-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
and Beyond—Inositol Utilization and Its Implications for the Emergence of Fungal Virulence
article has not abstract
Published in the journal: and Beyond—Inositol Utilization and Its Implications for the Emergence of Fungal Virulence. PLoS Pathog 8(9): e32767. doi:10.1371/journal.ppat.1002869
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002869Summary
article has not abstract
There are over one million fungal species in nature, but only a handful of them cause human diseases. A variety of distinct factors aid the virulence of fungi in their transition from environmental reservoirs to mammals. One important factor is their ability to acquire nutrients efficiently so that they can survive and thrive in a nutrient-limiting host environment. The human fungal pathogen Cryptococcus neoformans (C. neoformans) is the most common cause of fungal meningitis, yet the mechanisms of Cryptococcus neurotropism remain poorly understood. Recent studies have revealed that Cryptococcus has evolved sophisticated acquisition systems to utilize the carbohydrate inositol both in plant niches and in human brains, where abundant inositol is available. Inositol utilization in Cryptococcus and its likely contribution to Cryptococcus virulence may represent one example of a common trait for the emergence of pathogens from environmental reservoirs.
Cryptococcus Can Undergo Sexual Reproduction by Utilizing Inositol from Plants
C. neoformans and its sibling species Cryptococcus gattii (C. gattii) are basidiomycetes that cause systemic fungal infection in animals and humans. These two species have distinct, but also overlapping, environmental niches. C. gattii was traditionally considered to only exist in tropical and subtropical regions and was mostly associated with plants such as the Eucalyptus species [1]. In contrast, C. neoformans has a more global distribution, being isolated mostly in soil contaminated by plant debris and bird droppings. In addition, C. neoformans has been isolated from a variety of plant species [2], including indigenous African trees that have been proposed as the origin of C. neoformans in Africa [3], suggesting that this species also has an arboreal niche.
The details as to why Cryptococcus prefers tree or other environmental niches remain unclear. Cryptococcus can complete its sexual cycle by associating with plants, suggesting such association is beneficial for the fungus [4]. Because cryptococcosis is noncommunicable between humans, the initial infection is likely exclusively caused by environmental sources. Basidiospores are thought to be the initial infectious particles inhaled by the human host to cause cryptococcosis, as spores are small enough to lodge into the deep alveoli of the lung and are fully virulent [5], [6]. Hence, mating and recombination of Cryptococcus have to occur in nature, as supported by population studies of environmental isolates [7], [8]. However, neither mating nor basidiospores have yet been observed in the environment. The discovery of Cryptococcus mating on plants sheds light on the whereabouts of Cryptococcus spores in the environment. Recently it was found that inositol secreted from plants stimulates Cryptococcus mating [4]. The importance of inositol in the mating of Schizosaccharomyces pombe [9] and for fertility of plants and humans has also been reported [10], [11], suggesting a conserved contribution of inositol in sexual reproduction.
There are two main sources from which fungal cells acquire inositol. For one, intracellular glucose can be used to produce inositol in a multiple-step inositol biosynthetic pathway in which the inositol 3-phosphate synthase (Ino1) is the rate-determining enzyme [12]. Inositol can also be imported from the extracellular environment via inositol transporters (ITRs). Cryptococcus can use inositol both as a carbon source and as a precursor to generate secondary messages that are important for regulating cellular functions and for adapting to environmental signals. Interestingly, in contrast to only one or two inositol transporters present in most fungi, Cryptococcus contains an unusually large inositol transporter gene family with over ten members, derived in part from recent gene duplications, suggesting Cryptococcus has evolved by associating with the tree niches for inositol utilization [13] (Table 1). Among the transporters, Itr1 and Itr1a are two required for fungal mating [13]. The ability to sense and efficiently acquire inositol from plant surfaces could fuel Cryptococcus in its proliferation and sporulation.
Tab. 1. Number of inositol transporter (ITR) candidates in fungi. 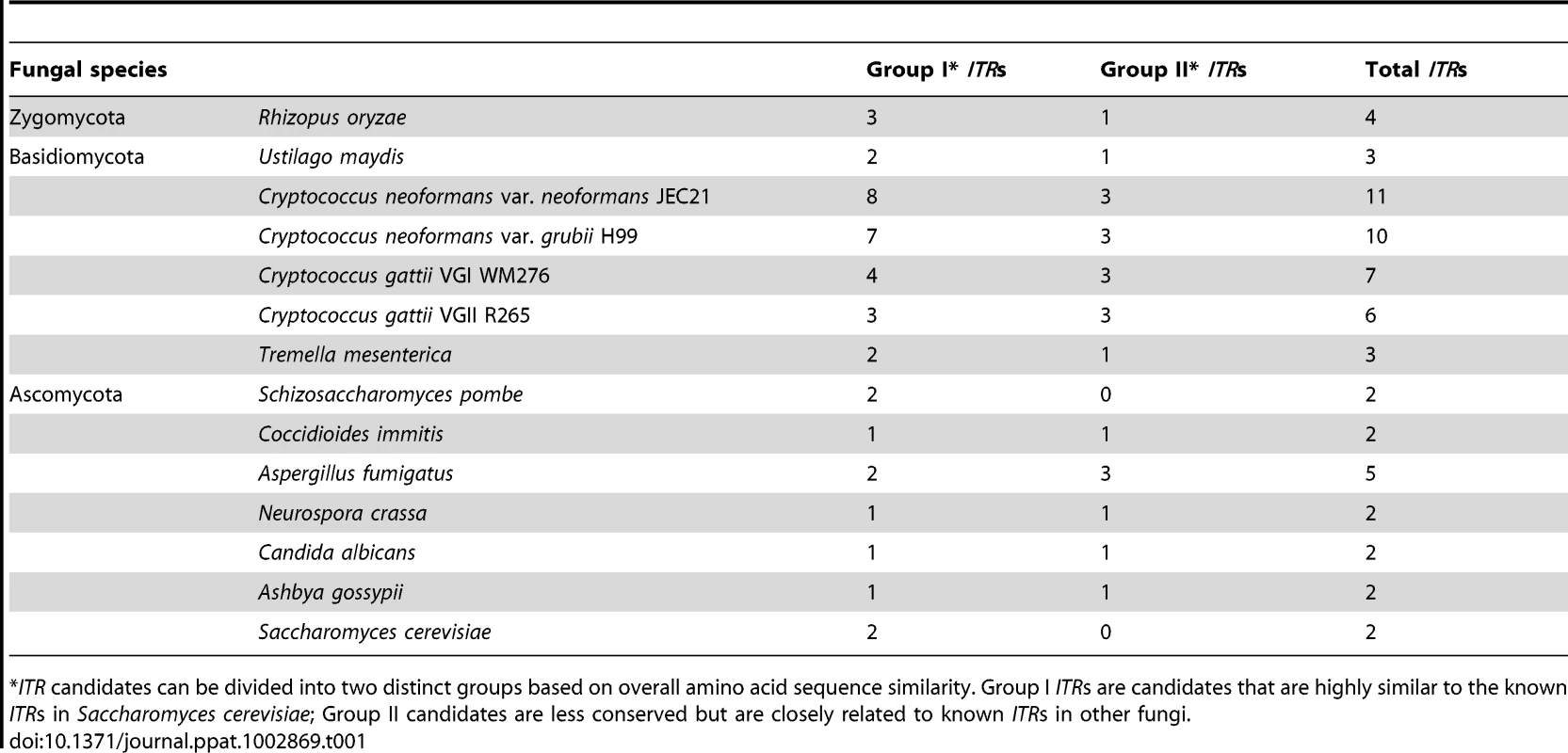
ITR candidates can be divided into two distinct groups based on overall amino acid sequence similarity. Group I ITRs are candidates that are highly similar to the known ITRs in Saccharomyces cerevisiae; Group II candidates are less conserved but are closely related to known ITRs in other fungi. Adapting to Trees and Other Niches May Contribute to the Emergence of Cryptococcus Virulence in Humans
The progenitor of Cryptococcus existed before humans or other warm-blooded mammals populated the world, and plants or plant materials could well represent the original niches for Cryptococcus, as suggested by a recent report [3]. The successful transmission from an environmental host to a warm-blood mammalian host defines a precondition for the success of a human pathogen. Mammals developed a sophisticated defense system to ward off the attack of deadly microbes, including physical barriers (high body temperature and epithelial surfaces) and immune response (innate and adaptive immunity). In addition, nutrient limitation is an important restricting factor for the growth of those microbes in vivo. Cryptococcus cells grow well at body temperature (37°C), and possess an enlarged polysaccharide capsule and thick melanized cell wall, which enable these cells to resist the hostile host environment. As an intracellular pathogen, the ability of Cryptococcus to survive and replicate in macrophages after phagocytosis has been proposed to be a consequence of adaptations that have evolved for protection against environmental predators in nature, like amoebae [14]. By associating with the plant niche, Cryptococcus may have developed a complex nutrient-acquisition system to acquire limited nutrients, including inositol, to support its growth and sexual reproduction. This efficient nutrient utilization system could also play an important role in using nutrients in mammalian hosts. It has been shown that enzymes involved in inositol metabolism and inositol sphingolipid biosynthesis are required for the pathogenesis of C. neoformans [15].
Inositol Acquisition May Contribute to Cryptococcus CNS Infection
The predominant clinical manifestation of cryptococcal infection is the development of fatal meningoencephalitis, especially in people living with AIDS/HIV. The cause of Cryptococcus neurotropism remains unclear. Several factors point to inositol as one of the potential host factors promoting the development of cryptococcal meningitis. First, both human and animal brains contain abundant inositol, which plays a critical role in regulating normal neurological responses and psychological feedback [16]. Inositol is a major osmolyte in the human and animal brains and is present in the human cerebellum (5.1 mM) at over 200-fold higher concentrations than are found in plasma (0.02 mM) [16]. Astrocytes that associate with the blood-brain barrier (BBB) contain over 8 mM inositol that can be rapidly released [16]. HIV-infected persons have increased brain inositol levels due to gliosis or increased cell membrane turnover [17]. Second, Cryptococcus can utilize inositol as a carbon source, which may provide a growth advantage during brain infection since glucose levels are generally low in brain [18], [19]. Third, Cryptococcus can efficiently acquire environmental inositol with its large inositol transporter gene family [4], [13], [20]. Mutants lacking two major fungal inositol transporters, Itr1a and Itr3c, showed attenuated virulence in multiple murine models, indicating that inositol acquisition is required for the Cryptococcus–host interaction, particularly during brain infection [4], [13], [20]. Recently, we found that inositol can directly increase the rate of Cryptococcus transversal across the human brain macrovascular endothelial cell monolayer in an in vitro model of the BBB, and the inositol effect is fungal inositol transporter–dependent (Liu et al., unpublished). This discovery demonstrates that inositol sensing and utilization could be an important virulence factor for the development of cryptococcal meningitis, which provides a direct biological connection between an environmental adaptation strategy and the emergence of its virulence during human infection (Figure 1).
Fig. 1. A model of how inositol affects the infection cycle of C. neoformans. 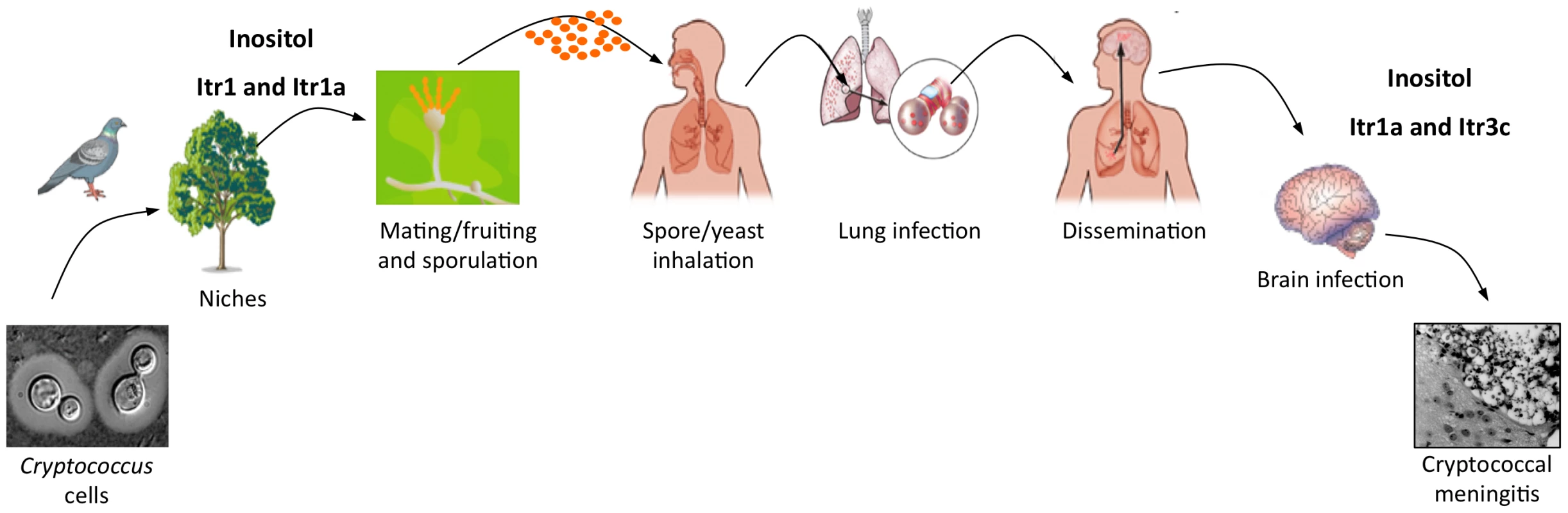
Cryptococcus cells commonly exist in the environment by associating with several niches, including birds, soil, and plants. Inositol is present on plant surfaces and can stimulate fungal mating (including fruiting) to produce infectious spores. Spores inhaled by humans can enter the lungs to cause lung infection. Fungal cells can also be disseminated to the central nervous system (CNS), where abundant inositol is present, and cause fungal meningitis. Inositol can be used as a precursor for both the energy source and the signaling molecule. Part of the model is adapted from Hull and Heitman [6]. Despite progress in understanding the role of inositol in Cryptococcus pathogenesis, many questions remain unanswered. It remains unclear how inositol promotes fungal cell transversal across the BBB and whether inositol is utilized as a signaling molecule, a carbon source, or both by the fungus during brain infection. It is also unknown whether inositol contributes to the development of capsule structure in Cryptococcus and other neurotropic pathogens, since the capsule is one common feature of those pathogens and contributes to their neurotropism. Addressing these questions could lead to a better understanding of the Cryptococcus CNS infection.
Contribution of Inositol to the Virulence of Other Pathogens
Inositol is the precursor for making phosphatidylinositol (PI) and is essential for cellular structure and regulation of intracellular signaling in all eukaryotes. The role of inositol acquisition in the development of virulence has been studied in a variety of fungi, protozoa, and certain eubacteria [12]. Similar to Cryptococcus, the yeast pathogens Candida albicans and Candida glabrata (C. glabrata) can acquire inositol through both de novo biosynthesis pathways and import via inositol transporters. Blocking either pathway does not affect fungal infection, but deleting both pathways is lethal, suggesting inositol acquisition is essential for Candida survival and either pathway is sufficient to support fungal growth and full virulence [21]. The inositol regulon is wired differently in C. albicans compared to the one in C. glabrata, suggesting complex inositol regulatory systems in different fungi [22].
Besides fungi, inositol also plays a role in pathogenicity of other parasitic microorganisms [12]. Interestingly, although parasites such as Trypanosoma brucei and Leishmania mexicana and mycobacteria such as Mycobacterium tuberculosis can both synthesize and import inositol, blocking inositol biosynthesis leads to growth defect and virulence attenuation, indicating inositol uptake itself is not sufficient [23], [24]. Inositol synthesized in cells has been suggested to be the source of PI used for GPI anchor assembly, which may explain the importance of inositol biosynthesis despite the ability of pathogens to import inositol.
Other Adaption Strategies Associated with Cryptococcus
Besides utilizing plants as one niche, Cryptococcus cells often associate with certain amoeba species in which the yeast cells can be taken up but survive inside the amoebae, a phenomenon similar to Cryptococcus–macrophage interactions. The interaction between Cryptococcus and amoebae has been shown to increase the resistance of Cryptococcus to phagocytosis during its infection in lung, suggesting that selective pressures placed by amoebae on Cryptococcus contribute to the maintenance of fungal virulence in animal hosts [14]. In addition, Cryptococcus cells can increase ploidy and significantly enlarge in cell size in vivo as a way of protecting yeast cells from phagocytosis [25]. Nitrogen-rich pigeon guano is another primary ecological niche of C. neoformans. Media made of pigeon guano has been shown to stimulate mating of C. neoformans but not C. gattii [26]. The availability of nitrogen, such as uric acid, has been shown to play a role in Cryptococcus virulence [27]. A recent study demonstrated a nitrogen-metabolite repression process to regulate the nitrogen acquisition [28]. Thus, understanding the environmental niches of a particular human pathogen can be very helpful in understanding its disease mechanism. In addition, the adaption of a pathogen to new environmental niches could result in the emergence of new virulence traits. The perfect example is the outbreak of cryptococcosis in otherwise healthy people caused by C. gattii in western North America where Eucalyptus trees do not exist and it is not a tropical climate. The most common C. gattii strain (VGIIa) showed higher proliferation rates in macrophages than other C. gattii isolates from around the world: an indication of the emergence of virulence since proliferation rate is correlated with fungal virulence [29]. The emergence of disease caused by C. gattii in immunocompetent individuals in temperate Vancouver Island, Canada and its expansion in western North America suggests an evolution of host range, geographic location, and virulence of this pathogen [30].
Zdroje
1. Casadevall A, Perfect JR (1998) Cryptococcus neoformans. Washington, DC: ASM Press.
2. Mitchell TG, Castaneda E, Nielsen K, Wanke B, Lazera MS (2011) Environmental niches for Cryptococcus neoformans and Cryptococcus gattii. In: Heiman J, Kozel TR, Kwon-Chung KJ, Perfect J, Casadevall A, editors. Cryptococcus: from human pathogen to model yeast. Washington DC: ASM Press. pp. 237–260.
3. LitvintsevaAP, MitchellTG (2012) Population genetic analyses reveal the African origin and strain variation of Cryptococcus neoformans var. grubii. PLoS Pathog 8: e1002495 doi:10.1371/journal.ppat.1002495.
4. XueC, TadaY, DongX, HeitmanJ (2007) The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1 : 263–273.
5. BottsMR, HullCM (2010) Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol 13 : 437–442.
6. HullCM, HeitmanJ (2002) Genetics of Cryptococcus neoformans. Annu Rev Genet 36 : 557–615.
7. CampbellLT, CurrieBJ, KrockenbergerM, MalikR, MeyerW, et al. (2005) Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot Cell 4 : 1403–1409.
8. LitvintsevaAP, MarraRE, NielsenK, HeitmanJ, VilgalysR, et al. (2003) Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot Cell 2 : 1162–1168.
9. NiederbergerC, GraubR, SchweingruberAM, FankhauserH, RusuM, et al. (1998) Exogenous inositol and genes responsible for inositol transport are required for mating and sporulation in Schizosaccharomyces pombe. Curr Genet 33 : 255–261.
10. TsuiMM, YorkJD (2010) Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv Enzyme Regul 50 : 324–337.
11. CarlomagnoG, NordioM, ChiuTT, UnferV (2011) Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol 159 : 267–272.
12. ReynoldsTB (2009) Strategies for acquiring the phospholipid metabolite inositol in pathogenic bacteria, fungi and protozoa: making it and taking it. Microbiology 155 : 1386–1396.
13. XueC, LiuT, ChenL, LiW, LiuI, et al. (2010) Role of an expanded inositol transporter repertoire in Cryptococcus neoformans sexual reproduction and virulence. mBio 1: e00084–00010.
14. SteenbergenJN, ShumanHA, CasadevallA (2001) Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 98 : 15245–15250.
15. SheaJM, HenryJL, Del PoetaM (2006) Lipid metabolism in Cryptococcus neoformans. FEMS Yeast Res 6 : 469–479.
16. FisherSK, NovakJE, AgranoffBW (2002) Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 82 : 736–754.
17. YiannoutsosCT, ErnstT, ChangL, LeePL, RichardsT, et al. (2004) Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage 23 : 928–935.
18. BarnettJA (1976) The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem 32 : 125–234.
19. HealyME, DillavouCL, TaylorGE (1977) Diagnostic medium containing inositol, urea, and caffeic acid for selective growth of Cryptococcus neoformans. J Clin Microbiol 6 : 387–391.
20. WangY, LiuTB, DelmasG, ParkS, PerlinD, et al. (2011) Two major inositol transporters and their role in cryptococcal virulence. Eukaryot Cell 10 : 618–628.
21. ChenYL, KauffmanS, ReynoldsTB (2008) Candida albicans uses multiple mechanisms to acquire the essential metabolite inositol during infection. Infect Immun 76 : 2793–2801.
22. BetheaEK, CarverBJ, MontedonicoAE, ReynoldsTB (2010) The inositol regulon controls viability in Candida glabrata. Microbiology 156 : 452–462.
23. MartinKL, SmithTK (2005) The myo-inositol-1-phosphate synthase gene is essential in Trypanosoma brucei. Biochem Soc Trans 33 : 983–985.
24. MovahedzadehF, SmithDA, NormanRA, DinadayalaP, Murray-RustJ, et al. (2004) The Mycobacterium tuberculosis ino1 gene is essential for growth and virulence. Mol Microbiol 51 : 1003–1014.
25. OkagakiLH, StrainAK, NielsenJN, CharlierC, BaltesNJ, et al. (2010) Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 6: e1000953 doi:10.1371/journal.ppat.1000953.
26. NielsenK, De ObaldiaAL, HeitmanJ (2007) Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell 6 : 949–959.
27. OlszewskiMA, NoverrMC, ChenGH, ToewsGB, CoxGM, et al. (2004) Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 164 : 1761–1771.
28. LeeIR, ChowEW, MorrowCA, DjordjevicJT, FraserJA (2011) Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans. Genetics 188 : 309–323.
29. MaH, HagenF, StekelDJ, JohnstonSA, SionovE, et al. (2009) The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A 106 : 12980–12985.
30. KronstadJW, AttarianR, CadieuxB, ChoiJ, D'SouzaCA, et al. (2011) Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 9 : 193–203.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2012 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- A Population Genomics Perspective on the Emergence and Adaptation of New Plant Pathogens in Agro-Ecosystems
- A Novel Rhabdovirus Associated with Acute Hemorrhagic Fever in Central Africa
- Ifitm3 Limits the Severity of Acute Influenza in Mice
- Copper at the Front Line of the Host-Pathogen Battle
- The Human Cytomegalovirus Assembly Compartment: A Masterpiece of Viral Manipulation of Cellular Processes That Facilitates Assembly and Egress
- Insights from Genomics into Bacterial Pathogen Populations
- Slc15a4, a Gene Required for pDC Sensing of TLR Ligands, Is Required to Control Persistent Viral Infection
- Relacin, a Novel Antibacterial Agent Targeting the Stringent Response
- Global Assessment of Genomic Regions Required for Growth in
- The Battle over mTOR: An Emerging Theatre in Host–Pathogen Immunity
- Very Long O-antigen Chains Enhance Fitness during -induced Colitis by Increasing Bile Resistance
- and Beyond—Inositol Utilization and Its Implications for the Emergence of Fungal Virulence
- Antifungal Drug Discovery: Something Old and Something New
- Deep Sequencing of Antiviral T-Cell Responses to HCMV and EBV in Humans Reveals a Stable Repertoire That Is Maintained for Many Years
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Insights from Genomics into Bacterial Pathogen Populations
- Relacin, a Novel Antibacterial Agent Targeting the Stringent Response
- Ifitm3 Limits the Severity of Acute Influenza in Mice
- Antifungal Drug Discovery: Something Old and Something New
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



