Treatment algorithm for post sternotomy wound infection – our experience
Authors:
K. N. Manjunath 1; M. S. Venkatesh; B. P. Sanmathi 1; S. Shanthakumar 1; G. Abhijit 1; S. Anam 1; S. Ravishankar 2
Authors‘ workplace:
Department of Plastic and Reconstructive Surgery, Ramaiah Medical College, Bangalore, India
1; Department of cardiothoracic and vascular surgery, Ramaiah Medical college, Bangalore, India
2
Published in:
ACTA CHIRURGIAE PLASTICAE, 65, 1, 2023, pp. 13-19
doi:
https://doi.org/doi: 10.48095/ccachp202313
Introduction
Over 90,000 cardiac surgeries are performed in the United States alone every year and the number is increasing at 5% per year [1]. In India, approx. 15,000 surgeries are performed per year. Median sternotomy introduced by Schumacher and Lure [2] is the most commonly used incision in open heart surgeries [3]. The 1–3% incidence of surgical site infections (SSI) have been observed in USA. Various factors can lead to sternal wound infection. These include old age, comorbidities such as diabetes, hypertension, high body mass index, and chronic obstructive pulmonary disease. Sternal wound infection developing into deep sternal wound infection (DSWI) increases the mortality and morbidity. Different studies have documented varied incidence of DSWI from 2% up to 47% [4]. Although infection rates are low, the severity of the infection determine the patient’s morbidity and mortality.
Superficial sternal wound infection can be conservatively managed with regular wound dressings, debridement, and secondary suturing. However, one of the most important steps in the management of deep sternal wound infections is obliteration of a dead space. This is achieved with the use of muscle flaps. Various local flaps have been used in sternal wound reconstruction of which the pectoralis major muscle flap is considered a workhorse flap. The aim of this study was to develop a treatment algorithm for sternal wound infection. The post sternotomy wound dehiscence will be divided into superficial and deep. Superficial wounds were treated with vinegar and deep wounds were treated with the pectoralis major muscle advancement flap. The treatment outcome was then studied.
Materials and methods
Clinical data
The patient cohort included in the study were 25 patients with sternal wound infections following sternotomy for cardiac surgeries, between the period of January 2016 and August 2021. The patients were then classified as superficial or deep wound infection. The ulcers with soft tissue cover over the sternal bone and steel wires were classified as superficial wound infection. If the ulcer extended till the sternal bone and if sternal wires were visible, then ulcers were classified as deep sternal wound infection.
The superficial wound dehiscence was treated with 1% acetic acid dressing and deep wound infections were treated with the pectoralis muscle flap. Treatment outcomes were measured as favorable or unfavorable. Patients who healed without any complication irrespective of time were considered having a favorable outcome. In superficial wounds, an outcome was termed as unfavorable if the culture growth increased from scanty to heavy and discharge increased in a wound. In deep wound infection, gaping and wound re-dehiscence were considered as an unfavorable outcome. The demographic data of the patients and the time duration are described as a mean and a median. Average time duration taken for wound healing will be compared with the available study average and statistical significance tested with the ANOVA test.
Superficial wounds were treated with diluted edible vinegar dressing at two-day intervals till they healed completely. 4% acetic acid (edible vinegar) was diluted to 1% acetic acid with normal saline solution. Wound dressing was changed at two-day intervals. Baseline wound culture and sensitivity was done at the beginning of the treatment and repeated at 10 and 20 days. If an ulcer grew organisms, sensitivity-based antibiotics were administered to the patient. At 10 days, if there was no growth, then the antibiotics were stopped. Dressing was discontinued once the ulcer epithelialized completely.
Treatment method for deep sternal wound infections
Ulcers which were bone deep and those with exposed sternal wires were classified as deep sternal infections. In all these ulcers, after thorough debridement, bilateral pectoralis muscle advancement flaps were used to cover the wound. These ulcers were covered as early as possible to prevent the chances of sternal osteomyelitis and spreading mediastinitis. Flap surgery was deferred only in cases where wound cultures had heavy growth of organisms. In these circumstances, regular dressing and systemic antibiotics continued till the growth was scanty and then flap was performed.
Surgical technique
Patients were adequately prepared for surgery with optimization of blood pressure, blood sugar, hemoglobin level, albumin content and cardiopulmonary function. After appropriate anesthesia, thorough debridement was done and nonviable tissue was removed. Bilateral pectoralis major advancement flaps were planned. The flaps were elevated in the avascular plane deep to pectoralis major muscle and elevated from medial to lateral side. Both pectoralis muscles were advanced till the midline covering the sternum and the sternal hardware was reached. Both advanced ends were sutured to each other in the midline. The pectoralis fascia and the proximal rectus sheath were included in the closure. Deep dermis and skin sutures were closed in layers.
Outcome measures
Treatment outcomes were measured as favorable or unfavorable. Patients who healed without any complication irrespective of time were considered having a favorable outcome. In superficial wounds, an outcome was termed as unfavorable if the culture growth increased from scanty to heavy and discharge increased in the wound. In deep wound infection, gaping and wound re-dehiscence were considered as an unfavorable outcome.
Statistical analysis
The descriptive statistics is presented as a mean; the time taken for treatment outcome is described as an average. The time duration taken to heal both superficial and deep sternal wound infection is compared with the population mean and the statistical significance is tested using the independent t-test.
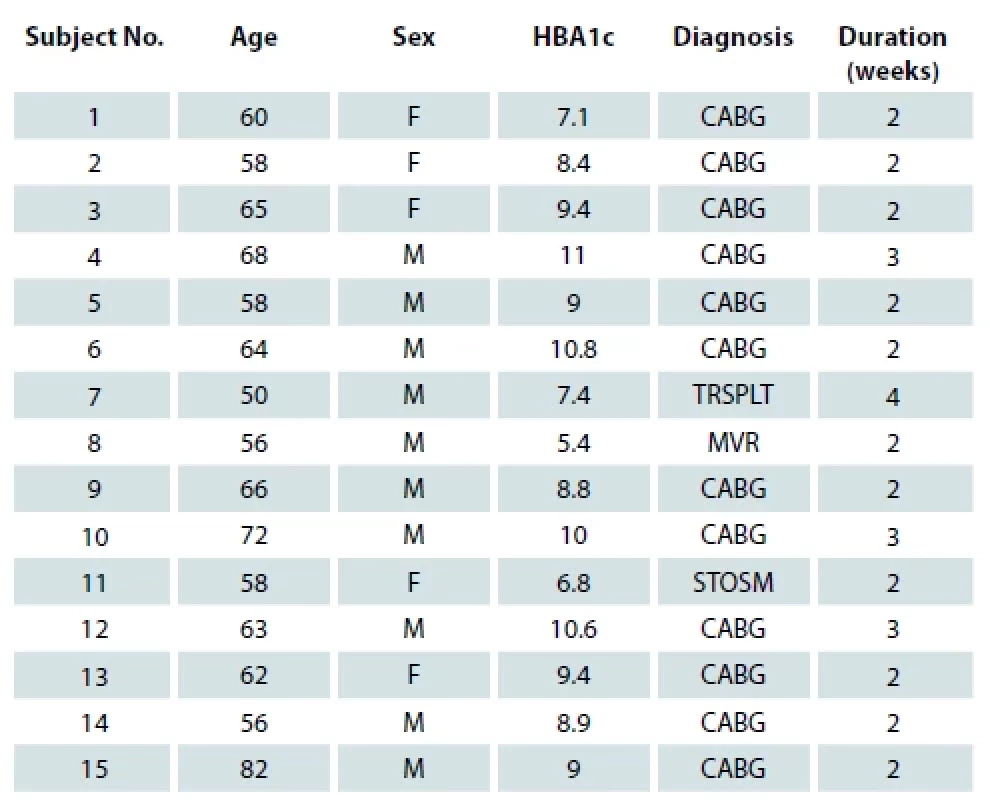
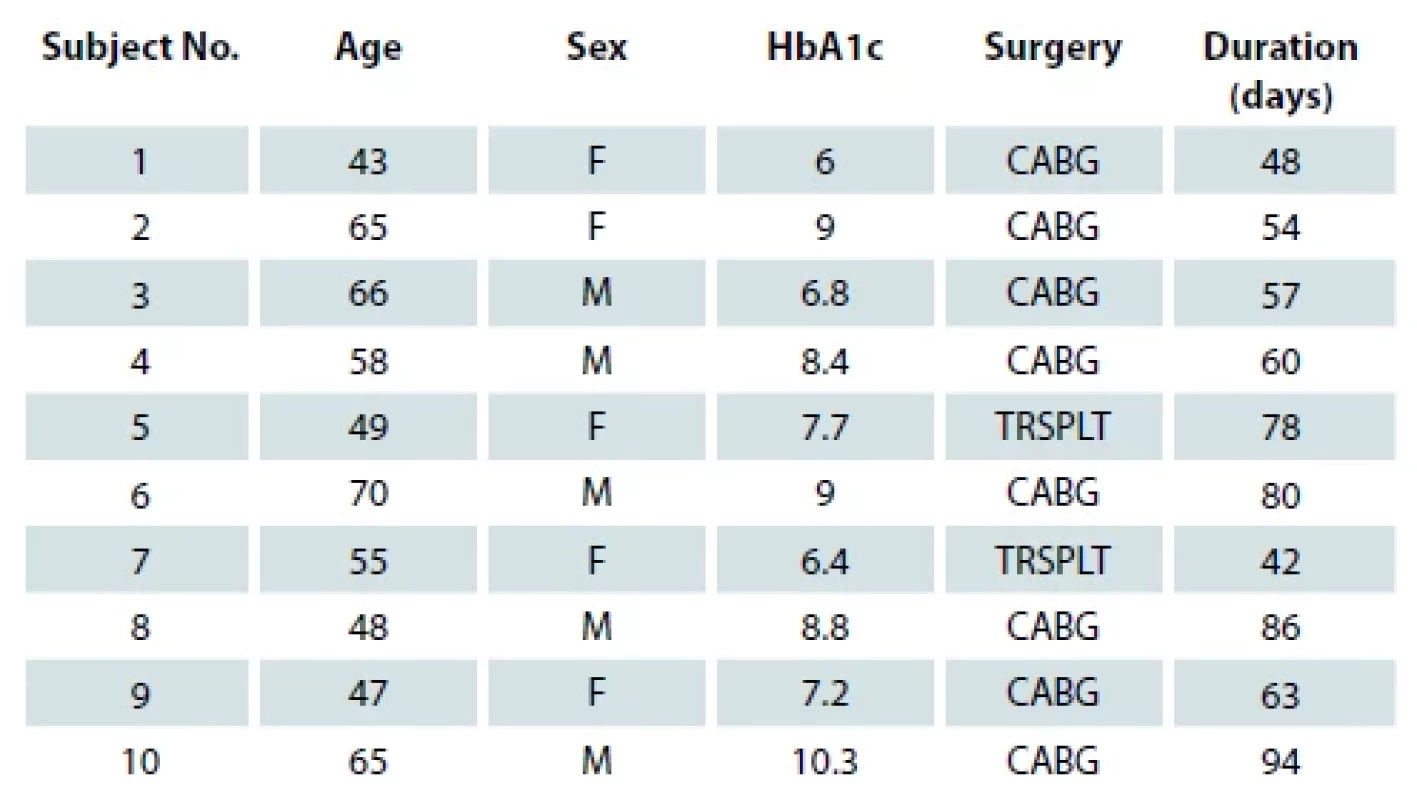
Results
Between the period of January 2016 till August 2021, a total of 25 post sternotomy wound infection cases were included in the study. Fifteen patients had deep sternal wound infection and 10 patients had superficial wound infection. Out of the 15 patients with deep sternal wound infection, 5 were female and 10 were male patients, the youngest patient was 50 years and the oldest 82 years old. The mean of glycated hemoglobin (HbA1c) of the patients was 10.8. In 12 out of 15 patients, wounds healed within 2 weeks after flap surgery and the rest healed within 4 weeks after flap surgery (Tab. 1). None of them reported re-dehiscence. Out of the 10 patients with superficial wound dehiscence, 5 were females and 5 were males; the youngest patient was 43 years and the oldest 70 years old. Mean HbA1c of the patients was 7.96 years. An average time duration for healing of a superficial wound was 66.2 days (Tab. 2). None of them reported an increase in bacterial load or discharge from the wound during the course of the treatment. As compared to population mean of 51.2 days, superficial sternal wound infection in our study took significantly more time. (The T-value is 2.760143 and the P-value is 0.011053. The result is significant at P < 0.05). In deep sternal wound infection, wound healing took less time compared to a population mean of 90 days. (The T-value is −68.026425 and the P-value < 0.00001. The result is significant at P < 0.05.)
Discussion
Median sternotomy approach is most commonly used for cardiac surgery because of its exposure and surgical convenience [3]. Surgical site infection is a known complication. As per the Centre for Disease Control (CDC) criteria, SSI is to be reported if infection occurs within 30 days after surgery or within 1 year if an implant was left in place. Various classifications of the surgical site infections are proposed. Pairolero and Arnold classified infected median sternotomies wounds into three types based on duration and clinical findings [5].
Type 1 infections occurred within the first week after surgery and presented with serosanguineous discharge only.
Type 2 infections occurred during the second to fourth week and presented with cellulitis and mediastinal suppuration.
Type 3 infections occurred many weeks to months after sternotomy and involved sinus tracts draining pus.
Another classification includes superficial and deep infections based on the involvement of the tissues. Superficial wound infections involve skin and soft tissue, whereas deep infections involve fascia and sternum [6]. Deep sternal infection represents more complex reconstructive problems. According to CDC, positive cultures from the mediastinal fluid, fever, sternal pain and instability, all these are considered as deep sternal wound infection (DSWI) [7,8]. However, in our study we classified only wounds which were bone deep with exposed bone and sternal wires as DSWI. Since positive wound cultures or fever can also be seen in post operative cases, these were not considered deep sternal wound infections for treatment.
The incidence of superficial sternal wound infections ranges from 0.5 to 8 % [9]. The diagnosis is usually made by the signs of erythema, drainage of pus or most commonly low-grade fever. Several techniques have been used for the management of superficial sternal wound infections. Surgical revision with open dressings or closed system has been proved to be effective. Martino A et al [10] presented their results of negative pressure wound therapy in managing patients with superficial sternal wound infection in their article. They concluded that superficial wound infection was effectively controlled with the negative pressure wound therapy dressing. A study done by Aygün et al showed a protective effect of locally applied rifamycin on sternal wound infections [11]. One more study indicated that if sternal wound infection/dehiscence was limited and superficial, the wound could be treated daily with iodine-soaked gauze followed by surgical closure (when the wound was judged to be clean and dry) [10] were sufficient. Studies have been performed to assess the effectiveness of vinegar in treating infected wounds [12]. The efficacy of vinegar in treating sternal wounds has not been done in a systematic manner. It is known that acidification of a wound increases the pO2 and reduces the histotoxicity of ammonia which may be present in bacterial infection (ammonia being less toxic in an acid environment) [13]. The majority of bacteria and fungi are alkaline in nature and acid media can definitely counter their infestation and thus bringing down the local wound infection. When used locally, edible vinegar (4% acetic acid), diluted to 1% with normal saline solution, can acidify the ulcer floor and promote granulation formation. It is also known to prevent bacterial growth / infestation and even some of fungal infection [14].
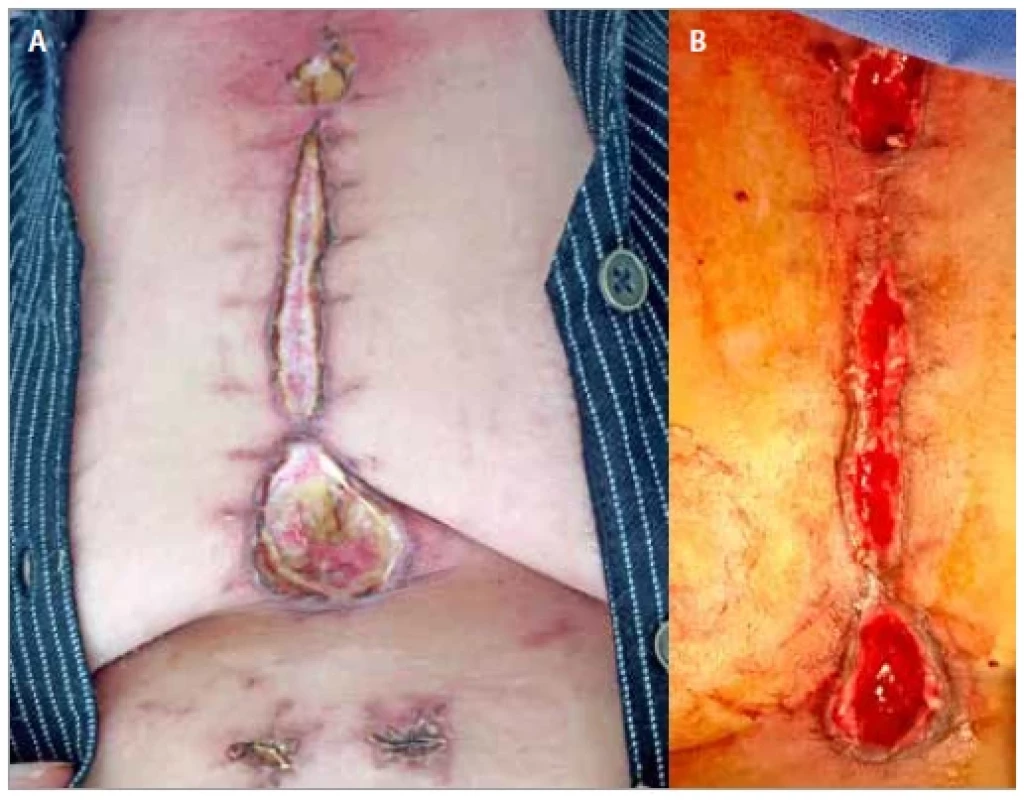
In our study, we used vinegar-based dressings for sternal wound optimization. Edible vinegar dressing (4% acetic acid (edible vinegar) was diluted to 1% acetic acid with saline solution) was done at 2-day intervals. First gauze layer of the wound was soaked with the vinegar solution. Baseline wound culture and sensitivity was done at the beginning of the treatment and repeated at 10 and 20 days. In 10 of our cases all the wounds improved in terms of granulation as well as bacterial load. Although our cases took 4–8 weeks (Fig. 1A,B), all the wounds healed completely. We could not find any study using vinegar dressing for sternotomy wounds. In comparison to other modalities of dressing, vinegar dressing was efficient, low cost and readily available. However, a study which used conservative method for treatment, opined that it took 51 days in average [15] days to convert a sternal wound to a healthy wound. Later, one more procedure was used to cover the wound. In our study, only vinegar dressing was used and in some cases secondary suturing was done.
Deep sternal wound infections are more complex problems and their incidence ranges from 0.7 to 2.3% [16]. It is important to distinguish DSWIs from superficial sternal wound infections because morbidity and mortality differed considerably among the two groups [17]. As per CDC, the diagnosis of deep sternal wound infection requires the presence of one of the following: (1) organism isolated from the culture of mediastinal tissue or fluid; (2) evidence of mediastinitis seen during surgery; or (3) presence of either sternal instability, chest pain, or fever (> 38° C), and either purulent drainage from the mediastinum, isolation of an organism present in a blood culture, or culture of the mediastinal area [18].
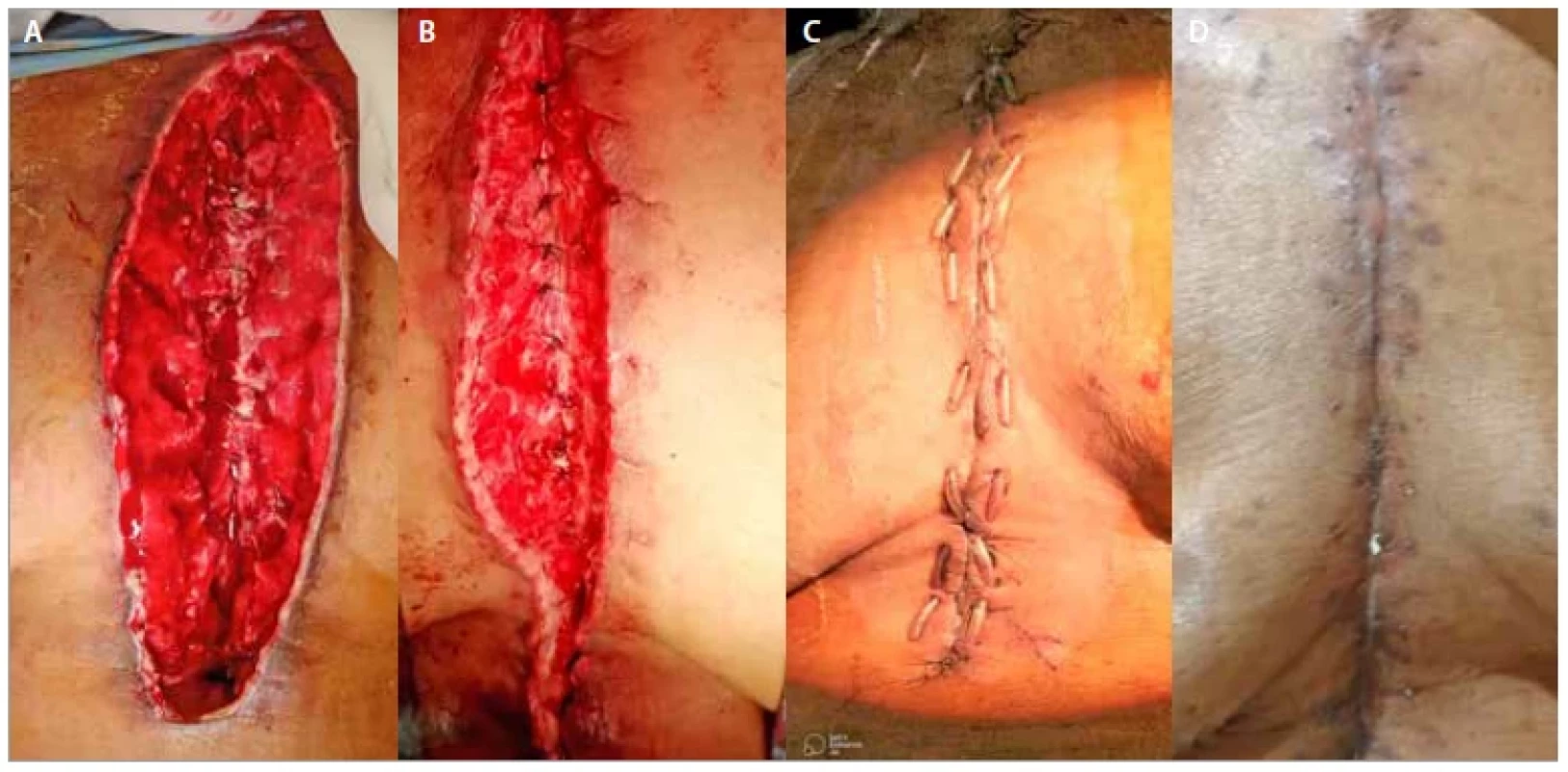
However, in our study wound dehiscence which was bone deep with exposed sternal wires were only considered deep sternal wound infection (Fig. 2A) as the wounds with discharge only were conservatively treated with dressings only. As per CDC, DSWI patients require much more aggressive treatment involving early debridement, tissue cover and long-term antibiotics. We followed the same protocol and approached the patients with similar treatment. Patients suspected of DSWI were subjected to a CT scan of the chest, which assesses the depth of dehiscence and looked for retrosternal collection [19]. On clinical evidence of DSWI, empiric antibiotic therapy involving broad spectrum antibiotics was initiated. Antibiotics based on wound culture was initiated as soon as culture report was ready. Closure of the dehiscence was planned as soon as possible; flap was deferred only when the wound grew heavy growth of multi-resistant organism. Various techniques have been described for DSWI: omental transposition [20], unilateral or bilateral pectoralis major muscle turnover flap in mediastinitis [21], pectoralis major muscle transposition [22], bilateral myocutaneous pectoralis major muscle flaps [23], rectus abdominus muscle flap [24], latissimus dorsi muscle flap [25], microsurgical free flap [26], primary sternal closure with titanium plate fixation [27], one--step radical sternal debridement and muscle flap reconstruction [28]. All these muscle flaps have an advantage. Muscle flaps increase the local blood flow, occlude the dead space. Muscles also deliver better antibiotic concentration and improve esthetic outcome [29]. Pectoralis major muscle has various advantages in post sternotomy cases. It is close to sternum and easy to detach without requiring additional incision, muscle has a rich blood supply and it is expendable. All these make it an ideal muscle for flap. Also, the bilateral pectoral muscle flap has shown better sternal stabilization than just rewiring the sternum [30]. The literature describes advantages and disadvantages of unilateral and bilateral pectoralis muscle flap. The main advantage of the unilateral pectoral muscle flap is that it preserves full strength in at least one arm but covers the sternum [3]. This needs at least one internal mammary artery to be intact. Spartalis et al [31] and Ortak et al [32] published a series of 55 patients and 48 patients, respectively, all had a successful outcome and not many functional deformities. At the same time, the advancement preserved both internal mammary arteries (IMA). In bilateral advancement of pectoralis major, the sternal origin is detached and it is reoriented to the midline where it is fixed to the opposite pectoralis muscle. This will not affect the function of the upper limb. Other studies conducted by Feng et al have reported the same. One of the disadvantages of bilateral pectoralis discussed in literature was that the muscle cannot cover the lower end of the sternum. In a study by Bongiolo junior et al, defects of the upper and middle thirds were covered with pectoralis major while the omentum and the rectus abdominis were used for lower third defects [33]. In our study, we did not use any additional method to cover the lower end of the sternum, only rectus fascia was utilized when required. We were successful in achieving tension free (Fig. 2B) repair and none of our cases had postoperative dehiscence. Davidson et al [34] also opined that the use of rectus abdominis was of no major advantage compared to bilateral pectoralis alone [35]. Some authors have proposed the refinements in techniques of bilateral pectoralis advancement [36]. They have proposed preservation of IMA perforators by isolating them carefully instead of cutting them. This prevented the likely thrombosis of the IMA, preserving it for the use in future. One of the less aggressive therapeutic options includes a fasciocutaneous flap based on internal thoracic artery perforators [37]. However, in our study, debridement of wound followed by bilateral pectoralis major muscle advancement flaps proved to be effective (Fig. 2C,D) as all the patients recovered without any unfavorable outcome [38].
Conclusion
Median sternotomy is one of the most commonly used incision used for cardiac surgeries. As in any other surgeries, surgical site infection is a common phenomenon. Timely and appropriate intervention, depending on the depth of infection can reduce the morbidity as well as mortality. Simple edible vinegar (1% acetic acid) dressings are effective for the management of superficial sternal wound infections. In patients where wound infections were bone deep or in cases where the sternal hardware was exposed, bilateral pectoralis major muscle advancement flaps reduced the morbidity of the patients. With this study, we could conclude that depth of the wound is a single most decision-making factor for muscle flap selection, irrespective of the bacterial culture. This proved to be an effective strategy for the management of superficial and deep sternal wound infections. Since the study had no comparison groups, the factors influencing the outcome are not completely evaluated. More patient studies are needed to ascertain this algorithm of treatment.
Roles of authors: All authors have been actively involved in the planning, preparation, analysis and interpretation of the findings, enactment, and processing of the article with the same contribution.
Declaration: All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as amended in 2008. Informed consent was obtained from all patients included in the study.
Conflict of interest: There is no conflict of interest to disclose.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not for profit sectors.
Assoc. Prof. K. N. Manjunath, MD
Department of plastic and reconstructive surgery
Ramaiah Medical college, Bangalore, India
Submitted: 25. 7. 2022
Accepted: 19. 3. 2023
Manjunath KN, Shanthakumar S, Abhijit G et al. Treatment algorithm for post sternotomy wound infection – our experience. Acta Chir Plast 2023; 65(1): 13–19.
Sources
1. iData Research. Intelligence behind the data. How many cardiac surgeries are performed each year? New study by iData Research. [online]. Available from: https://idataresearch.com/over-900000-cardiac-surgeries-performed-every-year-in-the-united-states.
2. Shumacker HB., Lurie PR. Pulmonary valvulotomy; description of a new operative approach with comments about diagnostic characteristics of pulmonic valvular stenosis. J Thorac Surg. 1953, 25(2): 173–186.
3. Wyckman A., Abdelrahman I., Steinvall I., et al. Reconstruction of sternal defects after sternotomy with postoperative osteomyelitis, using a unilateral pectoralis major advancement muscle flap. Sci Rep. 2020, 10(1): 8380.
4. Zhang H., Lin J., Yang H., et al. Bilateral partial pectoralis major muscle turnover flaps for the management of deep sternal wound infection following cardiac surgery. J Thorac Dis. 2020, 12(10): 6010–6015.
5. Pairolero PC., Arnold PG. Management of infected median sternotomy wounds. Ann Thorac Surg. 1986, 42(1): 1–2.
6. Zacharias A., Habib RH. Factors predisposing to median sternotomy complications: deep vs superficial infection. Chest. 1996, 110(5): 1173–1178.
7. Singh K., Anderson E., Harper JG. Overview and management of sternal wound infection. Semin Plast Surg. 2011, 25(1): 25–33.
8. Garner JS., Jarvis WR., Emori TG., et al. CDC definitions for noso-comial infections, 1988. Am J Infect Control. 1988, 16(3): 128–140.
9. Lee YP., Feng MC., Wu LC., et al. Outcome and risk factors associated with surgical site infections after cardiac surgery in a Taiwan medical center. J Microbiol Immunol Infect. 2010, 43(5): 378–385.
10. Martino A., Re FD., Falcetta G., et al. Sternal wound complications: results of routine use of negative pressure wound therapy. Braz J Cardiovasc Surg. 2020, 35(1): 50–57.
11. Aygün F., Kuzgun A., Ulucan S., et al. The protective effect of topical rifamycin treatment against sternal wound infection in diabetic patients undergoing on-pump coronary artery bypass graft surgery. Cardiovasc J Afr. 2014, 25(3): 96–99.
12. Ganjale SB., Dhamoji P. Study of effectiveness of local vinegar (Acetic Acid) in infected orthopaedic wound management. Indian J Orthop Surg. 2021, 7(2): 154–158.
13. El Fattah Morsi A., Mustafa FM., El Tokhy A. Vinegar simple method in dressing of pseudomonas infected wound. Int Invent J Med Medical Sci. 2016, 3(8): 143–146.
14. Agrawal KS., Sarda AV., Shrotriya R., et al. Acetic acid dressings: finding the Holy Grail for infected wound management. Ind J Plast Surg. 2017, 50(3): 273–280.
15. El Degwy M., Omar A., Elasheery AO., et al. Strategic management of deep sternal wound infection using vacuum assisted closure system. J Adv Pharm Edu Res. 2017, 7(4): 443–449.
16. Singh K., Anderson E., Harper JG. Overview and management of sternal wound infection. Semin Plast Surg. 2011, 25(1): 25–33.
17. Ridderstolpe L., Gill H., Granfeldt H., et al. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001, 20(6): 1168–1175.
18. Garner JS., Jarvis WR., Emori TG., et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988, 16(3): 128–140.
19. Gur E., Stern D., Weiss J., et al. Clinical-radiological evaluation of poststernotomy wound infection. Plast Reconstr Surg. 1998, 101(2): 348–355.
20. Konofaos P., Spartalis E., Karagkiouzis G., et al. An alternative technique for surgical management of poststernotomy osteomyelitis and reconstruction of the sternal defect. Case Rep Surg. 2013, 2013 : 451594.
21. Sahasrabudhe P., Jagtap R., Waykole P., et al. Our experience with pectoralis major flap for management of sternal dehiscence: a review of 25 cases. Indian J Plast Surg. 2011, 44(3): 405–413.
22. Van Huizum MA., van Egmond DB., Morshuis WJ. Simple pectoralis major myocutaneous advancement flaps for closure after sternal wound dehiscence. Eur J Plastic Surg. 2000, 23 : 195–199.
23. Reichenberger MA., Harenberg PS., Pelzer M.,
et al. Arteriovenous loops in microsurgical free tissue transfer in reconstruction of central sternal defects. J Thorac Cardiovasc Surg. 2010, 140(6): 1283–1287.
24. Spindler N., Kade S., Spiegl U., et al. Deep sternal wound infection – latissimus dorsi flap is a reliable option for reconstruction of the thoracic wall. BMC Surg. 2019, 19(1): 173.
25. Banic A., Ris HB., Erni D., et al. Free latissimus dorsi flap for chest wall repair after complete resection of infected sternum. Ann Thorac Surg. 1995, 60(4): 1028–1032.
26. Reichenberger MA., Harenberg PS., Pelzer M.,
et al. Arteriovenous loops in microsurgical free tissue transfer in reconstruction of central sternal defects. J Thorac Cardiovasc Surg. 2010, 140(6): 1283–1287.
27. Lee JC., Raman J., Song DH. Primary sternal closure with titanium plate fixation: plastic surgery effecting a paradigm shift. Plast Reconstr Surg. 2010, 125(6): 1720–1724.
28. Cabbabe EB., Cabbabe SW. Immediate versus delayed one-stage sternal debridement and pectoralis muscle flap reconstruction of deep sternal wound infections. Plast Reconstr Surg. 2009, 123(5): 1490–1494.
29. Neto AA., Coltro PS., Horácio GS., et al. Unilateral pectoralis major muscle flap for the treatment of sternal wounds due to Ludwig’s angina. Int Wound J. 2018, 15(1): 174–177.
30. Zeitani J., Pompeo E., Nardi P., et al. Early and long-term results of pectoralis muscle flap reconstruction versus sternal rewiring following failed sternal closure. Eur J Cardiothorac Surg. 2013, 43(6): e144–150.
31. Spartalis E., Markakis C., Moris D., et al. Results of the modified bi-pectoral muscle flap procedure for post-sternotomy deep wound infection. Surg Today. 2016, 46(4): 460–465.
32. Ortak T., Uraloglu M., Uysal A., et al. Reconstruction of sternal defects with pectoralis major muscle flap. Eur J Plast Surg. 2008, 30:
223–228.
33. Bongiolo MJ., Santos DN., Bittencourt RC., et al. Uso de retalho de músculo peitoral maior para o tratamento de osteomielite de esterno. Rev Bras Cir Plast. 2010, 25(1 Suppl): 67.
34. Davison SP., Clemens MW., Armstrong D., et al. Sternotomy wounds: rectus flap versus modified pectoral reconstruction. Plast Reconstr Surg. 2007, 120(4): 929–934.
35. Ascherman JA., Patel SM., Malhotra SM., et al. Management of sternal wounds with bilateral pectoralis major myocutaneous advancement flaps in 114 consecutively treated patients: refinements in technique and outcomes analysis. Plast Reconstr Surg. 2004, 114(3):
676–683.
36. Tomos P., Lachanas E., Michail PO., et al. Alternative bi-pectoral muscle flaps for postoperative sternotomy mediastinitis. Ann Thorac Surg. 2006, 81(2): 754–755.
37. Koulaxouzidis G., Orhun A., Stavrakis T., et al. Second intercostal internal mammary artery perforator (IMAP) fasciocutaneous flap as an alternative choice for the treatment of deep sternal wound infections (DSWI). J Plast Reconstr Aesthet Surg. 2015, 68(9): 1262–1267.
38. Lappa A., Malpieri MR., Cicco M., et al. An alternative inexpensive treatment for deep sternal wound infections after sternotomy. Interact Cardiovasc Thorac Surg. 2003, 2(4):
629–632.
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2023 Issue 1
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Safety and Tolerance of Metamizole in Postoperative Analgesia in Children
-
All articles in this issue
- Editorial
- Treatment algorithm for post sternotomy wound infection – our experience
- Role of perforator flaps in leg and foot reconstruction
- Pattern of invasion of oral squamous cell carcinoma and its relation to the presence of nodal metastases – a review
- Secondary reconstruction of the orbit and conjunctival sac – a case report
- Clinical outcomes of absorbable plates (hydroxyapatite-poly-l-lactide composites) for phalangeal fractures – case reports
- Prospective observational study of clinical outcomes in using posterior interosseous free flap for finger defects
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Pattern of invasion of oral squamous cell carcinoma and its relation to the presence of nodal metastases – a review
- Treatment algorithm for post sternotomy wound infection – our experience
- Prospective observational study of clinical outcomes in using posterior interosseous free flap for finger defects
- Role of perforator flaps in leg and foot reconstruction
