-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
Measles virus (MeV), a member of the paramyxovirus family of enveloped RNA viruses and one of the most infectious viral pathogens identified, accounts for major pediatric morbidity and mortality worldwide although coordinated efforts to achieve global measles control are in place. Target cell entry is mediated by two viral envelope glycoproteins, the attachment (H) and fusion (F) proteins, which form a complex that achieves merger of the envelope with target cell membranes. Despite continually expanding knowledge of the entry strategies employed by enveloped viruses, our molecular insight into the organization of functional paramyxovirus fusion complexes and the mechanisms by which the receptor binding by the attachment protein triggers the required conformational rearrangements of the fusion protein remain incomplete. Recently reported crystal structures of the MeV attachment protein in complex with its cellular receptors CD46 or SLAM and newly developed functional assays have now illuminated some of the fundamental principles that govern cell entry by this archetype member of the paramyxovirus family. Here, we review these advances in our molecular understanding of MeV entry in the context of diverse entry strategies employed by other members of the paramyxovirus family.
Published in the journal: Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry. PLoS Pathog 7(6): e32767. doi:10.1371/journal.ppat.1002058
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1002058Summary
Measles virus (MeV), a member of the paramyxovirus family of enveloped RNA viruses and one of the most infectious viral pathogens identified, accounts for major pediatric morbidity and mortality worldwide although coordinated efforts to achieve global measles control are in place. Target cell entry is mediated by two viral envelope glycoproteins, the attachment (H) and fusion (F) proteins, which form a complex that achieves merger of the envelope with target cell membranes. Despite continually expanding knowledge of the entry strategies employed by enveloped viruses, our molecular insight into the organization of functional paramyxovirus fusion complexes and the mechanisms by which the receptor binding by the attachment protein triggers the required conformational rearrangements of the fusion protein remain incomplete. Recently reported crystal structures of the MeV attachment protein in complex with its cellular receptors CD46 or SLAM and newly developed functional assays have now illuminated some of the fundamental principles that govern cell entry by this archetype member of the paramyxovirus family. Here, we review these advances in our molecular understanding of MeV entry in the context of diverse entry strategies employed by other members of the paramyxovirus family.
Paramyxoviruses: Receptors and Virus Entry
The Paramyxoviridae are enveloped, non-segmented, negative-strand RNA viruses that include major human pathogens belonging to two subfamilies. The Pneumonvirinae subfamily includes respiratory syncytial virus (RSV) and the metapneumoviruses, while the Paramyxovirinae subfamily includes, amongst others, measles virus (MeV), mumps virus, human parainfluenza viruses (hPIV1-4), and the recently emerged, highly pathogenic henipaviruses Hendra (HeV) and Nipah (NiV). Members of both subfamilies are responsible for significant human morbidity and mortality. MeV, in particular, remains a major cause of childhood mortality worldwide despite the availability of a live-attenuated vaccine [1].
Of the different paramyxovirus genera, only the morbilliviruses, including MeV, and the henipaviruses are known to bind to proteinaceous receptors displayed on the surface of target cells for infection. Consequently, their attachment proteins lack neuraminidase activity, while all other members of the Paramyxovirinae subfamily carry haemagglutinin-neuraminidase (HN) attachment proteins with high specificity for sialic acid-containing oligosaccharides or glycolipids [2]. Specifically, all MeV haemagglutinin (H) attachment proteins analyzed thus far are capable of high-affinity interaction with signaling lymphocytic activation molecule (SLAM/CD150 w) [3], [4]. H proteins derived from the attenuated vaccine strain Edmonston and some isolates also bind to the regulator of complement activation (CD46) [5]–[7]. Clinically, systemic spread and viremia may be supported by a third MeV receptor that has been hypothesized to be present on epithelial cells [8], [9]. The henipavirus attachment (G) proteins have adapted to bind ephrinB2 and B3 as receptors [10]–[12].
All paramyxoviruses gain entry into and spread between cells by promoting direct membrane fusion. Membrane merger is mediated by the viral fusion (F) protein, which, like other class I fusion proteins such as influenza HA and HIV env, first forms metastable homo-trimers that require proteolytic activation to gain functionality [2]. Receptor binding by the attachment protein is thought to then trigger major conformational changes in mature F, resulting first in insertion of a hydrophobic domain, the fusion peptide, into the target membrane and ultimately in formation of a fusion pore through juxtapositioning of the F transmembrane domain and fusion peptide in the thermodynamically stable postfusion conformation [13]–[17] (Figure 1). Unlike retro - or orthomyxovirus entry, the complexity of the paramyxovirus fusion triggering mechanism is raised to a higher level by the fact that the receptor binding and fusion-promoting functions are contributed by separately encoded envelope glycoproteins. This physical separation of the two functions necessitates a mechanism of posttranslational linkage, which is accomplished through the formation of virus-specific hetero-oligomer complexes between the two proteins [2]. However, the overall spatial organization of functional Paramyxovirinae fusion complexes and the molecular mechanism that links receptor binding with coordinated F protein refolding into the postfusion conformation remain largely unknown.
Fig. 1. Measles virus fusion model. 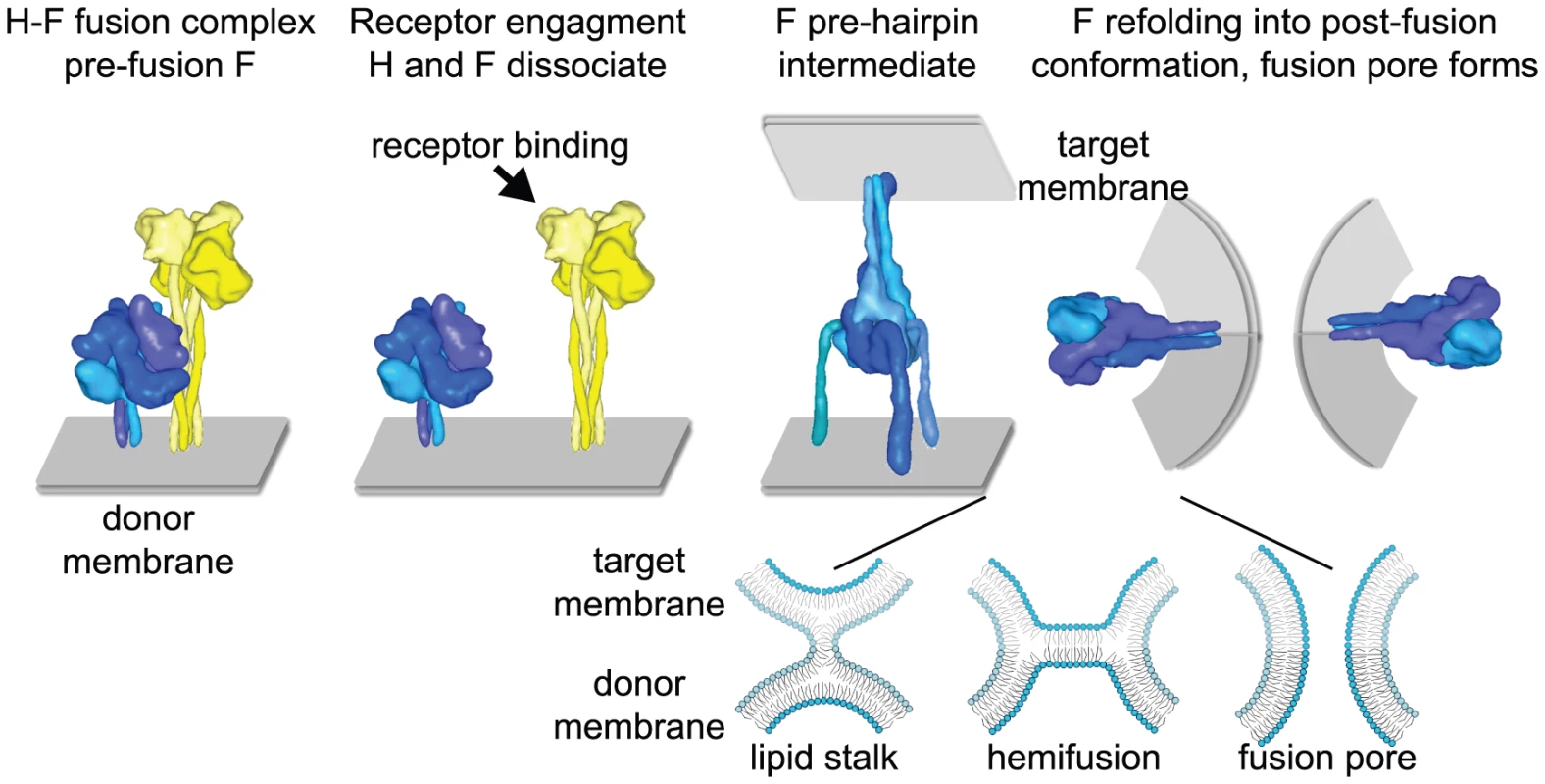
(Left panel) Model representation of the MeV envelope glycoprotein prefusion hetero-oligomer. The H and F complexes are aligned in a staggered head configuration in which the F head is thought to stand in contact with the H stalk [33], [57]. (Middle and right panels) Hypothetical dissociation model of F triggering. Upon binding to the cellular receptor, H and F dissociate, resulting in triggering of major conformational changes in metastable prefusion F. Refolding into the stable postfusion conformation is considered to occur through a series of intermediate conformations, including a hypothetical pre-hairpin intermediate [13], [56]. Likely, refolding of multiple F complexes is required to open a fusion pore and enable viral entry. For improved clarity, MeV H is represented as a single tetramer, and F as a single trimer in the hetero-oligomeric fusion complex. More than one F trimer may interact, however, with each individual H tetramer. The insert shows an enlarged representation of proposed lipid mixing intermediates. As F refolds, first the outer membranes are thought to fuse, creating a lipid stalk. Membrane merger is then thought to advance through hemifusion to pore formation. For clarity, F complexes have been eliminated from the lipid mixing representations. Structural renderings are based on original crystal structures (form I H head domains as in [31]), homology models of MeV F [55], [58] based on coordinates reported for pre- and post-fusion PIV5 and PIV3 F, respectively [56], [59], or hypothetical structural models (F pre-hairpin intermediate). H stalk domains are modeled in an assumed α-helical configuration [33]. High-resolution structural models were aligned at the level of the transmembrane domain (viral envelope) and then morphed into low resolution images using the Sculptor (resolution 12, voxel size 3) package [60]. Current evidence suggests that members of different Paramyxovirinae genera have developed distinct strategies by which the glycoprotein interaction regulates triggering of the F protein [18]–[20]. Based on endoplasmic reticulum (ER) co-retention studies with hPIV3 - and PIV5-derived glycoprotein pairs, which demonstrated that an ER-retained glycoprotein mutant is unable to co-retain its unmodified counterpart [21], and the characterization of receptor binding–deficient HN proteins [22], it is thought that HN attachment proteins do not interact intracellularly with F. For paramyxoviruses that display HN, then, receptor binding and HN tetramer rearrangement appear to induce tight interaction of the HN and F oligomers at the cell surface, ultimately lowering the energy barrier for F refolding in an association model [23].
By contrast, in the case of MeV, the H-F fusion complexes appear to be pre-formed intracellularly [24]. Fusion promotion appears to follow a dissociation model, in which receptor binding results in separation of the preassembled H and F hetero-oligomers. Henipavirus G-F-mediated fusion seems to be regulated by a mechanism similar to MeV, since for both systems the level of fusion was found to be inversely correlated to the avidity of the glycoproteins for each other [25]–[27]. Also in both MeV and NiV, decreased receptor binding activity strengthens the hetero-oligomers [28], [29].
Insight into the mechanism by which the MeV H protein translates receptor binding into the activation of its homologous F protein has emerged from the recent solution of the crystal structures of H in complex with its receptors [30], [31], as well as from advances concerning the organization of MeV H-F fusion complexes [32]–[34] and predictions about H oligomer rearrangements that may take place during fusion [31]. Here, we will summarize these advances and their impact on our understanding of the mechanism of paramyxovirus fusion. In addition, we will compare the mechanism of MeV fusion triggering with that of other paramyxoviruses.
Attachment Protein Receptors and Structure: The Structural Framework
The ectodomains of all Paramyxovirinae attachment proteins are composed of a membrane-proximal stalk, which supports a terminal globular head that mediates receptor binding. While the stalk regions are absent from all currently available crystal structures, circular dichroism analyses of PIV5 HN [35] and structure predictions for the stalks of MeV H and PIV5 HN [34], [35] support an α-helical coiled-coil configuration. It has been firmly established that the stalks of both HN and H determine F specificity [34], [36]–[38], and a domain in each that mediates the interaction with F has been identified [33], [39]. What remains unknown for any paramyxovirus attachment protein is the cascade of conformational and/or structural changes that translates receptor binding to the head region to its stalk domain, followed by triggering of F refolding.
Crystal structures of soluble head domains have been solved for several paramyxovirus attachment proteins, including MeV H, and reveal a common six-blade propeller fold typical of sialidase structures [40]–[44]. The HN attachment proteins interact with sialic acid through specific sites at the center of the β-propeller fold [43]–[46]. Although the H and henipavirus G proteins do not bind to sialic acid, they do both retain a vestigial central pocket analogous to the sialic acid binding pocket in HN [31], [40]–[42]. However, the two proteins have clearly adapted in different ways to be able to bind their respective receptors. While the ephrinB2/B3 binding sites in G localize to the top of the propeller and overlap with the sialic acid binding site in HN [40], [47], both known MeV receptor binding sites map to a position closer to the lateral surface of the β-propeller [30], [31] (Figure 2). Indeed, it appears that the MeV H receptor binding site must be located proximal to this position and away from the dimer interface in order to trigger fusion [48]. This use of a lateral surface of the β-propeller for receptor interaction was also confirmed by a mutational analysis of canine distemper virus H [49], which was guided by the data obtained for MeV H. Since canine distemper virus and MeV are closely related members of the morbillivirus genus, these observations suggest that lateral positioning of the receptor binding site is likely common to all morbillivirus H proteins.
Fig. 2. Representation of MeV H head domains complexed with soluble Slam receptor based on the coordinates reported by Hashiguchi and colleagues [31]. ![Representation of MeV H head domains complexed with soluble Slam receptor based on the coordinates reported by Hashiguchi and colleagues <em class="ref">[<b>31</b>]</em>.](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/8f3239f8f54997738bf716c55e7fac46.png)
Slam moieties (dark green) and covalently linked H dimers (cyan and light purple) in the tetrameric arrangement are highlighted. Receptor binding is proposed to trigger a significant reorganization of the non-covalent dimer-dimer interface (form I versus form II [31]). In the original X-ray analysis, form II was observed when an additional L482R mutation was introduced into MeV H. This mutation was found to enhance SLAM-dependent fusion and also appeared in a clinical MeV isolate of the D1 genotype [31]. Structural renderings were prepared as described for Figure 1. Dotted lines highlight the dimer–dimer intersection. Hypothetical positions of the H stalk domains are marked in the side view representations. In addition, H head crystals assumed an overall more cube-like structure when compared with the largely spherical folds of head domains of the related Paramyxovirinae HN and G proteins [41], [42]. As noted by Bowden and colleagues [40], this is consistent with: 1) the morbilliviruses and henipaviruses having adapted independently to proteinaceous receptors; and, 2) morbillivirus H being more distantly related to both HN and henipavirus G than HN and G are to each other. Experimental results indicate that a tetramer (dimer-of-dimers) may constitute the physiological oligomer for henipavirus G and several paramyxovirus HN attachment proteins [35], [44], [50]. The initial crystal structures of soluble MeV H head domains showed a monomeric or, when one of two intermolecular disulfide bonds in the H stalk domain was present, dimeric organization of the head domains [41], [42]. A more recent crystal structure of MeV H head domains complexed with CD46 confirmed the lateral position of the receptor binding site [30]. The co-crystals spontaneously assumed a homo-dimer configuration, despite the absence of stabilizing intermolecular disulfide bonds from the H head construct. This suggests that the presence of the ligand exerts a stabilizing effect on the H head arrangement. However, full-length H complexes are found in a predominantly tetrameric organization when subjected to mild-detergent extraction and native PAGE analysis [32], indicating that native MeV H, like HN, is tetrameric.
Compared to HN dimers, the MeV H head domains are twisted relative to each other in dimeric configuration and the buried protein–protein interface amounts to only approximately 1300 Å2, considerably smaller than the 1800–2000 Å2 calculated for HN. This may explain the largely monomeric nature of soluble H head domains when expressed in the absence of a stabilizing intermolecular disulfide bond. Most importantly, with respect to the mechanism of fusion, the structures of free and CD46-bound H head domains are virtually identical, arguing against receptor-induced conformational changes of the head domain as the basis for F triggering. Rather, similar to propositions for HN [44], a general spatial reorganization of the H oligomers upon receptor binding was suggested as a possible mechanism of fusion initiation. If correct, this may indeed constitute a fundamentally conserved theme of paramyxovirus entry.
The recently reported co-crystals of soluble H head domains with SLAM receptor provide groundbreaking new insight into the possible mechanism of F triggering. Unlike in previous H structures, H:SLAM co-crystals spontaneously assumed tetrameric configurations [31]. Two discrete spatial organizations were found: the first form places the four SLAM binding sites easily accessible on a relatively planar field, suggesting that all binding sites are arranged perpendicular to the viral envelope; in contrast, the second form, which was assumed by an H variant harboring an L482R mutation, shifts two of the SLAM binding sites closely into the structure (Figure 2). Form I is thought to correspond to prefusion H immediately after receptor binding, whereas form II may represent receptor-bound postfusion H that no longer interacts with F [31]. Transition between the two configurations leaves the H dimer structure itself largely intact, but results in the reorganization of the dimers relative to each other. It also involves expansion of the dimer–dimer interface (from 1312 Å2 in form I to 2099 Å2 in form II). This, in turn, would reorganize the membrane-proximal stalks from a predicted tightly grouped four-helix arrangement to an open configuration in which the stalk domains of the two H dimers are separated from one another. This dissociation of the tetrameric stalk into the two dimers then presumably releases the F protein, resulting in the triggering of the conformational changes in F by an as yet undetermined mechanism.
In another recent study, the possibility of a requirement for an alteration in the association between the monomers in each dimer in the head of MeV H was explored by the introduction of disulfide bonds across the dimer interface [48]. Such disulfide bonds eliminated the ability of the protein to trigger the homologous F protein. However, overall expression levels of this mutated H were low compared to the standard protein, complicating conclusions at this point. It has been hypothesized [51] that the disulfide bonds could prevent minor adjustments in dimer organization that may precede the significant tetramer rearrangement proposed by Hashiguchi and colleagues [31]. Interestingly, opposite results were obtained when a similar dimer stabilization approach was applied previously to the Newcastle disease virus HN protein: fusion was slightly enhanced [52].
The Physiological MeV Fusion Complex: Mechanism of F Triggering
While the X-ray structures of partial paramyxovirus ectodomains, especially in complex with their receptors, constitute a framework for our understanding of viral entry, they lack proof that the physiological organization of native fusion complexes is accurately represented. Furthermore, little light is shed on the spatial arrangement of the functional hetero-oligomer consisting of attachment and fusion protein spikes. Interfacing structural with functional information will be required to dissect the mechanistic principles of the functional paramyxovirus fusion complex.
As discussed above, data from attachment protein chimeras and co-immunoprecipitation studies with site-directed mutants of HN and H indicate that the attachment protein stalk domains mediate the interaction with F. More recently, biochemical assessments and in silico alignments of H and F structures [33], [34] have suggested that the MeV attachment and fusion protein head domains are positioned at different levels relative to the viral envelope, resulting in a staggered head model (Figure 1) rather than the originally assumed lateral arrangement [33]. This model assumes an α-helical conformation of the H stalk domains, which is supported by secondary structure predictions [34], [35], mutagenesis results [33], and circular dichroism analysis of the related PIV5 HN [35]. Further experimental testing confirmed that H stalk elongations membrane-distal, but not proximal, to the proposed F binding domain are compatible with the formation of functional fusion complexes, consistent with the “staggered head” arrangement [33]. Membrane-proximal stalk extensions of up to 50% of its predicted normal length (∼60 Å of additional length in α-helical configuration) were well tolerated, arguing against direct functional contacts involving the MeV H and F head domains.
Systematic mutagenesis of a domain in the H stalk membrane-proximal to the postulated F contact zone revealed additional residues that, when mutated, block F triggering without affecting physical interaction of H and F and receptor binding [53], suggesting that receptor binding and F triggering can be separated. This was tested in a novel bi-molecular complementation assay [32] of discrete H functional defects (Figure 3), which led to three mechanistic conclusions: I) F interaction, receptor binding and F triggering constitute discrete functions that can be complemented in trans; II) efficient fusion promotion does not mandate simultaneous high-affinity docking of receptor moieties to all binding sites in an H oligomer; III) the functional H fusion oligomer is a tetramer.
Fig. 3. Schematic of bi-molecular H complementation to explore the organization of the physiological complex. 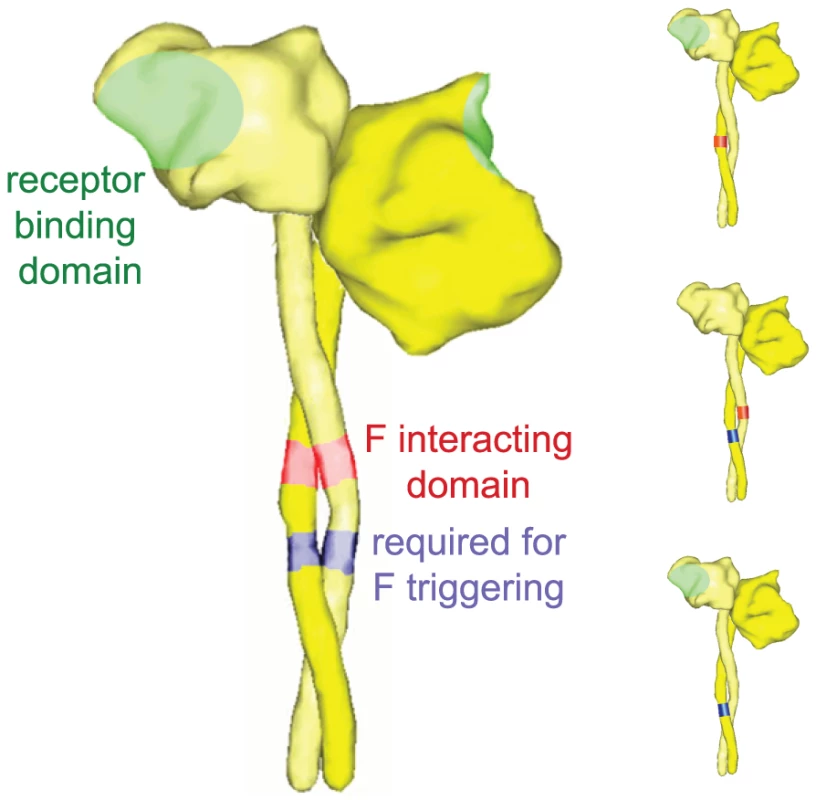
(Left panel) Overview of previously identified functional domains in H, responsible for interaction with F [33], [34], receptor binding [29], [61], or required for F triggering [53]. For simplicity, an H dimer is shown representing form I as described in [31]. (Right panel) Co-expression of H variants defective in individual functions in all possible combinations restores F fusion promotion activity through trans-complementation of functionality [32]. Structural renderings were generated as outlined for Figure 1. Remarkably, the F-interactive domains in MeV H and NiV/HeV G may not fully overlap, since point mutations in the corresponding stalk positions of HeV G, unlike similar mutations in HN and H, do not abolish the physical interaction with F [54]. This suggests that the mechanisms used by G and H to regulate fusion may not be completely equivalent. The henipaviruses may have developed a more elaborate strategy to hold their F proteins in the metastable pre-fusion conformation in contrast to morbillivirus fusion complexes. While unknown at present, this could possibly also involve G head contacts with F in addition to the G stalk interactions.
Considering, however, that residues in the stalk domains of H, HN, and G proteins have been implicated in determining F triggering and that the disulfide backbone and hence the overall architecture of prefusion F [55], [56] are highly conserved among the Paramyxovirinae, we propose an overall largely conserved spatial organization that positions the functional paramyxovirus hetero-oligomer in a staggered head arrangement. The stoichiometry of the physiological hetero-oligomer remains unclear at present. Space constraints very likely prevent the formation of F3/(H4)3 or (F3)4/H4 complexes. However, an (F3)2/H4 hetero-oligomer configuration appears as a structurally plausible alternative to a simple F3/H4 arrangement. Morbillivirus - and henipavirus-derived F proteins may feature a lower inherent activation energy barrier for refolding than F proteins of parainfluenza viruses, rendering them dependent on an interaction with their attachment protein oligomer to stabilize the prefusion conformation.
Independent of an association or dissociation mechanism of F triggering, however, reorganization of the non-covalent head domain dimer–dimer interface in a tetrameric attachment protein complex upon receptor binding emerges as the common denominator among the Paramyxovirinae to transmit receptor binding to the F contact zone in the attachment protein stalk domain. Short-range changes in the microenvironment between H and F, either through receptor-induced transient association and dissociation, or receptor-induced dissociation of preassembled hetero-oligomer complexes, may then drive irreversible conformational changes in F that ultimately must result in dissolution of the hetero-oligomer and, in turn, membrane fusion.
Summary and Perspectives
A combination of structural and functional assays has illuminated central mechanistic principles of paramyxovirus entry. Differences exist among the Paramyxovirinae with regard to morphology and relative orientation of the attachment protein head domains, position of the receptor binding site on the head β-propeller, and the strategies employed to control refolding of the mature fusion protein. However, the overall spatial organization of the paramyxovirus fusion hetero-oligomer and the transmission of receptor binding from the attachment to the fusion protein emerge as largely conserved. Receptor binding does not alter the conformation of individual H monomers but likely results in realignment of the non-covalent head domain dimer–dimer interface. By altering the attachment protein stalk configuration, the latter may change the microenvironment of the F contact zone.
Conceptually, “trigger microdomains” at the interface of functional fusion complexes constitute attractive targets for the design of novel antivirals. However, the stoichiometry of the functional hetero-oligomer, the detailed structure of the overall complex, and the molecular nature of the F residues mediating H specificity remain largely unknown, precluding structure-based drug target identification efforts. Novel approaches such as cryo-electron tomographic analysis of intact, native complexes overlayed with the available partial X-ray data in pseudoatomic structures may likely be required to address these questions. Combined with further refined functional and biochemical analyses, such procedures have the potential to advance our molecular insight into the organization and functional foundation of the fusion complex to a degree where in silico identification of druggable sites for the development of future therapeutics and prophylactics becomes meaningful.
Zdroje
1. GriffinDE 2007 Measles Virus. KnipeDMHowleyPM Fields Virology 5 ed. Philadelphia Wolters Kluwer/Lippincott Williams & Wilkins 1551 1585
2. LambRAParksGD 2007 Paramyxoviridae: The viruses and their replication. KnipeDMHowleyPM Fields Virology 5 ed. Philadelphia Wolters Kluwer/Lippincott Williams & Wilkins 1449 1496
3. TatsuoHOnoNYanagiY 2001 Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol 75 5842 5850
4. TatsuoHOnoNTanakaKYanagiY 2000 SLAM (CDw150) is a cellular receptor for measles virus. Nature 406 893 897
5. DorigREMarcilAChopraARichardsonCD 1993 The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75 295 305
6. NanicheDVarior-KrishnanGCervoniFWildTFRossiB 1993 Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol 67 6025 6032
7. ManchesterMEtoDSValsamakisALitonPBFernandez-MunozR 2000 Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol 74 3967 3974
8. TaharaMTakedaMShiroganeYHashiguchiTOhnoS 2008 Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J Virol 82 4630 4637
9. LeonardVHSinnPLHodgeGMiestTDevauxP 2008 Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J Clin Invest 118 2448 2458
10. BonaparteMIDimitrovASBossartKNCrameriGMungallBA 2005 Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A 102 10652 10657
11. NegreteOALevroneyELAguilarHCBertolotti-CiarletANazarianR 2005 EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436 401 405
12. NegreteOAWolfMCAguilarHCEnterleinSWangW 2006 Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog 2 e7 doi:10.1371/journal.ppat.0020007
13. LambRAJardetzkyTS 2007 Structural basis of viral invasion: lessons from paramyxovirus F. Curr Opin Struct Biol 17 427 436
14. MelikyanGBMarkosyanRMHemmatiHDelmedicoMKLambertDM 2000 Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol 151 413 423
15. PeisajovichSGSamuelOShaiY 2000 Paramyxovirus F1 protein has two fusion peptides: implications for the mechanism of membrane fusion. J Mol Biol 296 1353 1365
16. Ben-EfraimIKligerYHermeshCShaiY 1999 Membrane-induced step in the activation of Sendai virus fusion protein. J Mol Biol 285 609 625
17. ZhaoXSinghMMalashkevichVNKimPS 2000 Structural characterization of the human respiratory syncytial virus fusion protein core. Proc Natl Acad Sci U S A 97 14172 14177
18. IorioRMMahonPJ 2008 Paramyxoviruses: different receptors - different mechanisms of fusion. Trends Microbiol 16 135 137
19. SmithECPopaAChangAMasanteCDutchRE 2009 Viral entry mechanisms: the increasing diversity of paramyxovirus entry. FEBS J 276 7217 7227
20. IorioRMMelansonVRMahonPJ 2009 Glycoprotein interactions in paramyxovirus fusion. Future Virol 4 335 351
21. PatersonRGJohnsonMLLambRA 1997 Paramyxovirus fusion (F) protein and hemagglutinin-neuraminidase (HN) protein interactions: intracellular retention of F and HN does not affect transport of the homotypic HN or F protein. Virology 237 1 9
22. LiJQuinlanEMirzaAIorioRM 2004 Mutated form of the Newcastle disease virus hemagglutinin-neuraminidase interacts with the homologous fusion protein despite deficiencies in both receptor recognition and fusion promotion. J Virol 78 5299 5310
23. ConnollySALeserGPJardetzkyTSLambRA 2009 Bimolecular complementation of paramyxovirus fusion and hemagglutinin-neuraminidase proteins enhances fusion: implications for the mechanism of fusion triggering. J Virol 83 10857 10868
24. PlemperRKHammondALCattaneoR 2001 Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J Biol Chem 276 44239 44246
25. PlemperRKHammondALGerlierDFieldingAKCattaneoR 2002 Strength of envelope protein interaction modulates cytopathicity of measles virus. J Virol 76 5051 5061
26. AguilarHCMatreyekKAChoiDYFiloneCMYoungS 2007 Polybasic KKR motif in the cytoplasmic tail of Nipah virus fusion protein modulates membrane fusion by inside-out signaling. J Virol 81 4520 4532
27. AguilarHCMatreyekKAFiloneCMHashimiSTLevroneyEL 2006 N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J Virol 80 4878 4889
28. BishopKAStantchevTSHickeyACKhetawatDBossartKN 2007 Identification of Hendra virus G glycoprotein residues that are critical for receptor binding. J Virol 81 5893 5901
29. CoreyEAIorioRM 2009 Measles virus attachment proteins with impaired ability to bind CD46 interact more efficiently with the homologous fusion protein. Virology 383 1 5
30. SantiagoCCelmaMLStehleTCasasnovasJM 2010 Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat Struct Mol Biol 17 124 129
31. HashiguchiTOseTKubotaMMaitaNKamishikiryoJ 2011 Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat Struct Mol Biol 18 135 141
32. BrindleyMAPlemperRK 2010 Blue native PAGE and biomolecular complementation reveal a tetrameric or higher-order oligomer organization of the physiological measles virus attachment protein H. J Virol 84 12174 12184
33. PaalTBrindleyMASt ClairCPrussiaAGausD 2009 Probing the spatial organization of measles virus fusion complexes. J Virol 83 10480 10493
34. LeeJKPrussiaAPaalTWhiteLKSnyderJP 2008 Functional interaction between paramyxovirus fusion and attachment proteins. J Biol Chem 283 16561 16572
35. YuanPLeserGPDemelerBLambRAJardetzkyTS 2008 Domain architecture and oligomerization properties of the paramyxovirus PIV 5 hemagglutinin-neuraminidase (HN) protein. Virology
36. DengRWangZMahonPJMarinelloMMirzaA 1999 Mutations in the Newcastle disease virus hemagglutinin-neuraminidase protein that interfere with its ability to interact with the homologous F protein in the promotion of fusion. Virology 253 43 54
37. TsurudomeMKawanoMYuasaTTabataNNishioM 1995 Identification of regions on the hemagglutinin-neuraminidase protein of human parainfluenza virus type 2 important for promoting cell fusion. Virology 213 190 203
38. TanabayashiKCompansRW 1996 Functional interaction of paramyxovirus glycoproteins: identification of a domain in Sendai virus HN which promotes cell fusion. J Virol 70 6112 6118
39. MelansonVRIorioRM 2004 Amino acid substitutions in the F-specific domain in the stalk of the newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J Virol 78 13053 13061
40. BowdenTAAricescuARGilbertRJGrimesJMJonesEY 2008 Structural basis of Nipah and Hendra virus attachment to their cell-surface receptor ephrin-B2. Nat Struct Mol Biol 15 567 572
41. ColfLAJuoZSGarciaKC 2007 Structure of the measles virus hemagglutinin. Nat Struct Mol Biol 14 1227 1228
42. HashiguchiTKajikawaMMaitaNTakedaMKurokiK 2007 Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci U S A 104 19535 19540
43. CrennellSTakimotoTPortnerATaylorG 2000 Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat Struct Biol 7 1068 1074
44. YuanPThompsonTBWurzburgBAPatersonRGLambRA 2005 Structural studies of the parainfluenza virus 5 hemagglutinin-neuraminidase tetramer in complex with its receptor, sialyllactose. Structure 13 803 815
45. ZaitsevVvon ItzsteinMGrovesDKiefelMTakimotoT 2004 Second sialic acid binding site in Newcastle disease virus hemagglutinin-neuraminidase: implications for fusion. J Virol 78 3733 3741
46. LawrenceMCBorgNAStreltsovVAPillingPAEpaVC 2004 Structure of the haemagglutinin-neuraminidase from human parainfluenza virus type III. J Mol Biol 335 1343 1357
47. XuKRajashankarKRChanYPHimanenJPBroderCC 2008 Host cell recognition by the henipaviruses: crystal structures of the Nipah G attachment glycoprotein and its complex with ephrin-B3. Proc Natl Acad Sci U S A 105 9953 9958
48. NavaratnarajahCKOezguenNRuppLKayLLeonardVH 2011 The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat Struct Mol Biol 18 128 134
49. von MesslingVOezguenNZhengQVongpunsawadSBraunW 2005 Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J Virol 79 5857 5862
50. BossartKNCrameriGDimitrovASMungallBAFengYR 2005 Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J Virol 79 6690 6702
51. SaphireEOOldstoneMB 2011 Measles virus fusion shifts into gear. Nat Struct Mol Biol 18 115 116
52. MahonPJMirzaAMMusichTAIorioRM 2008 Engineered intermonomeric disulfide bonds in the globular domain of the Newcastle disease virus hemagglutinin-neuraminidase protein: Implications for the mechanism of fusion promotion. J Virol 82 10386 10396
53. CoreyEAIorioRM 2007 Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J Virol 81 9900 9910
54. BishopKAHickeyACKhetawatDPatchJRBossartKN 2008 Residues in the stalk domain of the Hendra virus G glycoprotein modulate conformational changes associated with receptor binding. J Virol 82 11398 11409
55. LeeJKPrussiaASnyderJPPlemperRK 2007 Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge. J Virol 81 8821 8826
56. YinHSWenXPatersonRGLambRAJardetzkyTS 2006 Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439 38 44
57. PrussiaAJPlemperRKSnyderJP 2008 Measles virus entry inhibitors: a structural proposal for mechanism of action and the development of resistance. Biochemistry 47 13573 13583
58. PlemperRKLakdawalaASGernertKMSnyderJPCompansRW 2003 Structural features of paramyxovirus F protein required for fusion initiation. Biochemistry 42 6645 6655
59. YinHSPatersonRGWenXLambRAJardetzkyTS 2005 Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci U S A 102 9288 9293
60. BirmannsSRusuMWriggersW 2011 Using Sculptor and Situs for simultaneous assembly of atomic components into low-resolution shapes. J Struct Biol 173 428 435
61. PattersonJBScheiflingerFManchesterMYilmaTOldstoneMB 1999 Structural and functional studies of the measles virus hemagglutinin: identification of a novel site required for CD46 interaction. Virology 256 142 151
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous SystemsČlánek The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral ReleaseČlánek Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1Článek High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The N-Terminus of the RNA Polymerase from Infectious Pancreatic Necrosis Virus Is the Determinant of Genome Attachment
- Evolutionary Analysis of Inter-Farm Transmission Dynamics in a Highly Pathogenic Avian Influenza Epidemic
- Glycosaminoglycan Binding Facilitates Entry of a Bacterial Pathogen into Central Nervous Systems
- Endemic Dengue Associated with the Co-Circulation of Multiple Viral Lineages and Localized Density-Dependent Transmission
- The SV40 Late Protein VP4 Is a Viroporin that Forms Pores to Disrupt Membranes for Viral Release
- The -glycan Glycoprotein Deglycosylation Complex (Gpd) from Deglycosylates Human IgG
- The Lipid Transfer Protein CERT Interacts with the Inclusion Protein IncD and Participates to ER- Inclusion Membrane Contact Sites
- Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-β-Dependent Induction of Pro-Apoptotic Noxa
- Environmental Constraints Guide Migration of Malaria Parasites during Transmission
- HIV-1 Efficient Entry in Inner Foreskin Is Mediated by Elevated CCL5/RANTES that Recruits T Cells and Fuels Conjugate Formation with Langerhans Cells
- Kupffer Cells Hasten Resolution of Liver Immunopathology in Mouse Models of Viral Hepatitis
- Highly Pathogenic Avian Influenza Virus H5N1 Infects Alveolar Macrophages without Virus Production or Excessive TNF-Alpha Induction
- Uses Host Triacylglycerol to Accumulate Lipid Droplets and Acquires a Dormancy-Like Phenotype in Lipid-Loaded Macrophages
- Compensatory T Cell Responses in IRG-Deficient Mice Prevent Sustained Infections
- Detection of Inferred CCR5- and CXCR4-Using HIV-1 Variants and Evolutionary Intermediates Using Ultra-Deep Pyrosequencing
- A Dynamic Landscape for Antibody Binding Modulates Antibody-Mediated Neutralization of West Nile Virus
- HSV-2 Infection of Dendritic Cells Amplifies a Highly Susceptible HIV-1 Cell Target
- “Rational Vaccine Design” for HIV Should Take into Account the Adaptive Potential of Polyreactive Antibodies
- Impact of Endofungal Bacteria on Infection Biology, Food Safety, and Drug Development
- Infection Reduces B Lymphopoiesis in Bone Marrow and Truncates Compensatory Splenic Lymphopoiesis through Transitional B-Cell Apoptosis
- The Intrinsic Antiviral Defense to Incoming HSV-1 Genomes Includes Specific DNA Repair Proteins and Is Counteracted by the Viral Protein ICP0
- Molecular Interactions that Enable Movement of the Lyme Disease Agent from the Tick Gut into the Hemolymph
- Pathogen Recognition Receptor Signaling Accelerates Phosphorylation-Dependent Degradation of IFNAR1
- A Freeze Frame View of Vesicular Stomatitis Virus Transcription Defines a Minimal Length of RNA for 5′ Processing
- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Tipping the Balance: Secreted Oxalic Acid Suppresses Host Defenses by Manipulating the Host Redox Environment
- Bacteria-Induced Dscam Isoforms of the Crustacean,
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Insertion of an Esterase Gene into a Specific Locust Pathogen () Enables It to Infect Caterpillars
- A Role for TLR4 in Infection and the Recognition of Surface Layer Proteins
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
- Low CCR7-Mediated Migration of Human Monocyte Derived Dendritic Cells in Response to Human Respiratory Syncytial Virus and Human Metapneumovirus
- HIV/SIV Infection Primes Monocytes and Dendritic Cells for Apoptosis
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- Productive Parvovirus B19 Infection of Primary Human Erythroid Progenitor Cells at Hypoxia Is Regulated by STAT5A and MEK Signaling but not HIFα
- Identification of DNA-Damage DNA-Binding Protein 1 as a Conditional Essential Factor for Cytomegalovirus Replication in Interferon-γ-Stimulated Cells
- The Influenza Virus Protein PB1-F2 Inhibits the Induction of Type I Interferon at the Level of the MAVS Adaptor Protein
- Passively Administered Pooled Human Immunoglobulins Exert IL-10 Dependent Anti-Inflammatory Effects that Protect against Fatal HSV Encephalitis
- Infection of Induces Antifungal Immune Defenses
- Merozoite Invasion Is Inhibited by Antibodies that Target the PfRh2a and b Binding Domains
- Cyclic di-GMP is Essential for the Survival of the Lyme Disease Spirochete in Ticks
- Cross-Neutralizing Antibodies to Pandemic 2009 H1N1 and Recent Seasonal H1N1 Influenza A Strains Influenced by a Mutation in Hemagglutinin Subunit 2
- Spatial Dynamics of Human-Origin H1 Influenza A Virus in North American Swine
- Coronavirus Gene 7 Counteracts Host Defenses and Modulates Virus Virulence
- Clathrin Facilitates the Morphogenesis of Retrovirus Particles
- Contribution of Intrinsic Reactivity of the HIV-1 Envelope Glycoproteins to CD4-Independent Infection and Global Inhibitor Sensitivity
- Functional Analysis of Host Factors that Mediate the Intracellular Lifestyle of
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- High Affinity Nanobodies against the VSG Are Potent Trypanolytic Agents that Block Endocytosis
- Structural and Mechanistic Studies of Measles Virus Illuminate Paramyxovirus Entry
- Sporangiospore Size Dimorphism Is Linked to Virulence of
- The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



