RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
Authors:
HF. Mayer; MC. Colman Perez; I. Stoppani
Authors‘ workplace:
Buenos Aires, Argentina
; University of Buenos Aires, Hospital Italiano de Buenos Aires, Plastic Surgery Department
Published in:
ACTA CHIRURGIAE PLASTICAE, 62, 1-2, 2020, pp. 50-53
INTRODUCTION
Some patients undergoing implant-based breast reconstruction (IBBR) with acellular dermal matrices (ADMs) develop a postoperative erythema overlying their ADM grafts named red breast syndrome (RBS). The entity was first described in 2010 in a correspondence between Nahabedian1 and Newman et al.2. RBS is characterized by a blanching erythema, which is non-infectious and self-limited, and it is alleged to be due to an immunological response to the ADM (either related to the sterilization process, process of eliminating immunogens from the cadaveric dermis, or other) and its incorporation to the surrounding tissue. The incidence of RBS is yet unknown, and the long-term sequelae of RBS remain to be seen3.
To the best of our knowledge, this entity has never been related to the use of a synthetic mesh. Herein we report the first case in the medical literature of RBS associated to the use of a polyglycolic acid mesh.
CASE REPORT
We present a 61-year-old patient, with a BRCA-1 gene mutation diagnosed after her sister’s breast cancer diagnosis. She underwent a prophylactic bilateral nipple-sparing mastectomy reconstructed with 390cc Mentor® anatomical textured implants (Mentor Corp., Leyden, the Netherlands) placed in submuscular position with the aid of a polyglycolic acid mesh (Safil® Mesh, Braun Surgical, Barcelona, Spain).
The patient received an intramuscular deposit steroid injection prior to leaving the hospital, 24 hours after the procedure. She also received perioperative IV antibiotics, and oral antibiotics (cephalexin 1g twice daily) were continued until drain removal on the 7th postoperative day when the daily output was less than 30 cc. Twenty days after the procedure, the patient developed a right-sided blanching erythema starting at the inframammary fold (IMF) which extended towards the nipple areola complex (NAC). Three days later she developed an erythema on her left breast that followed the same pattern starting at the IMF and rapidly progressing towards the NAC (Figure 1). The patient denied having fever or sweats, she had no pain or tenderness on either breast. Laboratory tests showed normal white blood cell count (7386/mm3) and the ultrasound revealed no fluid collections (Figure 2).
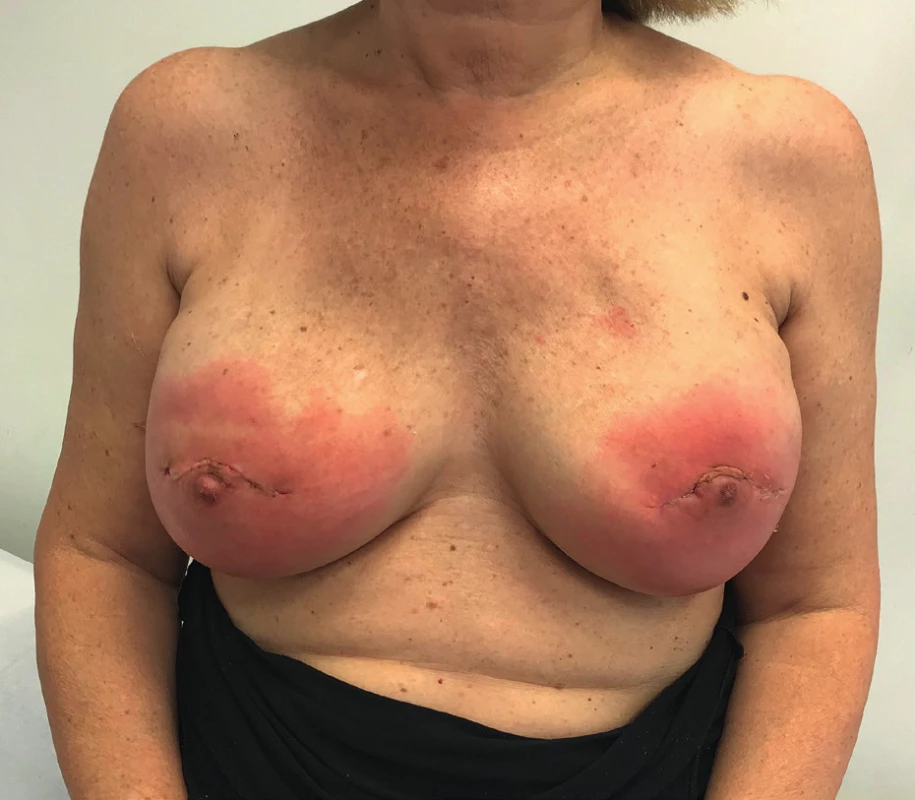
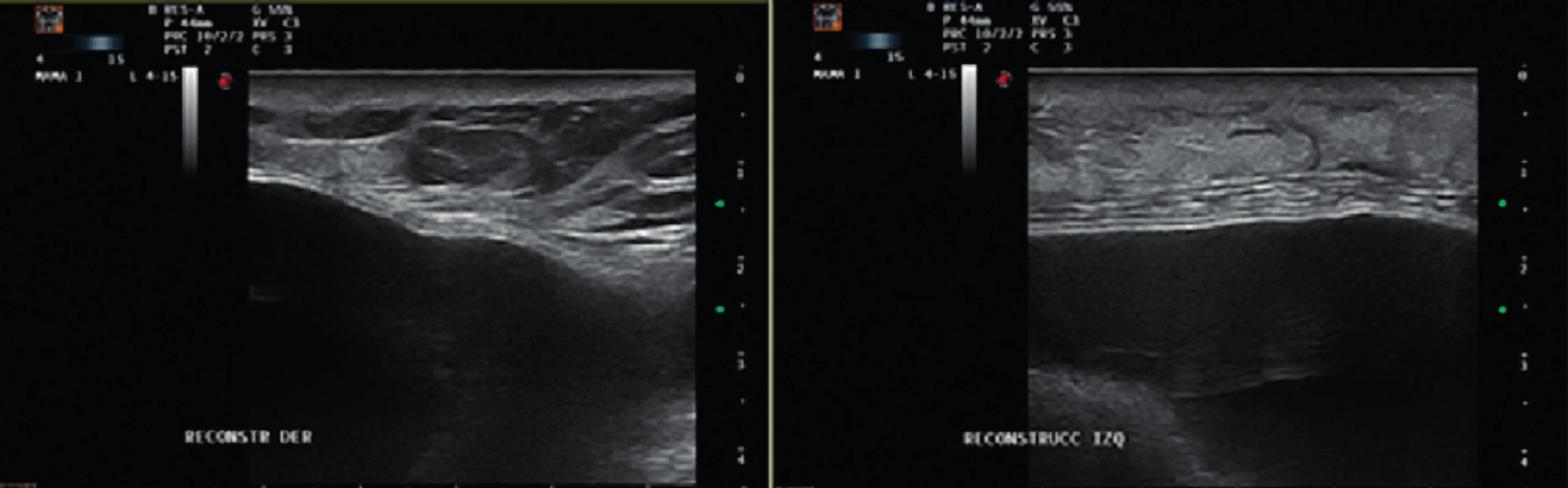
Given the fact that the patient presented with no clinical signs of infection, normal white blood cell count and an ultrasound negative for collections we decided to watch and wait. The compromise of both breasts and the pattern of the erythema also was suggestive of an allergic reaction to the synthetic mesh. The patient continued strict controls in the outpatient setting. Gradually the erythema begun to disappear and resolved spontaneously. (Figure 3.)
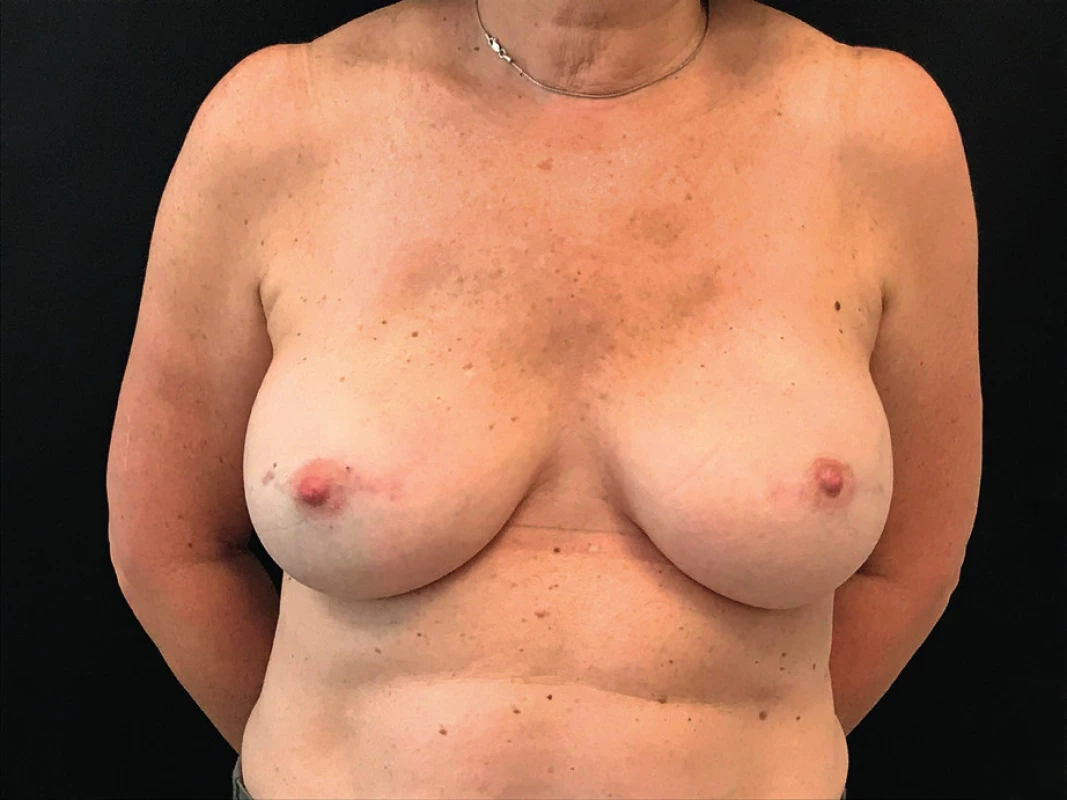
DISCUSSION
RBS, as a clinical entity, is characterized by erythema and the absence of infectious manifestations such as fever, tenderness or pain, increased local temperature, abscess or response to antibiotics. The erythema appears days to weeks after the reconstruction using ADM, located over the areas where the ADM has been placed (generally involving the lower pole of the breast). It blanches with pressure and resolves spontaneously without intervention. This reaction is supposed to be immunologic in origin, due to the sterilization method or the presence of biological antigens in the ADM3. Neither of these factors are usually present in a polyglycolic acid mesh, which might explain the absence of related reports to a synthetic mesh so far.
ADMs work as biocompatible scaffolds for cellular ingrowth that allows its incorporation to the surrounding tissue providing more elasticity to the tissue. ADMs were introduced in 1995 for treatment of full thickness burn injuries4 and were first used by Breuing and Warren in 2005 to reestablish the continuity of the pectoralis major in IBBR and reduce rippling5. ADMs are used to provide load support and improve the aesthetic result by pulling the muscle down to cover the incision, allowing for effective lower pole expansion and achieving a better definition of the IMF6. Other meshes, both biological and synthetic, have been also used for IBBR with similar purposes7,8,9,10. In Argentina and many other countries, given the high cost of ADMs, synthetic meshes are often used, being a popular alternative in IBBR11,12. Polyglycolic meshes were first described for breast reconstructive purposes by the first author and Hugo Loustau in 200713,14. Since then, we have used this absorbable mesh in more than 800 patients.
After revision of the available literature, we have not found a description of RBS associated with any synthetic mesh. This case in point, the mesh we used was Safil® mesh which is a polyglycolic acid mesh, when we generally use polyglactin 910 Vicryl® knitted mesh (Ethicon, Sommerville, NJ, USA) suggesting that there is a difference between those two brands despite it being the similar type of thread. We conjecture that this mesh may have a different immune response in the body, the same way aseptic ADMs have been shown to have more incidence of RBS than sterile ADMs15.
Even though, in the case presented, no antibiotics were used due to the clear absence of infectious manifestations, the risk of not treating a true infection in the breast outweighs the morbidity of unnecessary use of antibiotics, thus the recommendation is still empiric antibiotic coverage if there is any clinical doubt3.
To date, the diagnosis of RBS remains a diagnosis of exclusion, and further casuistry of RBS associated to polyglycolic meshes is needed to determine its link. RBS is considered an idiopathic erythema and the exact etiology remains unknown. Ganske et al have studied the origin of RBS through punch biopsies demonstrating eosinophilic infiltration as seen in delayed-type (type IV) allergic reactions and showed a response to steroids and chemotherapy16. These facts may suggest an immune response to the presence of the mesh.
Our patient received intramuscular steroids prior to hospital discharge, this possibly influenced the timing of the erythema to 20 days after the procedure. However, we did not treat the RBS with steroids at time of appearance of the erythema. If there is any doubt of infection, steroids would exacerbate the condition.
In conclusion, to the best of our knowledge, this is the first case of RBS associated to the use of a polyglycolic mesh in IBBR. New cases that may arise are awaited to aid in the investigation of probable causes and to determine the best treatment.
Author contribution: Conception and design, acquisition of data, and analysis and interpretation of data: Mayer HF; writing, review and edition: Mayer HF and Stoppani I; writing original draft: Perez Colman MC.
Conflict of Interests: The authors declare that they have no conflict of interest.
Financial support: This study did not receive any specific grant from funding agencies in the public, commercial and not-for-profit sectors.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent: The patient gave her informed consent in writing prior to inclusion in the study.
Corresponding author:
Horacio F. Mayer, MD, FACS
Plastic Surgery Department, Hospital Italiano de Buenos Aires, University of Buenos Aires, Plastic Surgery Department
Peron 4190, 1st. floor (1181)
Buenos Aires, Argentina
Sources
1. Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation: reply. Plast Reconstr Surg. 2010, 126 : 1120–1.
2. Newman MI., Hanabergh E., Samson MC. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2010, 126 : 1120–1. (author reply)
3. Wu PS., Winocour S., Jacobson SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg. 2015, 39 : 227–30.
4. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995, 21 : 243–8.
5. Breuing KH., Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005, 55 : 232–9.
6. Ortiz JA. Clinical Outcomes in Breast Reconstruction Patients Using a Sterile Acellular Dermal Matrix Allograft. Aesthetic Plast Surg. 2017, 41 : 542–50.
7. Jacobs JM., Salzberg CA. Implant-based breast reconstruction with meshes and matrices: biological vs synthetic. Br J Hosp Med (Lond). 2015, 76 : 211–6.
8. Hallberg H., Lewin R., Søfteland, M.B. et al. Complications, long-term outcome and quality of life following Surgisis® and muscle-covered implants in immediate breast reconstruction: a case-control study with a 6-year follow-up. Eur J Plast Surg. 2019, 42 : 33–42.
9. Quinn EM., Barry M., Kell M. Immediate implant reconstruction using absorbable TIGR mesh after nipple-sparing mastectomy. Eur J Plast Surg. 2019, 42 : 1–6; https://doi.org/10.1007/s00238-019-01603-0
10. Chen G., Zhang Y., Xue J., Zhu X., Liu C., Sun L., Gu X., Zhang H., Liu C. Surgical Outcomes of Implant-based Breast Reconstruction Using TiLoop Bra Mesh Combned with Pectoralis Major Disconnection. Ann Plast Surg. 2019, 83(4): 396–400.
11. Mayer HF., de Belaustegui EA., Loustau HD. Current status and trends of breast reconstruction in Argentina. J Plast Reconstr Aesthet Surg. 2018, 71 : 607–9.
12. Sharma S., Van Barsel S., Barry, M. et al. De novo experience of resorbable woven mesh in immediate breast reconstruction post-mastectomy. Eur J Plast Surg. 2017, 40 : 17.
13. Loustau H., Mayer HF., Sarrabayrouse M. Immediate prosthetic breast reconstruction: the ensured subpectoral pocket (ESP). J Plast Reconstr Aesthet Surg. 2007, 60 : 1233–8.
14. Loustau HD., Mayer HF. Beyond biologics: absorbable mesh as a low-cost, low-complication sling for implant-based breast reconstruction. Plast Reconstr Surg. 12014, 34 : 323e–4e.
15. Lewis P., Jewell J., Mattison G, et al. Reducing postoperative infections and Red Breast Syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg. 2015, 74(Suppl.1):30–2.
16. Ganske I., Hoyler M., Fox SE., Morris DJ., Lin SJ., Slavin SA. Delayed hypersensitivity reaction to acellular dermal matrix in breast reconstruction: the red breast syndrome? Ann Plast Surg.2014, 73(Suppl 2):139–43.
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2020 Issue 1-2
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- ENHANCED RECOVERY PROTOCOL FOLLOWING AUTOLOGOUS FREE TISSUE BREAST RECONSTRUCTION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- INDICATION AND IMPORTANCE OF RECONSTRUCTIVE SURGERIES OF FACIAL SKELETON IN MAXILLOFACIAL SURGERY: REVIEW
- Editorial
- CURRENT OPTIONS IN PHARMACOLOGICAL INTERVENTIONS FOR MICROVASCULAR ANASTOMOSIS PATENCY: REVIEW
- ACCIDENTAL FINDING OF SYNCHRONOUS BILATERAL DUCTAL CARCINOMA IN SITU IN A YOUNG MAN REFERRED TO MASTECTOMY DUE TO GYNECOMASTIA – AND WHAT IF LIPOSUCTION HAVE BEEN USED? CASE REPORT
- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
