Bilateral latissimus dorsi for breast reconstruction in one stage
Authors:
Dražan L. 1,2; Knoz M. 1,2; Streit L. 1,2; Cheimaris A. 1,2
Authors‘ workplace:
Department of Plastic and Aesthetic Surgery, St. Anne’s University Hospital Brno, Czech Republic
1; Faculty of Medicine, Masaryk University Brno, Czech Republic
2
Published in:
ACTA CHIRURGIAE PLASTICAE, 64, 2, 2022, pp. 82-85
doi:
https://doi.org/10.48095/ccachp202282
Introduction
Unilateral breast reconstruction using latissimus dorsi myocutaneous (LDM) flap has been well established [1–4].
Bilateral use of the LDM flaps seems to be impractical, because of the difficulties to harvest two LDM flaps simultaneously by two surgical teams while the patient is positioned in lateral decubitus position during the procedure. Another obstacle could be a possibility of the first flap pedicle compression when harvesting the second one. We present our technique in case series of 5 patients, allowing simultaneous harvest of both LDM flaps for the purpose of bilateral breast reconstruction.
Materials and methods
The authors present a retrospective study of 5 patients undergoing bilateral breast reconstruction from February 2016 to April 2020.
Three patients underwent immediate bilateral LDM flap reconstruction with implants, 1 patient in combination with local mastectomy cutaneous flaps, and 1 patient in combination with lipografting.
Three patients underwent bilateral immediate reconstruction after prophylactic mastectomy; pedicled LDM flap was used in one patient as a rescue procedure after prophylactic mastectomy and reconstruction with implants followed by bilateral necrosis of the skin flaps. One patient underwent delayed reconstruction after bilateral mastectomy.
Surgical technique
Surgical planning and marking of the LDM flaps are performed while the patient is standing. The midline of the chest, the inframammary fold line and the anterior axillary fold line are marked. The lateral edge of the LDM and the posterior axillary fold line, the contour line of the bra on the back are then marked. The skin island is usually oriented horizontally, so that the resulting scar is hidden under the bra. The outer quarter to one third of the flap extends beyond the outer edge of the LDM. At the surgical theatre, the patient is placed in a prone position. The LDM flap is harvested simultaneously by two surgical teams. The dissection is performed over the thoracodorsal fascia, including as much subcutaneous fat overlying LDM flap as possible. At the lateral border, the LDM is separated from the anterior serratus muscle, the paraspinal fascia, the vertebral fascia, superomedial from the trapezius muscle and the teres major muscle in the axillar area. When dissecting the LDM flap cranially, we identify and dissect the insertion of the muscle attaching laterally on the humerus and LDM pedicle running medially. We separate both structures and slide our finger under the LDM insertion when using the cautery to detach the structure. This creates enough space to dissect the LDM pedicle, identify the LDM motoric nerve and resect a 2–3 cm segment. The key level of the procedure is creation of subcutaneous pockets on the lateral side of the chest in the subaxillar area. These pockets must be large enough to accommodate and temporarily "store" the LDM flaps. The medial margin of the skin island is marked with a temporary suture to facilitate the orientation of the flap when it is later pulled to the front of the chest. The LDM flap slides into the subcutaneous pockets, allowing the surgeon to close the defects on the back. The skin defects after LDM flap harvest are closed in three layers with the application of Redon's drains. Subsequently, the patient is turned to a supine position, and the operation continues in two teams. According to the previous indication, prophylactic mastectomy or other preparations for breast reconstruction are performed. The LDM flap is pulled through a subcutaneous tunnel from the axillary pocket to the mastectomy site. Great care must be taken to prevent the malrotation of the flap pedicle when pulled out, which would result in disturbance in the flap circulation. Shaping of the flap and other reconstruction procedures (implants, expanders, local mastectomy flaps, or lipografting) are performed in the same manner as in the case of one-sided reconstruction technique [5].
Results
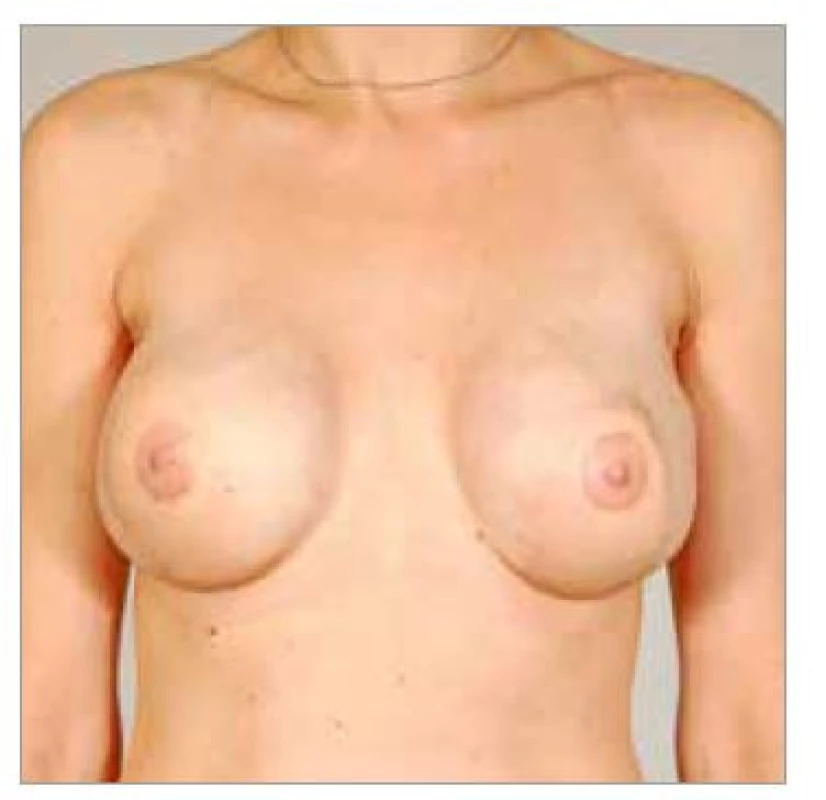
Five patients were operated using the bilateral LDM flap reconstruction technique. Three patients underwent bilateral immediate reconstruction after prophylactic mastectomy (Fig. 1–4),




1 patient underwent delayed reconstruction (Fig. 5–6), and in 1 patient LDM was used after prophylactic mastectomy and reconstruction with implants followed by bilateral necrosis of the skin flaps (Fig. 7–9). The youngest patient was 40 years old; the oldest patient was 72 years old; the average age of the patients was 52.8 years. The average operative time was 4 hours and 4 minutes. All 10 LDM flaps survived completely. There was seroma formation in 6 out of 10 LDM donor sites in 4 patients. Seromas had been aspirated in outpatient basis repeatedly.




Discussion
The LDM flap was first introduced by Tansini in 1896 and later by d'Este in 1912 using LDM flap for chest defect reconstruction [3,4]. Wider use of the LDM flap for breast reconstruction has been achieved by the introduction of musculocutaneous flaps following the works of Olivari, Schneider and Muhlbauer [6–8]. A lower volume in comparison to the original breast is a usual disadvantage of the LDM flap use for breast reconstruction. Thus, the use of the pedicled LDM flap technique requires the use of an implant in case of insufficient volume of the reconstructed breast. In 1998,
Mc Craw and Papp published an expanded form of "fleur-de-lis" flap with overlying subcutaneous fat, increasing the flap volume in order to make it more suitable for autologous breast reconstruction [9]. Bilateral LDM flap procedure has been described by Chiaramonte and Smith in 2001 [10,11]. The use of transverse rectus abdominus myocutaneous , deep inferior epigastric perforator or superficial inferior epigastric artery free flaps has become a today's gold standard for autologous breast reconstruction. However, these free tissue transfer reconstructions require a surgical team trained in a microsurgical technique of free tissue transfer; moreover, long-lasting surgical procedures are not suitable for all patients, especially for those with severe comorbidities, or for heavy smokers.
The combination of reliability and simplicity of LDM flap transfer and the possibility of simultaneous bilateral flap harvest and thus achieving the operation time of about 4 hours belong to the advantages of the described method of bilateral breast reconstruction. The reconstruction can be performed in combination with an expander/implant, lipografting, local flaps, or just as a LDM flap itself. This eliminates the complicated positioning of the patient on her side when harvesting the LDM flap. However, when harvesting the LDM flap by our preferred method in prone position, it is necessary to consider that muscular structures, including the neurovascular pedicle, are located more laterally, compared to the method of LDM flap harvest on the side. It should also be considered, that due to the two-phase pull out of the LDM flap, i.e. pocketing in the prone position and subsequent pulling in the patient's position on the back, there is an increased risk of malrotation of the flap and kinking of the flap pedicle. To ease the proper direction of the flap poles, we mark the lateral pole of the skin island with a visible stitch. During the rotation, this “stitch pole” moves to the medial part of the reconstructed breast (rotation of the skin island in 180 degrees).
Conclusion
The described technique of bilateral breast reconstruction using LDM flaps enables safe breast reconstruction in a single stage procedure. The average reconstruction time is about 4 hours, which corresponds to unilateral prophylactic mastectomy and LDM flap reconstruction in comparison. The operation is suitable for the patients with a lack of the skin cover of future breasts and for patients contraindicated for long microsurgical procedures. This places the bilateral LDM procedure to the centre of the breast reconstructions range, between the reconstructions with double free tissue transfer and the breast implants.
Role of authors: Luboš Dražan – first author; Martin Knoz – corresponding author; Libor Streit – consulting author; Antreas Cheimaris – consulting author.
Conflict of interests: Luboš Dražan M.D., Ph.D., Martin Knoz M.D., Ph.D., Libor Streit M.D., Ph.D., and Antreas Cheimaris M.D., declare that they have no conflict of interest.
All authors have read and approved the final version of the manuscript.
All authors declare that this paper or its part is not concurrently under review in another journal or publication.
Compliance with ethical requirements: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study, formal consent is required and was received from all patients included in the study.
Martin Knoz, MD, PhD
Department of Plastic and Aesthetic Surgery,
St. Anne’s University Hospital Brno
Berkova 34
612 00 Brno
Czech Republic
Submitted: 20. 12. 2021
Accepted: 6. 6. 2022
Sources
1. Maxwell GP, McGibbon BM, Hoopes JE. Vascular considerations in the use of a latissimus dorsi myocutaneous flap after a mastectomy with an axillary dissection. Plast Reconstr Surg. 1979; 64(6): 771–780. doi: 10.1097/00006534-197912000-00004.
2. Bostwick J. 3rd, Nahai F., Wallace J.G., et al. Sixty latissimus dorsi flaps. Plast Reconstr Surg. 1979; 63(1): 31–41. doi: 10.1097/00006534-197901000-00006.
3. Tansini I. Sopra il mio nuovo processo di amputazione della mamella [in Italian]. Riforma Med. 1906; 12 : 757.
4. Maxwell G.P. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980; 65(5): 686–692. doi: 10.1097/00006534-198005000-00027.
5. Drazan L., Castagnetti F. Keeping extra skin for nipple-areola reconstruction during mastectomy. Plast Reconstr Surg. 2007; 120(3): 815–816. doi: 10.1097/01.prs.0000278814.71726.b5.
6. Olivari N. The latissimus flap. Br J Plast Surg. 1976; 29(2): 126–128. doi: 10.1016/0007-1226(76)90036-9.
7. Schneider W.J., Hill H.L. Jr, Brown R.G. Latissimus dorsi myocutaneous flap for breast reconstruction. Br J Plast Surg. 1977; 30(4): 277–281. doi: 10.1016/0007-1226(77)90117-5.
8. Mühlbauer W. Mastektomie und primäre Brustrekonstruktion [Mastectomy and primary breast reconstruction]. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1989 : 915–920. German.
9. Papp C., McCraw J.B. Autogenous latissimus breast reconstruction. Clin Plast Surg. 1998; 25(2): 261–266.
10. Chiaramonte MF, Nahabedian MY. Bilateral breast reconstruction with the latissimus dorsi musculocutaneous flap: the importance of patient positioning. Ann Plast Surg. 2001; 46(2): 163–166. doi: 10.1097/00000637-200102000-00014.
11. Smith B.K., Cohen B.E., Biggs T.M., et al. Simultaneous bilateral breast reconstruction using latissimus dorsi myocutaneous flaps: a retrospective review of an institutional experience. Plast Reconstr Surg. 2001; 108(5): 1174–1181; discussion 1182–1183. doi: 10.1097/00006534-200110000-00011.
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2022 Issue 2
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Safety and Tolerance of Metamizole in Postoperative Analgesia in Children
-
All articles in this issue
- Editorial
- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Temporary skin closure in extremity soft tissue sarcoma – our indications
- Modified harvesting technique for pedicled pectoralis major muscle flap after extended manubrial resection in case of recurrent cervicothoracic junction tumors
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Subcutaneous shoulder hibernoma presenting as an atypical lipomatous tumor – a case report
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Single-stage reconstruction of a large lower eyelid defect using a full-thickness bilamellar autograft
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
