-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Small bowel adenocarcinoma diagnosed by video capsule endoscopy in a patient with celiac disease: a case report and review of literature
Adenokarcinom tenkého střeva diagnostikovaný pomocí video kapslové endoskopie u pacientky s celiakií: kazuistika a přehled literatury
Celiakie je imunitně zprostředkovaná enteropatie, rozvíjející se při požití lepku u geneticky predisponovaných osob. Je spojena se zvýšeným rizikem rozvoje malignit trávicího traktu. Tyto komplikace jsou většinou diagnostikovány v pozdním stádiu, což je jedním z faktorů jejich nepříznivé prognózy. Naše kazuistika se týká ženy, jíž byla celiakie diagnostikována v 53 letech. Po 2 letech se u ní náhle rozvinula sideropenická anémie. Esofagogastroduodenoskopie ani kolonoskopie neprokázala krvácení do gastrointestinálnho traktu. Video kapslová endoskopie odhalila exulcerovanou stenózu v jejunu se známkami krvácení, před kterou kapsle retinovala. Na simultánně provedené CT enterografii nebyla infiltrace patrná. Byla provedena operační revize s resekcí infiltrace jejuna. Histologicky byl prokázán adenokarcinom tenkého střeva, vzhledem k rizikovým faktorům byla zahájena adjuvantní chemoterapie. Znalost maligních komplikací celiakie, jejich rizikových faktorů a možnosti využití moderních enteroskopických metod může přispět k časné diagnostice a zlepšení prognózy těchto onemocnění. Vzhledem k nedostatku dat a absenci doporučených postupů se léčba adenokarcinomu tenkého střeva řídí názory expertů a doporučenými postupy léčby kolorektálního karcinomu. Chirurgická resekce je jediná potenciálně kurativní léčba. U stádia II s rizikovými faktory a stádia III by měla být zvážena adjuvantní chemoterapie.
Klíčová slova:
adenocarcinoma – video capsule endoscopy – Celiac disease – small bowel – surgery
Authors: Barbora Packová 1; Lumír Kunovský 1,2; Michal Eid 3; Radek Kroupa 1; Milan Dastych 1; Michal Šenkyřík 1; Tomáš Grolich 2; Jakub Hustý 4; Petr Jabandžiev 5; Václav Kubeš 6; Vladimír Prochazka 2; Jiří Dolina 1
Published in the journal: Vnitř Lék 2020; 66(7): 39-42
Category: Kazuistika
Summary
Celiac disease is an immune mediated entheropathy triggered by gluten in genetically predisposed individuals. Patients with celiac disease are at a higher risk of gastrointestinal malignancies. Diagnosis at an advance stage is one of the factors of an unfavorable prognosis of these complications. Our patient is a woman who was diagnosed with celiac disease at 53 years of age. After two years on a gluten-free diet she developed sideropenic anemia. No source of bleeding was found on the esophagogastroduodenoscopy or colonoscopy. Video capsule endoscopy revealed exulcerated bleeding stenosis in the jejunum, in front of which the capsule lodged. There were no signs of infiltration on simultaneous CT enterography. The patient was operated on and the infiltration of the jejunum was resected. The specimen was evaluated by a histopathologist as a moderately differentiated adenocarcinoma. Due to the risk factors, the patient received adjuvant chemotherapy. The knowledge of the malignant complications of celiac disease, their risk factors and the possibilities of modern enteroscopic methods could help in the early diagnosis and improvement of the prognosis of these diseases. Due to a lack of data and an absence of guidelines, treatment of a small bowel adenocarcinoma is based on an expert agreement and guidelines for colon cancer. Surgical treatment is the only potentially curative option. For stage II with risk factors and stage III adjuvant chemotherapy should be considered.
Keywords:
Adenocarcinoma – video capsule endoscopy – Celiac disease – small bowel – surgery
Introduction
Celiac disease (CD) is a chronic multiorgan autoimmune disease triggered by exposure to gluten in genetically predisposed individuals (1). Patients with CD are at a higher risk of malignant complications such as malignant lymphoma, small bowel adenocarcinoma and others (2). Diagnosis of these complications at an advanced stage is one of the factors of their unfavorable prognosis. The use of modern enteroscopic methods such as video capsule endoscopy (VCE) or double balloon enteroscopy has been helpful in the diagnosis of small intestinal malignancies during recent years (3, 4). Due to rarity of these diagnoses there is a lack of a standardized therapeutic approach.
Case report
Our patient is a woman who was diagnosed with CD at 53 years of age. She was initially asymptomatic, with Sjögren syndrome in her past medical history and a positive family history of CD in her daughter. We performed targeted screening, according to recommendations (5), which showed a positivity of specific autoantibodies anti-tissue transglutaminase (anti-TTG) in IgA and anti-deaminated gliadin peptides (anti-DGP) in IgA and IgG. A biopsy of the distal duodenum showed villous atrophy Marsh 3b according to the Marsh-Oberhuber classification. No anemia was detected at the time of the diagnosis of CD (hemoglobin 125g/L, MCV 88 fL, ferritin 41.4μg/L). The patient started a strict gluten-free diet and was in stable condition, without any signs of malnutrition during the follow-up visits. Anti-TTG IgA and anti-DGP IgA decreased to normal levels in seven months with a slight persistent positivity of anti-DGP IgG.
After two years on a gluten-free diet the patient started to suffer from artralgies, for which prednisolone, hydroxychloroquine and non - -steroid-anti-inflammatory drugs were prescribed. She experienced slight intermittent epigastric pain and one episode of black stool. Moderate anemia (hemoglobin 97g/L, MCV 84 fL, ferritin 22.1μg/L) was detected on a follow-up visit to rheumatology. We performed an esophagogastroduodenoscopy and colonoscopy in search of bleeding with physiological findings. In accordance with ESGE guidelines (6) VCE was scheduled, an additional push enteroscopy (as required before VCE by the insurance company in our region) with biopsy showed stable findings without villous atrophy (Marsh 1, CD3+ CD8+ as shown by immunohistochemistry). The VCE revealed exulcerated, stenotized infiltration of the small bowel, with signs of bleeding (Fig. 1a, 1b), in front of which the capsule lodged. The capsule was visualized on an abdominal x-ray (Fig. 2). No infiltration was evident on a simultaneously performed CT enterography, only the position of the lodged capsule indicated the site of stenosis (Fig. 3). The patient was operated upon with a finding of infiltration of the jejunum at the mesenterial site, with bilateral lymphadenopathy. A resection of 60cm of the jejunum (Fig. 4) with side-to-side anastomosis was performed. The patient was discharged 7 days after surgery without any postoperative complications. The specimen was evaluated by a histopathologist as moderately differentiated adenocarcinoma (Grade 2), with angioinvasion and a positive lymph node (1/13), R0 resection, pT3N1M0 (Fig. 5). The patient received adjuvant chemotherapy XELOX (capecitabine plus oxaliplatin) due to risk factors (positive lymph node and angioinvasion).
Fig. 1. VCE showing malignant tumor of the small bowel (a, b), with apparent bleeding (c) 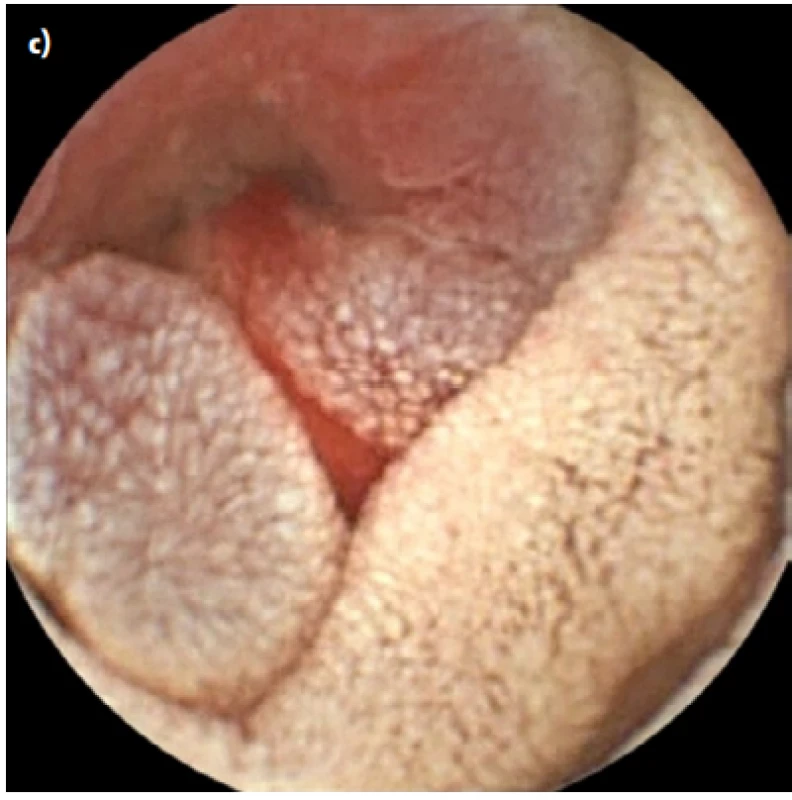
Fig. 2. A visible capsule on the abdominal x-ray 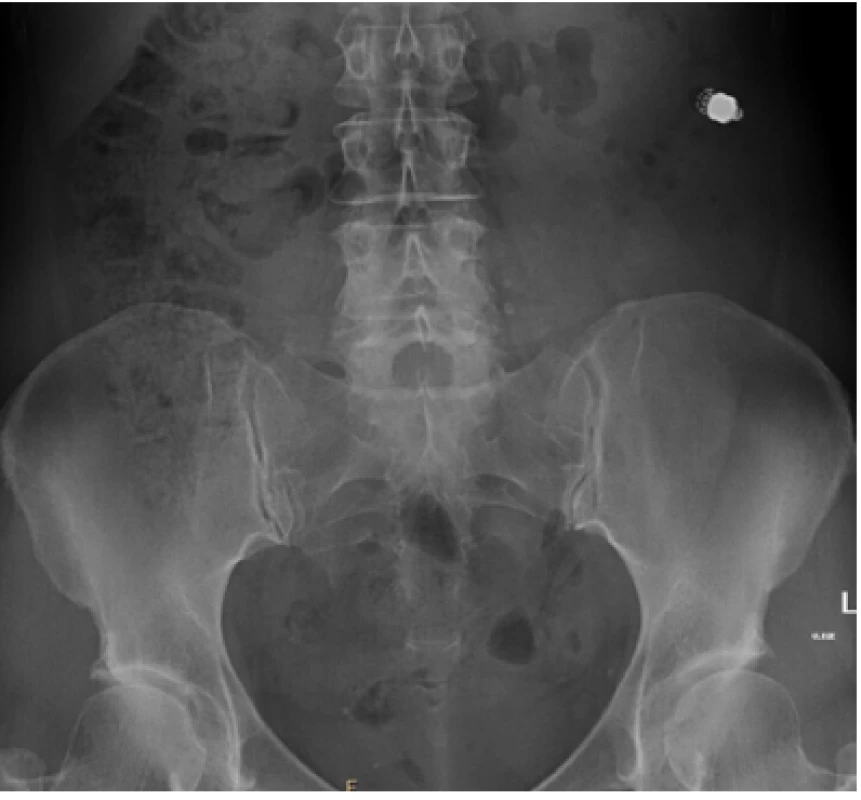
Fig. 3. CT enterography (coronal scan) – VCE lodged in the jejunum with no evident CT signs of tumor infiltration 
Fig. 4. Peroperative view of infiltration of jejunum (red arrow) with the lodged capsule which was extracted by enterotomy (green arrow) 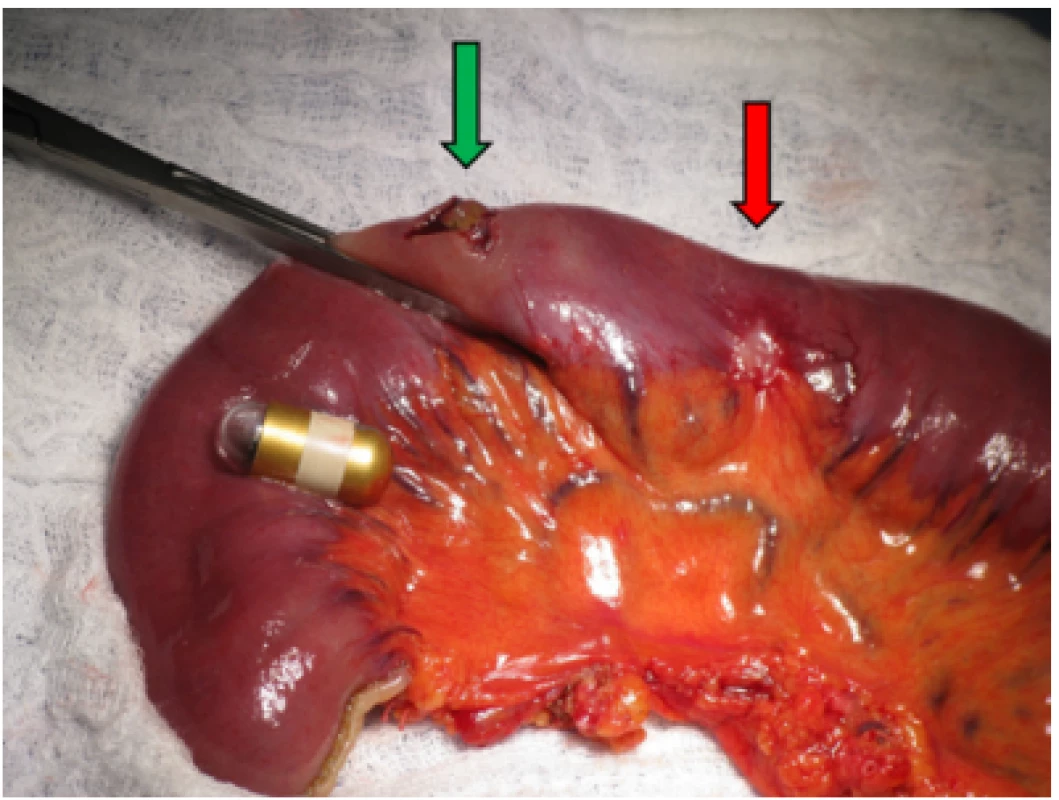
Fig. 5. Histological section: invasive adenocarcinoma infiltrating submucosal tissue, spreading under small intestine epithelium, HE staining, 100× 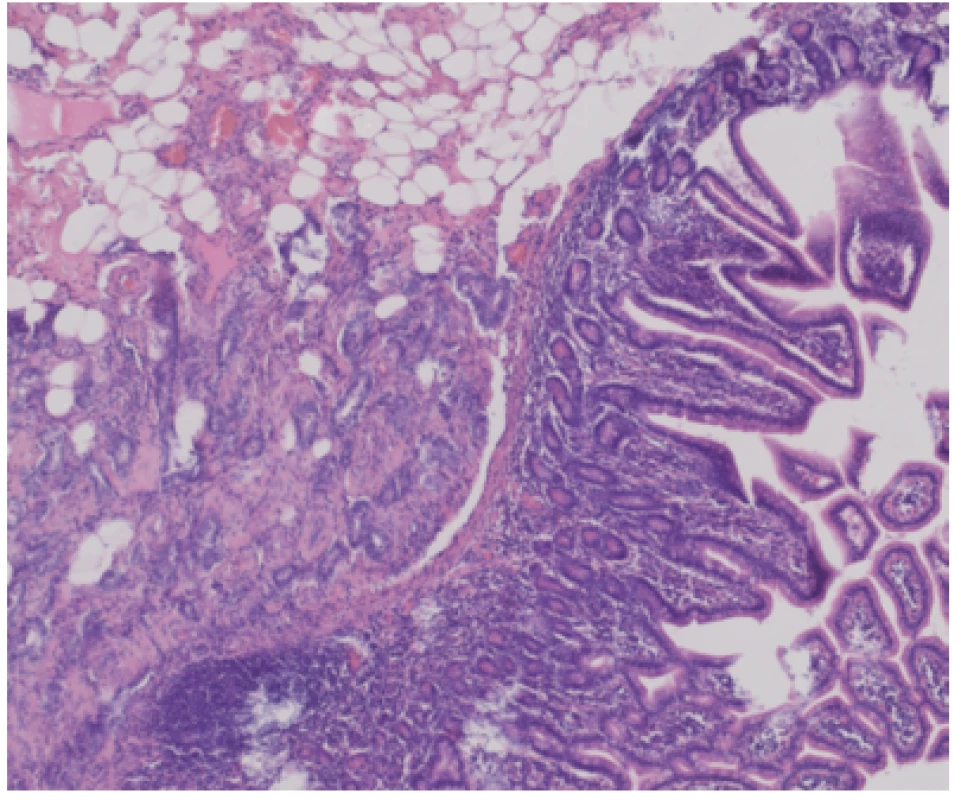
Discussion
Malignant tumors of the small bowel represent only 1–3% of gastrointestinal malignancies. Adenocarcinomas are the most frequent finding (35–50%) followed by carcinoid tumors (30%), lymphomas (15%) and gastrointestinal stromal tumors other than sarcomas (10%) (7–9). Nevertheless, patients with CD are at an increased risk of some of these malignancies. Studies investigating the risk of development of carcinoma in patients with CD bring very different results, ranging from a 10-fold increased risk (2) to an 82.6-fold increased risk (7). Although the relative risk of lymphoma dramatically decreases after one year of gluten-free diet (RR 157.6 to 12.7), the decrease in cases of carcinoma is not so expressed (RR 38.0 to 6.4) and the p-value for the trend doesn’t reach statistical significance (10).
Diagnosis of CD at an advanced age, untreated CD and possibly also persisting villous atrophy and male gender were described as risk factors of malignant complication development in celiac patients (11–14), but the number of patients in studies is usually small and the results aren’t uniform. Our patient was diagnosed with CD at the age of 53 years. Due to the lack of symptoms it is possible that CD was undiagnosed and untreated for a long time in our patient, highlighting the importance of family screening in such cases. A high turnover of the inflammatory population with mucosal lymphocyte infiltration, an increased permeability to oncogenic factors, a malabsorption of protective substances such as vitamins A and E, or an impaired immune surveillance are considered as pathogenetic mechanisms of cancerogenesis in these settings (14). Another question is the course of development of the carcinoma. A majority of opinions lean toward adenoma-carcinoma evolution sequence (15, 16), but there are some studies which question this approach, providing cases suggestive of dysplasia development in the flat mucosa (3). In contrast to small bowel adenocarcinoma in general, where the duodenum is affected most frequently, carcinomas tend to develop in the jejunum in most cases of CD patients, which corresponds with our case. Vomiting, anemia, weight loss, intestinal bleeding, abdominal mass, and perforation are the most frequent symptoms (17). Unfortunately, a diagnosis is usually made in the advanced stages of the disease (74% in stage III-IV) (14). In most cases the carcinoma isn’t accessible to esophagogastroduodenoscopy. CT or MR enterography and PET/CT are used in the diagnostic approach. The availability of VCE and device assisted enteroscopy could help in early diagnosis. In our case VCE was the only examination which revealed the infiltration of the jejunum, when even the CT enterography was negative. However, a recent study showed that the diagnostic implementation of new techniques did not yield a significant advantage in terms of an early diagnosis and better outcome, but there is a need for further investigation (18). Due to a lack of data and an absence of guidelines, treatment of a small bowel adenocarcinoma is based on expert agreement and guidelines for colon cancer. A prospective phase III clinical trial PRODIGE 33-BALLAD comparing adjuvant chemotherapy vs observation among patients with small bowel adenocarcinoma with stage I-III is still ongoing. Surgical treatment is the only potentially curative option. However, 40% of patients have a relapse after primary tumor resection. The main prognostic factors are lymph node invasion and localization, with duodenal tumors having a worse prognosis. Five-year survival in cases of lymph node invasion is poor (28–32%) (19–21). For stage II with risk factors (pT4) and stage III (N+) adjuvant chemotherapy should be considered. Regimens are based on fluoropyrimidine in combination with oxaliplatin (22).
Conclusion
We presented the case of a woman who was diagnosed with atypical CD at the age of 53 years. The disease was complicated by small bowel adenocarcinoma, which was detected by VCE. The patient underwent surgical resection and received adjuvant chemotherapy. The knowledge of the risk factors of CD, malignant complications and the use of modern enteroscopic methods could improve the outcomes of these patients in the future.
Grants or financial support
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (FNBr, 65269705, Sup 16/19).
CORRESPONDING AUTHOR:
Lumír Kunovský, M.D., Ph.D.,
Department of Gastroenterology and Internal Medicine,
University Hospital Brno,
Faculty of Medicine,
Masaryk University,
Jihlavska 20
625 00 Brno,
Czech Republic
Citation: Vnitř Lék 2020; 66(7): e39–e42
Received: 17. 7. 2019
Accepted: 17. 2. 2019
Zdroje
1. Bureš J. Celiakie v roce 2018. Vnitř Lék 2018; 64 : 602–610.
2. Askling J, Linet M, Gridley G, et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123 : 1428–1435.
3. Caio G, Volta U, Ursini F, et al. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol 2019; 19 : 45–53.
4. Tachecí I. Kapslová endoskopie. Hradec Králové: Nucleus HK, 2008.
5. Cílený screening celiakie (metodický pokyn MZ ČR 2011) Věstník MZ ČR, částka 3, 2011.
6. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47 : 352–376.
7. Bilimoria KY, Bentrem JD, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009; 249 : 63–71.
8. Yi PS, Howard M. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 2011; 3 : 33–42.
9. Kunovský L, Dastych M, Robek O, et al. Multiple neuroendocrine tumor of the small bowel: a case report and a review of literature. Vnitř Lék 2018; 64 : 966–969.
10. Van Gils T, Nijeboer P, Overbeek LIH, et al. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up. United Eur Gastroent 2018; 6 : 1485–1495.
11. Malamut G, Cellier C. Complications of coeliac disease. Best Pract Res Clin Gastro 2015; 29 : 451–458.
12. Goddard CJR, Gillett HR. Complications of coeliac disease: are all patients at risk? Postgrad Med J 2006; 82 : 705–712.
13. Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep 2011; 6 : 231–240.
14. El Zouhairi M, Venner A, Charabaty, et al. Small bowel adenocarcinoma. Curr Treat Option On 2008; 9 : 388–399.
15. Rampertab SD, Forde KA, Green PHR. Small bowel neoplasia in coeliac disease. Gut 2003; 52 : 1211–1214.
16. Brousse N, Meijer WR. Malignant complications of coeliac disease. Best Practice and Research: Clinical Gastroenterology 2005; 19 : 401–412.
17. Benhammane H, El M’rabet FZ, Serhouchni KI. Small Bowel Adenocarcinoma Complicating Coeliac Disease: A Report of Three Cases and the Literature Review. Case Reports in Oncological Medicine 2012; doi:10.1155/2012/935183.
18. Legué LM, Bernards N, Gerritse SL, et al. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in the Netherlands. Acta Oncol 2016; 55 : 1183–1189.
19. Talamonti MS, Goetz LH, Rao S, et al. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg 2002; 137: 564–570.
20. Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004; 101: 518–526.
21. Zar N, Holmberg L, Wilander E, et al. Survival in small intestinal adenocarcinoma. Eur J Cancer 1996; 32A: 2114–2119.
22. Locher Ch, Batumona B, Afchain P, et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Digestive and Liver Disease 2018; 50 : 15–19.
Štítky
Diabetológia Endokrinológia Interné lekárstvo
Článek Hypoglykemie u nediabetiků
Článok vyšiel v časopiseVnitřní lékařství
Najčítanejšie tento týždeň
2020 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Rizikové období v léčbě růstovým hormonem: přechod mladých pacientů k lékařům pro dospělé
- Statinová intolerance
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Koagulopatie asociovaná s onemocněním COVID-19
- Současná diagnostika a terapie sarkoidózy
- Obštrukčné spánkové apnoe a arteriálna hypertenzia: úloha črevného mikrobiómu
- CAR T-lymfocyty: horká novinka v léčbě nádorů
- Renální selhání u mnohočetného myelomu a jeho léčba
- Kostní metabolismus u idiopatických střevních zánětů 2
- Metformin -asociovaná laktátová acidóza
- Tangierská nemoc v rodině s fenotypem familiární hypercholesterolemie
- Hypoglykemie u nediabetiků
- Screening a krátká intervence u uživatelů nelegálních drog
- Kostní metabolismus u idiopatických střevních zánětů 1
- Etické konotace léčby onemocnění covid-19
- Biomarkery pro neendoskopické vyšetření sliznice jícnu
- Riziko kardiovaskulárních komplikací v závislosti na hladině glykemie: od diabetes mellitus k prediabetu
- ERCP u pacientů po choledochoduodenoanastomóze
- Přidělovaná ošetřovatelská péče jako jeden z indikátorů výskytu medikačního pochybení
- Small bowel adenocarcinoma diagnosed by video capsule endoscopy in a patient with celiac disease: a case report and review of literature
- Raritní koincidence chylothoraxu a hydrothoraxu při adenokarcinomu žaludku – kazuistika
- Muž s dysthymií (převážně negativním hodnocením všeho prožitého) indukoval depresi u senzitivní blízké osoby
- Vnitřní lékařství
- Archív čísel
- Aktuálne číslo
- Iba online
- Informácie o časopise
Najčítanejšie v tomto čísle- Hypoglykemie u nediabetiků
- Metformin -asociovaná laktátová acidóza
- Koagulopatie asociovaná s onemocněním COVID-19
- CAR T-lymfocyty: horká novinka v léčbě nádorů
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




