). Although we did not observe an appreciable decrease in LL-37 detection following uptake into intact cells, we demonstrated that LL37, like hBD-3, was susceptible to peptidase activity in cytoplasm-enriched fractions (Figure 4). These data suggest differential kinetics of uptake and degradation, likely dependent upon AMP structure and charge. In support of differential kinetic uptake of AMP molecules, we previously demonstrated that LL-37 and hBD3 differ in their abilities to bind and displace the iron-containing compound heme, also shown to bind SapA [39]. Consistent with these studies, cytoplasmic accumulation of LL-37 appeared delayed, relative to hBD3 (Figure 3C). Although we have not, as of yet, identified the binding sites for these SapA ligands, our data indicate that hBD3, which is highly cationic, may preferentially bind SapA based upon charge (unpublished). Additionally, it has been shown that the cathelicidin LL-37 contains proline-rich sequences that resist degradation by serine proteases [50] and NTHI lack PgtE and OmpT homologues, shown to mediate degradation of alpha-helical peptides [51]–[54], which may explain our observation that LL-37 appears more resistant to proteolysis within the bacterial cytoplasm. NTHI encodes homologues of bacterial cytoplasmic proteases, ClpX and Lon proteases, shown to contribute to AMP resistance in other microorganisms [55], [56]. The contribution of these proteases to AMP degradation in NTHI is currently unknown, but remains the focus of future work.
Fig. 6. Model of Sap-dependent import of AMP molecules and subsequent degradation. 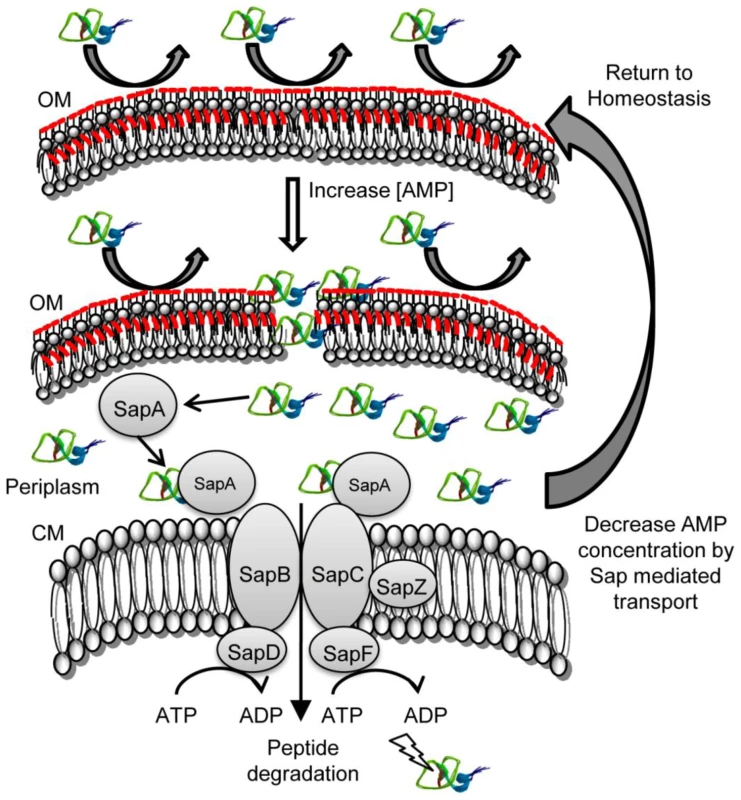
In the presence of low concentrations of AMP, NTHI are able to resist lethality by modification of the outer membrane (OM; ChoP and Lipid A acylation, red) and subsequent repulsion of cationic charged peptides away from the bacterial cell surface. An increase in local concentrations of AMP increases production of the Sap transporter which functions to bind and transport periplasmic AMPs across the NTHI cytoplasmic membrane (CM). Transported AMPs are susceptible to proteolytic degradation. A reduction in the critical threshold concentration of AMPs in the periplasm returns NTHI to a homestatic state of innate immune resistance. An alternative hypothesis is that an MTR drug efflux pump functions to export AMPs that have accumulated in the bacterial inner membrane and in the cytoplasm. It was recently shown that the MtrC periplasmic membrane fusion protein conferred resistance to LL-37 and β-defensins in Haemophilus ducreyi [47]. Interestingly, the H. ducreyi Sap transporter does not mediate β-defensin resistance [36], [47]. Although NTHI strain 86-028NP contains week homologs of the MTR system, our evidence suggests that this system is not functional or does not function to confer resistance to defensin or cathelicidin peptides, a well described function of the NTHI Sap transporter [37]–[39]. Further, we demonstrated that in vivo neutralization of native cBD-1 was sufficient to rescue competitive growth of the sapA mutant, suggesting that SapA was sufficient to mediate resistance to this defensin molecule in the host. Finally, a functional MTR system would be expected to export AMP molecules thus avoiding periplasmic, membrane and cytoplasmic accumulation of AMPs. We observed, in the absence of a functional Sap permease complex, AMP accumulation (Figure 5) and increased sensitivity to AMP-mediated killing (data not shown). We would expect, based upon these published reports that a functional MTR system would not result in this phenotype.
Development of antibiotic resistant strains secondary to conventional antibiotic use has prompted interest in the use of AMPs as therapeutic alternatives to treat bacterial infections [57]. The use of AMPs in therapeutic regimens may be hindered due to bacterial subversion mechanisms of AMP lethality [54]. Since the Sap transporter functions to protect against the accumulation of AMPs in the bacterial periplasm, and subsequent interaction with the cytoplasmic membrane, a mechanism to block transporter function may provide novel therapeutic supplements for NTHI infections. Delivery of small molecule inhibitors to block substrate binding and transport would confound an important AMP resistance mechanism in NTHI, rendering NTHI susceptible to innate immune attack while preserving the normal flora that are often disrupted by conventional antibiotic use.
The described novel mechanism of AMP import and subsequent degradation expands our understanding of host-pathogen interactions, particularly those that mediate resistance to key components of the host innate immune response. Future studies to identify AMP specific intracellular peptidases, AMP degradation products, and metabolic consequences of targeted peptide degradation, are needed to better understand bacterial survival strategies in the hostile host environment. Further, a better understanding of bacterial mechanisms used to transition from commensal to that of an opportunistic pathogen will better equip the design of therapeutics to combat disease.
Materials and Methods
Ethics statement
All animal experiments were carried out in strict accordance with the accredited conditions in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (Welfare Assurance Number A3544-01) at The Research Institute at Nationwide Children's Hospital, AR08-00027.
All experimental procedures were performed under xylazine and ketamine anesthesia, and all efforts were made to minimize suffering.
Animals
Healthy adult chinchillas (Chinchilla lanigera), purchased from Rauscher's Chinchilla Ranch (LaRue, OH), were used for these studies, after allowing them to acclimate to the vivarium for 7 to 10 days. Chinchillas were anesthetized with xylazine (2 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and ketamine (10 mg/kg), Phoenix Scientific Inc., St. Joseph, MO), and nasopharyngeal lavage fluids were obtained by passive inhalation of 500 µl of pyrogen-free sterile saline into one naris with recovery of lavage fluid from the contralateral naris as liquid was exhaled. Middle ear fluids were recovered via epitympanic tap through the superior bullae, and directly obtained from the inferior bullae behind the tympanic membrane. All animal experiments were performed using accredited conditions for animal welfare approved by the Institutional Animal Care and Use Committee (Welfare Assurance Number A3544-01) at The Research Institute at Nationwide Children's Hospital, AR08-00027.
Bacterial strains and culture conditions
Nontypeable Haemophilus influenzae strain 86-028NP is a minimally passaged clinical isolate obtained at Nationwide Children's Hospital in Columbus, Ohio. This prototypic wild type strain has been sequenced and extensively characterized in chinchilla models of otitis media [58], [59]. The parental NTHI strain 86-028NP:: rpsL is a streptomycin resistant strain constructed as previously described [60]. The sapBC permease deletion mutant was constructed as an unmarked, non-polar deletion mutation of the sapB and sapC genes by recombineering strategy as previously described [39]. Bacterial strains were grown on chocolate II agar (Becton Dickinson, Sparks, MD) or in brain heart infusion broth supplemented with 2 µg heme/mL and 1 µg NAD/mL (sBHI). Bacteria were cultured from overnight growth on chocolate II agar, resuspended in sBHI to OD490 = 0.65 (equivalent to 1×108 CFU/ml), diluted 1 : 5 into fresh sBHI medium and grown to mid-log phase (3 hours) at 37°C, 5% CO2, static.
Antibody-induced neutralization of native cBD-1 in vivo
In vivo neutralization of chinchilla β-defensin 1 (cBD-1) was performed as previously described with the following modifications [41]. We prepared and purified (r)cBD-1 based upon previously published methods [41], [48]. A HiTrap protein G HP column (GE healthcare, Pittsburgh, PA) was used to affinity purify total IgG from rabbit anti-(r)cBD-1 and the cognate pre-immune serum. One milliliter of serum was dialyzed [3.5 kDa molecular weight cutoff (MWCO), EMD Chemicals Inc., San Diego, CA] at 4°C against 20 mM sodium phosphate buffer, pH 7.0 and applied to the affinity column. Immunoglobulins were eluted from the affinity matrix with 0.1 M glycine-HCl, pH 2.7 in one milliliter fractions into eppendorf tubes that contained 200 µl of 1.0 M Tris-HCl, pH 9.0, to neutralize the acidic elution conditions. Samples that contained the greatest amount of protein (fractions 1 and 2) were pooled and dialyzed overnight at 4°C against sterile saline. Protein concentrations of the anti-(r)cBD-1 and the pre-immune serum were determined using Coomassie Plus Protein Assay Reagent (Thermo Scientific, Rockford, IL). We confirmed that anti-(r)cBD-1 antiserum, and not the pre-immune antiserum, bound purified (r)cBD-1 by immunoblot (data not shown). Five adult chinchillas were administered 90 µg anti-cBD-1 or pre-immune rabbit immunoglobulin, in a total volume of 200 µl. Antisera were administered by passive inhalation of droplets of the solution delivered to the nares or direct transbullar inoculation of the middle ears of anesthetized chinchillas. Animals were then placed in a prone position for 20 minutes prior to intranasal challenge with approximately 5.0×107 cfu NTHI wild type strain 86-028NP mixed with 5.0×107 cfu sapA mutant (co infection) in a 100 µl volume or transbullarly with 2.5×103 cfu NTHI wild type strain 86-028NP mixed with 2.5×103 cfu sapA mutant in a 200 µl volume. Nasopharyngeal lavage and epitympanic taps were performed 1, 2, 4, 7, 10, 14 days after NTHI co-challenge, and bacterial counts were determined by dilution plating of bacteria on chocolate agar.
NTHI fractionation
Parental NTHI strain 86-028NP::rpsL was grown to mid-log phase in brain heart infusion broth supplemented with 2 µg heme/ml and 2 µg NAD/ml (sBHI). Cells were pelleted by centrifugation and resuspended in 1X phosphate buffered saline containing 2 mg Polymyxin B sulfate (Sigma-Aldrich, St. Louis, MO)/ml, 0.05% glycerol, to bind lipooligosacharide and generate spheroplasts, thereby releasing periplasmic contents to the supernatant. Following incubation for 1 hour at 37°C on a rotating platform (100 rpm), spheroplasts were pelleted by centrifugation and resuspended in 1 ml 10 mM HEPES pH 7.4 (Sigma-Aldrich, St. Louis, MO), 0.05% glycerol and subsequently lysed by freeze-thaw method (10 cycles, −78°C to 37°C). Lysed spheroplasts were then incubated at room temperature, on an orbital rocker, with 1 µl Benzonase Nuclease (Novagen, Darmstadt, Germany) and 5 µl 1 M MgCl2 (Ambicon, Alamo, CA)) to a final concentration of 5 µM. Bacterial membranes were pelleted by ultracentrifugation (40,000 rpm for 1 hour, 4°C) and cytoplasmic proteins were removed in the supernatant. Cytoplasm and periplasm-enriched fractions were observed by two dimensional gel electrophoresis (Bio-Rad, Hercules, CA) of enriched fractions, stained with SYPRO Ruby Protein Gel Stain (Invitrogen, Carlsbad, CA) and imaged at 535 nm (Kodak Image Station 2000, New York, NY). To ensure successful NTHI fractionation, cytoplasm and periplasm-enriched fractions were analyzed by silver stain (Pierce, Rockford, IL) following SDS-PAGE separation. In addition, NTHI were exposed to a sub-lethal concentration of 15 µg ampicillin/mL to induce expression and trafficking of β-lactamase to the NTHI periplasm. Periplasm and cytoplasm-enriched fractions were separated by SDS-PAGE and transferred to nitrocellulose (Bio-Rad, Hercules, CA). Membranes were then blocked in 3% nonfat dried milk and probed for the periplasmic protein, β-lactamase, by incubating with mouse anti - β-lactamase for 45 minutes or the cytoplasmic protein, SapD, by incubating with rabbit anti-SapD overnight. Membranes were then washed, incubated with goat anti-mouse IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA) for 30 minutes or goat anti-rabbit IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA) for 1 hour, washed, and peroxidase activity was detected using Amersham ECL Western Blotting Detection Reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
In vivo pulse-chase analysis to determine AMP localization
Parental NTHI strain 86-028NP::rpsL were grown to mid-log phase in sBHI, normalized for cell number, and pelleted. Pellets were resuspended in 10 mM sodium phosphate buffer pH 7.4, supplemented with 2% sBHI. Cultures were incubated with sublethal concentrations of LL-37 or hBD3 as previously described, for a pulse period of 30 minutes. Cells were then pelleted by centrifugation, washed, and resuspended in 10 mM sodium phosphate buffer pH 7.4, supplemented with 2% sBHI. Cultures were incubated at 37°C, 5% CO2, static for a chase period of 0, 2, or 4 hours. Samples were transfered to ice and fractionated to obtain periplasm and cytoplasm-enriched fractions as described above. Enriched fractions were lyophilized (Labconco, Kansas City, MO) overnight and subsequently resuspended in 10 mM HEPES pH 7.4, 0.05% glycerol, then normalized for equal amounts of protein using Coomassie Plus Protein Assay Reagent (Thermo Scientific, Rockford, IL) prior to separation by SDS-PAGE (16.5% Ready Gel Tris-Tricine Precast Gels, Bio-Rad, Hercules, CA). Equal detection of NTHI lipopetide [61] by silver stain (Pierce, Rockford, IL) confirmed normalization of samples. Samples containing LL-37 or hBD3 were transferred to PVDF (Bio-Rad, Hercules, CA) or nitrocellulose (Bio-Rad, Hercules, CA), and blocked in 3% skim milk or 5% BSA, respectively. Membranes were incubated with rabbit anti-LL-37 (Phoenix Pharmaceuticals, Burlingame, CA) or goat anti-hBD3 (Leinco Technologies, St. Louis, MO) overnight at 4°C, washed, and incubated with goat anti-rabbit IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA) or rabbit anti-goat IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA), respectively. Membranes were washed and peroxidase activity was detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
Degradation of antimicrobial peptides in cytoplasm-enriched fractions
NTHI cells were fractionated as described above. Cytoplasm-enriched fractions were concentrated in Amicon centrifuge filters (3.0kDa MWCO) and normalized for equal amounts of protein using Coomassie Plus Protein Assay Reagent (Thermo Scientific, Rockford, IL). Cytoplasmic peptidase activity was monitored by incubating 8 µg of cytoplasm-enriched fractions with LL37 (40 ng) or hBD-3 (20 ng) in siliconized glass vials and incubated at 37°C for 2, 5.5 and 13 hours. In parallel, a protease inhibitor cocktail set was included (Calbiochem, LaJolla, CA). Following incubation, samples were separated on a SDS-PAGE gel (16.5% Ready Gel Tris-Tricine Precast Gels, Bio-Rad, Hercules, CA). Samples containing LL-37 or hBD3 were transferred to PVDF (Bio-Rad, Hercules, CA) or nitrocellulose (Bio-Rad, Hercules, CA), and blocked in 3% skim milk or 5% BSA, respectively. Membranes were incubated with rabbit anti-LL-37 (Phoenix Pharmaceuticals, Burlingame, CA) or goat anti-hBD3 (Leinco Technologies, St. Louis, MO) overnight at 4°C, washed, and incubated with goat anti-rabbit IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA) or rabbit anti-goat IgG (H+L) HRP conjugate (Invitrogen, Carlsbad, CA), respectively. Membranes were washed and peroxidase activity was detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Rockford, IL).
Cryo Immunoelectron microscopy
Parental strains NTHI 86-028NP::rpsL and the isogenic sapBC mutant strain were grown to mid-log phase in sBHI, normalized for cell number, and pelleted. Pellets were resuspended in 10 mM sodium phosphate buffer pH 7.4, supplemented with 2% sBHI. Human LL-37 cathelicidin (Phoenix Pharmaceuticals, Burlingame, CA) or human beta defensin 3 (PeproTech, Rocky Hill, NJ) were added to cultures at a final concentration of 0.25 µg LL-37/ml or 1 µg hBD3/ml. Cultures containing AMPs or cells alone were incubated at 37°C, 5% CO2, static for 30 minutes. Samples were transfered to ice and cells were pelleted by centrifugation, washed in 100 mM PIPES pH 7.0, and then resuspended in 100 mM PIPES. For immunolocalization of AMPs at the ultrastructural level, bacteria were fixed in 4% paraformaldehyde/0.05% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM PIPES/0.5 mM MgCl2, pH 7.2 (or PBS) for 1 hr at 4°C. Samples were then embedded in 10% gelatin and infiltrated overnight with 2.3 M sucrose/20% polyvinyl pyrrolidone in PIPES/MgCl2 at 4°C. Samples were trimmed, frozen in liquid nitrogen, and sectioned with a Leica Ultracut UCT cryo-ultramicrotome (Leica Microsystems Inc., Bannockburn, IL). 50 nm sections were blocked with 5% FBS/5% NGS for 30 min and subsequently incubated with rabbit anti-hBD3 (Leinco Technologies, Inc.) or rabbit anti-CAP-18 (LL-37) antibody for 1 hr at room temperature. Sections were then washed in block buffer and probed with 18 nm colloidal gold-conjugated anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hr at room temperature. Sections were washed in PIPES buffer followed by a water rinse, and stained with 0.3% uranyl acetate/2% methyl cellulose. Samples were viewed with a JEOL 1200EX transmission electron microscope (JEOL USA Inc., Peabody, MA). All labeling experiments were conducted in parallel with controls omitting the primary antibody. These controls were consistently negative at the concentration of colloidal gold conjugated secondary antibodies used in these studies.
Accession numbers
Proteins discussed in this manuscript are listed followed by their corresponding UniProtKB (Universal Protein Knowledgebase) number. These included: SapA (Q4QL73_HAE18), SapB (Q4QL74_HAE18), SapC (Q4QL75_HAE18), SapD (Q4QL76_HAE18), SapF (Q4QL77_HAE18), OmpT (OMPT_ECOLI), PgtE (PGTE_SALTY), ClpX (Q4QMK4_ HAE18), Lon (Q4QN81_ HAE18), HtrB (Q4QKN9_ HAE18), β-Lactamase (A720C2_ HAE18).
Zdroje
1. BrogdenKA 2005 Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3 238 250
2. SitaramNNagarajR 2002 Host-defense antimicrobial peptides: importance of structure for activity. Curr Pharm Des 8 727 742
3. StolzenbergEDAndersonGMAckermannMRWhitlockRHZasloffM 1997 Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A 94 8686 8690
4. HarderJBartelsJChristophersESchroderJM 2001 Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276 5707 5713
5. SorensenOArnljotsKCowlandJBBaintonDFBorregaardN 1997 The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90 2796 2803
6. BalsRWangXZasloffMWilsonJM 1998 The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 95 9541 9546
7. SalzmanNHGhoshDHuttnerKMPatersonYBevinsCL 2003 Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422 522 526
8. SelstedMEOuelletteAJ 2005 Mammalian defensins in the antimicrobial immune response. Nat Immunol 6 551 557
9. LehrerRI 2004 Paradise lost and paradigm found. Nat Immunol 5 775 776
10. LaubeDMYimSRyanLKKisichKODiamondG 2006 Antimicrobial peptides in the airway. Curr Top Microbiol Immunol 306 153 182
11. NizetVOhtakeTLauthXTrowbridgeJRudisillJ 2001 Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414 454 457
12. PeschelA 2002 How do bacteria resist human antimicrobial peptides? Trends Microbiol 10 179 186
13. ErnstRKGuinaTMillerSI 2001 Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect 3 1327 1334
14. KawasakiK 2006 [Outer membrane remodeling of Salmonella typhimurium and host innate immunity]. Yakugaku Zasshi 126 1227 1234
15. ShaferWMQuXWaringAJLehrerRI 1998 Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95 1829 1833
16. Sieprawska-LupaMMydelPKrawczykKWojcikKPukloM 2004 Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48 4673 4679
17. IslamDBandholtzLNilssonJWigzellHChristenssonB 2001 Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7 180 185
18. AttiaASBensonMAStauffDLTorresVJSkaarEP 2010 Membrane damage elicits an immunomodulatory program in Staphylococcus aureus. PLoS Pathog 6 e1000802
19. FrickIMAkessonPRasmussenMSchmidtchenABjorckL 2003 SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J Biol Chem 278 16561 16566
20. KleinJO 1997 Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr Infect Dis J 16 S5 8
21. KilpiTHervaEKaijalainenTSyrjanenRTakalaAK 2001 Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr Infect Dis J 20 654 662
22. PatelPBDiazMCBennettJEAttiaMW 2007 Clinical features of bacterial conjunctivitis in children. Acad Emerg Med 14 1 5
23. BrookI 2002 Bacteriology of acute and chronic frontal sinusitis. Arch Otolaryngol Head Neck Surg 128 583 585
24. SethiS 2000 Bacterial infection and the pathogenesis of COPD. Chest 117 286S 291S
25. RomanFCantonRPerez-VazquezMBaqueroFCamposJ 2004 Dynamics of long-term colonization of respiratory tract by Haemophilus influenzae in cystic fibrosis patients shows a marked increase in hypermutable strains. J Clin Microbiol 42 1450 1459
26. LysenkoESGouldJBalsRWilsonJMWeiserJN 2000 Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun 68 1664 1671
27. StarnerTDSwordsWEApicellaMAMcCrayPBJr 2002 Susceptibility of nontypeable Haemophilus influenzae to human beta-defensins is influenced by lipooligosaccharide acylation. Infect Immun 70 5287 5289
28. GroismanEAParra-LopezCSalcedoMLippsCJHeffronF 1992 Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci U S A 89 11939 11943
29. Parra-LopezCBaerMTGroismanEA 1993 Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. Embo J 12 4053 4062
30. Parra-LopezCLinRAspedonAGroismanEA 1994 A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. Embo J 13 3964 3972
31. EswarappaSMPanguluriKKHenselMChakravorttyD 2008 The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology 154 666 678
32. Lopez-SolanillaEGarcia-OlmedoFRodriguez-PalenzuelaP 1998 Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and animal bacterial pathogenesis. Plant Cell 10 917 924
33. ChenHYWengSFLinJW 2000 Identification and analysis of the sap genes from Vibrio fischeri belonging to the ATP-binding cassette gene family required for peptide transport and resistance to antimicrobial peptides. Biochem Biophys Res Commun 269 743 748
34. HarmsCDomotoYCelikCRaheEStumpeS 2001 Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems Trk(H) and Trk(G) from Escherichia coli K-12. Microbiology 147 2991 3003
35. RodasPIContrerasIMoraGC 2010 Salmonella enterica serovar Typhi has a 4.1 kb genetic island inserted within the sapABCDF operon that causes loss of resistance to the antimicrobial peptide protamine. J Antimicrob Chemother 65 1624 1630
36. MountKLTownsendCARinkerSDGuXFortneyKR 2010 Haemophilus ducreyi SapA contributes to cathelicidin resistance and virulence in humans. Infect Immun 78 1176 1184
37. MasonKMBruggemanMEMunsonRSBakaletzLO 2006 The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 62 1357 1372
38. MasonKMMunsonRSJrBakaletzLO 2005 A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect Immun 73 599 608
39. MasonKMRaffelFKRayWCBakaletzLO 2011 Heme Utilization by Nontypeable Haemophilus influenzae is Essential and Dependent on Sap Transporter Function. J Bacteriol 193 2527 35
40. ChromekMSlamovaZBergmanPKovacsLPodrackaL 2006 The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12 636 641
41. McGillivaryGMasonKMJurcisekJAPeeplesMEBakaletzLO 2009 Respiratory syncytial virus-induced dysregulation of expression of a mucosal beta-defensin augments colonization of the upper airway by non-typeable Haemophilus influenzae. Cell Microbiol 11 1399 1408
42. McGillivaryGRayWCBevinsCLMunsonRSJrBakaletzLO 2007 A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media. Mol Immunol 44 2446 2458
43. LimDJChunYMLeeHYMoonSKChangKH 2000 Cell biology of tubotympanum in relation to pathogenesis of otitis media - a review. Vaccine 19 Suppl 1 S17 25
44. GunnJSMillerSI 1996 PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol 178 6857 6864
45. McPheeJBBainsMWinsorGLewenzaSKwasnickaA 2006 Contribution of the PhoP-PhoQ and PmrA-PmrB two-component regulatory systems to Mg2+-induced gene regulation in Pseudomonas aeruginosa. J Bacteriol 188 3995 4006
46. BengoecheaJASkurnikM 2000 Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol Microbiol 37 67 80
47. RinkerSDTrombleyMPGuXFortneyKRBauerME 2011 Deletion of mtrC in Haemophilus ducreyi increases sensitivity to human antimicrobial peptides and activates the CpxRA regulon. Infect Immun 79 2324 34
48. HarrisRHWilkDBevinsCLMunsonRSJrBakaletzLO 2004 Identification and characterization of a mucosal antimicrobial peptide expressed by the chinchilla (Chinchilla lanigera) airway. J Biol Chem 279 20250 20256
49. IimuraMGalloRLHaseKMiyamotoYEckmannL 2005 Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol 174 4901 4907
50. ShinnarAEButlerKLParkHJ 2003 Cathelicidin family of antimicrobial peptides: proteolytic processing and protease resistance. Bioorg Chem 31 425 436
51. SugimuraKHigashiN 1988 A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol 170 3650 3654
52. SodeindeOASubrahmanyamYVStarkKQuanTBaoY 1992 A surface protease and the invasive character of plague. Science 258 1004 1007
53. GuinaTYiECWangHHackettMMillerSI 2000 A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol 182 4077 4086
54. YeamanMRYountNY 2003 Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55 27 55
55. BotosIMelnikovEECherrySKhalatovaAGRasulovaFS 2004 Crystal structure of the AAA+ alpha domain of E. coli Lon protease at 1.9A resolution. J Struct Biol 146 113 122
56. McGillivraySMEbrahimiCMFisherNSabetMZhangDX 2009 ClpX contributes to innate defense peptide resistance and virulence phenotypes of Bacillus anthracis. J Innate Immun 1 494 506
57. HancockRE 1997 Peptide antibiotics. Lancet 349 418 422
58. HarrisonADyerDWGillaspyARayWCMungurR 2005 Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol 187 4627 4636
59. HarrisonARayWCBakerBDArmbrusterDWBakaletzLO 2007 The OxyR regulon in nontypeable Haemophilus influenzae. J Bacteriol 189 1004 1012
60. TracyEYeFBakerBDMunsonRSJr 2008 Construction of non-polar mutants in Haemophilus influenzae using FLP recombinase technology. BMC Mol Biol 9 101
61. WangBClearyPPXuHLiJD 2003 Up-regulation of interleukin-8 by novel small cytoplasmic molecules of nontypeable Haemophilus influenzae via p38 and extracellular signal-regulated kinase pathways. Infect Immun 71 5523 5530
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



