Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
Authors:
R. I. Pongeluppi 1; R. A. Monteiro Cardoso 1; E. L. Zucoloto Jr 1; M. F. M. Ballestero 2; R. Santos De Oliveira 1; D. G. Abud 3; J. A. Farina Jr 4; B. O. Colli 1
Authors‘ workplace:
Department of Medicine, Federal University of São Carlos, Brazil
2; Department of Neuroscience and Behavioral Sciences, University Hospital, Medical School of Ribeirão Preto, University of São Paulo, Brazil
3; Division of Plastic Surgery, University Hospital, Medical School of Ribeirão Preto, University of São Paulo, Brazil
4
Published in:
ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 106-111
doi:
https://doi.org/10.48095/ccachp2023106
Introduction
Cirsoid aneurysm or scalp arteriovenous malformation (SAVM) are an arteriovenous fistulas of the scalp. Dilated draining veins produce cosmetic concerns, with a throbbing growing mass. Local pain, audible bruits, headache, tinnitus, and haemorrhage are other symptoms. Treatment includes surgery, embolization or a combination of both [1].
The term cirsoid aneurysms were introduced and described by Brecht in 1833 [2,3]. However, “cirsoid aneurysm” is an incorrect nomenclature from the pathological point of view, since it refers to multiple scalp arteriovenous connections, with no capillary bed between them [4]. “Cirsoid” refers to the ivy-leaf, varicose appearance [1]. “Aneurysma sepertinum,” “plexiform angioma” and “aneurysm racemosum” are other nomenclatures describing this condition [1,2,5]. Thus, the term SAVM is preferred and used in this article.
The gold standard test for identifying anomalous arteriovenous communications is digital subtraction angiography. However, MRI and CT can help in anatomical delimitation and understanding the involvement of underlying tissues [6].
The therapeutic options available for treating SAVM include endovascular, surgical, or mixed treatment [7].
Endovascular treatment can be performed using the transarterial or transvenous approach, using different materials for embolization, such as coils and Onyx®, or sclerosing materials, such as cyanoacrylate [1]. However, several complications associated with this approach such as hyperemia, skin necrosis, release of embolization material into the systemic circulation, and a higher rate of recurrence have been described. In addition, multiple treatment sessions are often required [6,8].
It is an extremely uncommon disease, with only about 200 reported cases in 15 years [9]. Due to the rarity of this disease, the objective of the present study is to describe the experience of a single reference neurosurgery service over the last 20 years.
Methods
This was a descriptive and retrospective analysis in which all cases of SAVM, which were treated at our institution between 2001 and 2022, were included.
Descriptive statistical methods were used. Analysed data included – age at diagnosis, sex, clinical presentation, aetiology, feeding vessels, endovascular and surgical treatment, complications, outcomes and recurrence.
The medical records and all available images were systematically reviewed and evaluated by the researchers. Some preand postoperative photographic documentation was also reviewed. The identity of patients was not disclosed, making procurement of signed informed consent form unnecessary [10].
Results
Case series
Seven patients were treated at our institution for SAVM between 2001 and 2022. The patients included five males and two females. The median age at diagnosis was 25 years, (range 3−42 years), with the mean age of 23.3 years.
The aetiologies of the lesions were identified through clinical histories of the patients. In order of prevalence, the main causes of the aneurysms identified were: spontaneous (without apparent cause) in four patients (56.4%), traumatic in two patients (28.7%), and congenital in one patient (14.2%). The most frequent locations of lesions were the frontal (28.7%) and parietal (28.7%) regions, followed by the fronto-temporo-parietal (14.2%), convexity (14.2%), and occipital regions (14.2%).
All patients included in our review underwent selective arteriography by digital subtraction, making it possible to identify the arteries involved in the malformation. More than one feeder was involved in the lesions of all patients. Nourishment via the superficial temporal artery was visualized in all seven patients, with the most prevalent association being with the occipital artery as visualized in five cases (71.5%), and the ophthalmic artery was implicated in three cases (43%). The vertebral artery fed the lesion in only one patient (14.2%).
The symptoms that led to the diagnosis of the lesions were: presence of a pulsating mass on the scalp in six patients (85.8%), history of minor local bleeding before diagnosis in one patient and chronic headache in one patient.
Six patients were treated with one and five embolization sessions before resection. The agents used for embolization were N-butyl-cyanoacrylate (NBCA) and ethylene-vinyl-alcohol copolymer (EVOH) (Onyx®).
One of the patients (No. 3) had a residual lesion after embolization. However, resection was contraindicated since the patient became bedridden after endovascular treatment of a small cerebellar arteriovenous malformation and has been receiving clinical radiological treatment for the past 102 months. Another patient (No. 6) who underwent two embolization sessions became bedridden after suffering a car accident in between, which contraindicated surgical treatment.
Surgical resection was performed in five patients. Only one patient was operated without preoperative endovascular treatment. After embolization, three patients suffered from scalp necrosis and required reconstruction, which was performed using a multidisciplinary approach with plastic surgery. No postoperative infections were observed in any patient. After gross total resection, no recurrence was observed. Mean follow-up was 42.6 months.
The results of our series are summarized in Tab. 1.
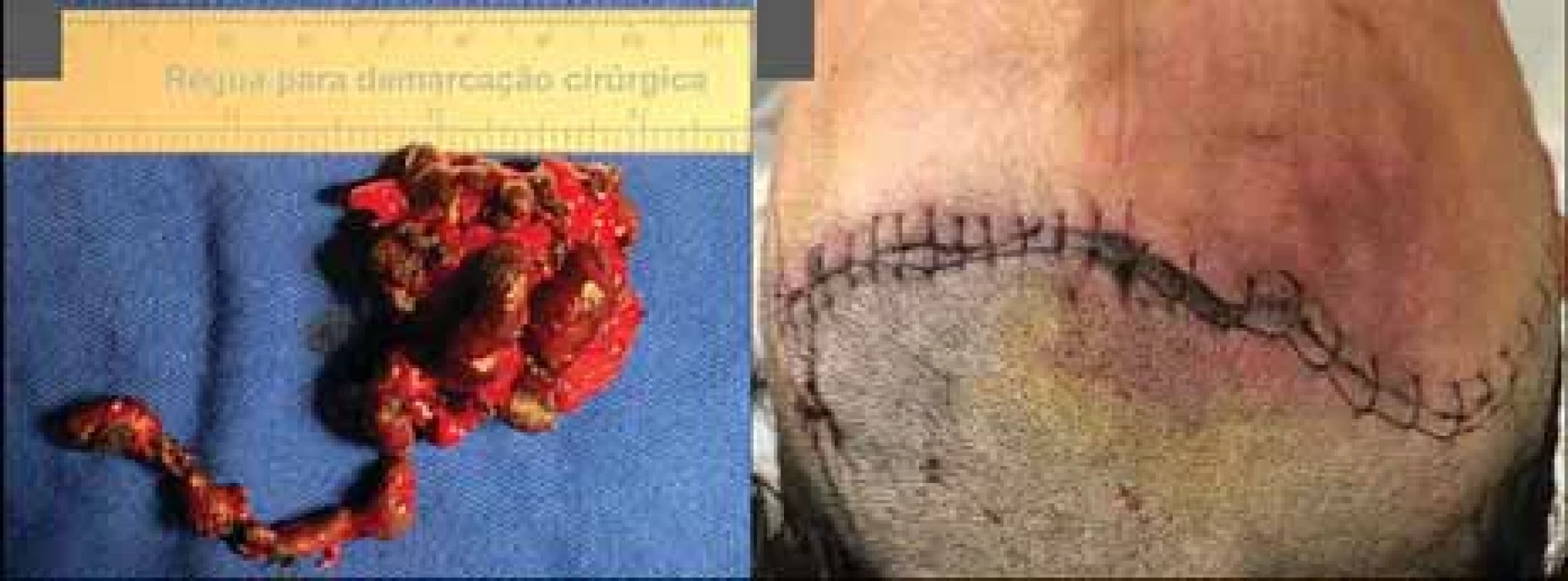
Illustrative case
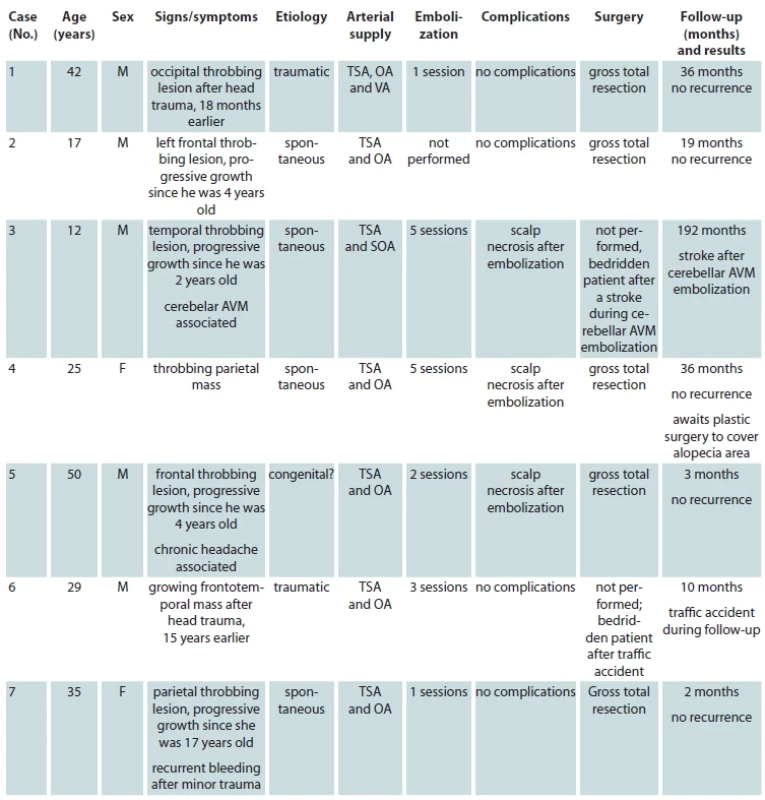
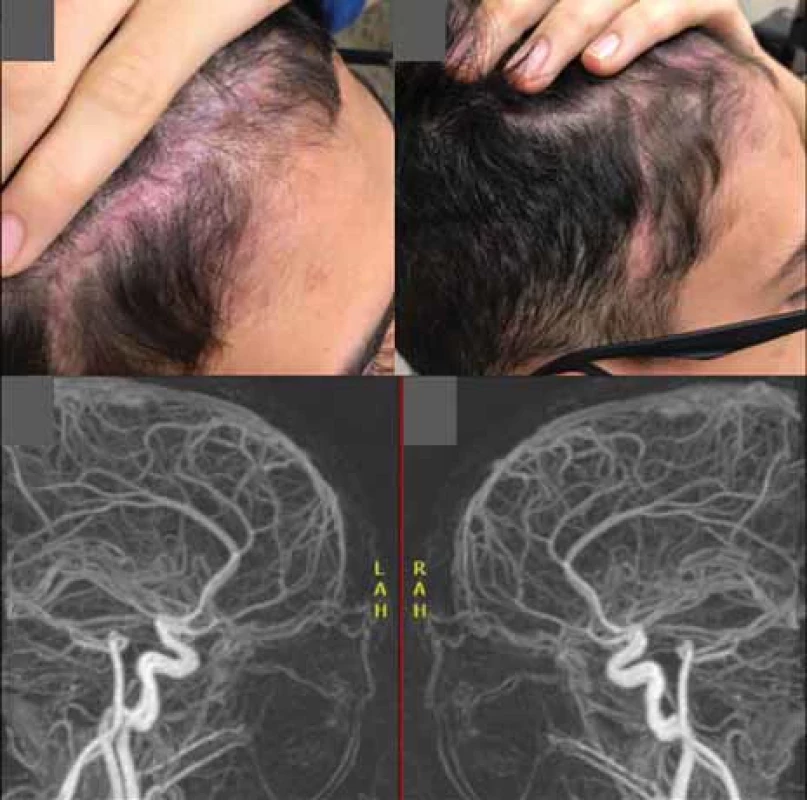
A 13-years-old male presented to our hospital with the complaint of a painless swelling in the right frontal region, which had been present since his early childhood, with an objective and subjective complaint of local pulsation. The injury had a psychosocial impact and adversely affected patient’s quality of life, for aesthetic reasons. Arteriography confirmed the diagnosis of a SAVM (Fig. 1).
The lesion was approached in a multidisciplinary manner, after joint planning involving interventional neuroradiology, neurosurgery, and plastic surgery.
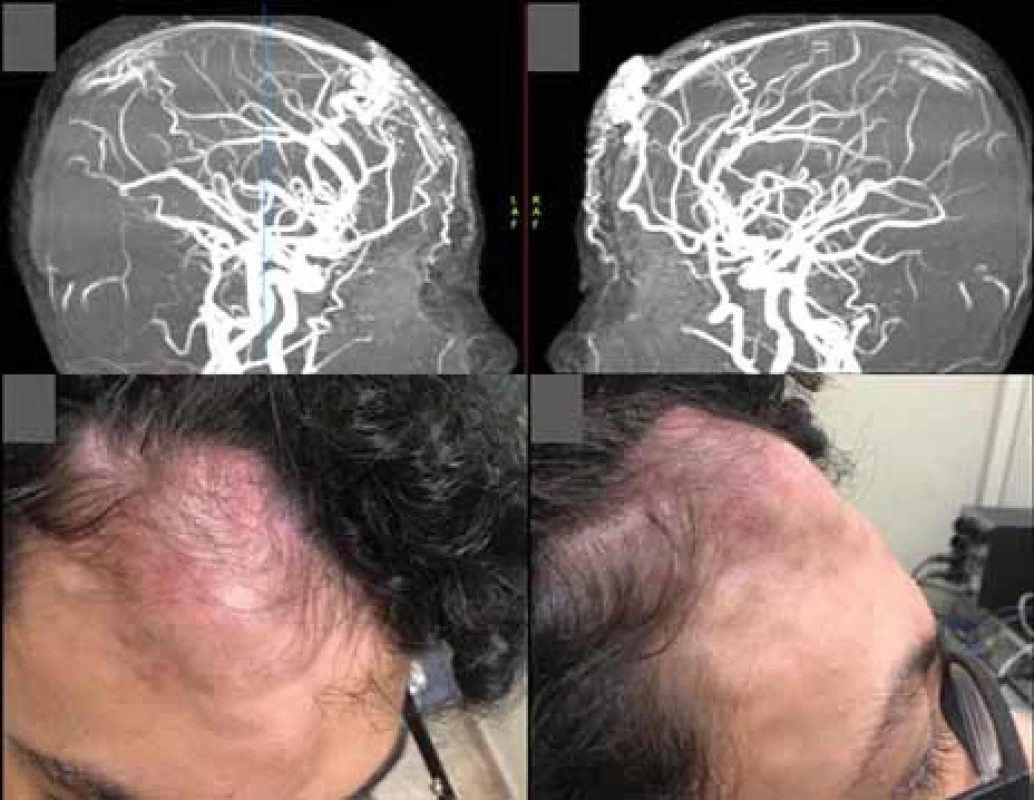
Preoperative embolization was performed 6 days before the procedure, in which 20 mL of EVOH copolymer was injected in the distal branches of the right superficial temporal artery, the main vessel supplying the nidus. In the control examination carried out at the end of the procedure, a reduction of approximately 90% in blood flow was observed (Fig. 2).
After embolization, an area of about 2 cm radius of ischemic skin was observed, which evolved with a necrotic appearance (Fig. 3).
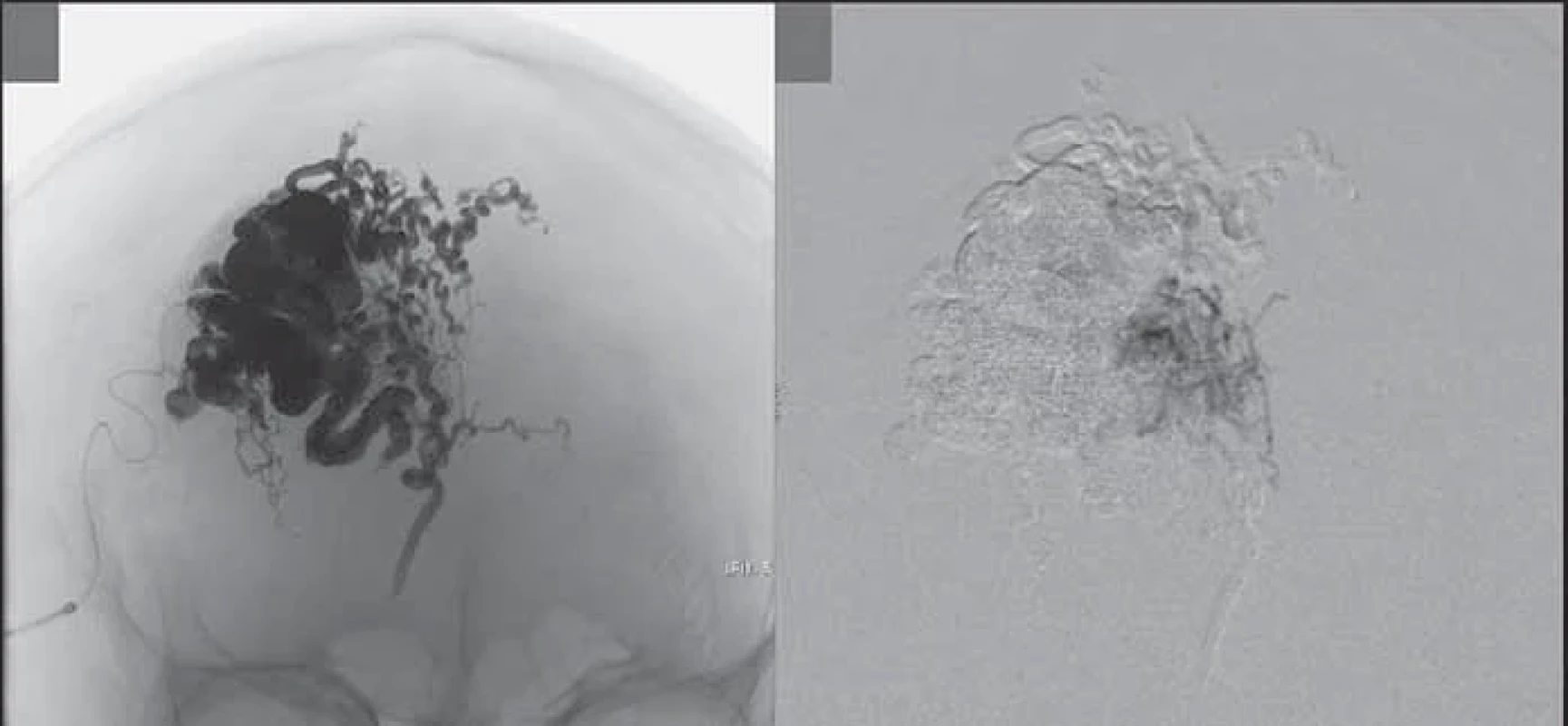
During surgery, a careful three-quarter bicoronal skin incision and anatomical dissection of the subgaleal plane were performed and the malformation was completely exposed. The lesion was in the aponeurotic galea of the right frontal region, with predominant irrigation obtained from branches of the right superficial temporal artery and to a lesser extent by branches of the contralateral equivalent artery, in addition to irrigation by small intracranial perforating branches.
The arteries supplying the nidus were meticulously and circumferentially coagulated, taking care to preserve the most proximal portions of the vessels, since they had no anatomical correlation with the malformation. After complete coagulation of the arteries, the coagulation of the draining veins of the nidus was performed and, finally, the lesion was completely excised. The procedure lasted for 150 min and involved negligible bleeding.
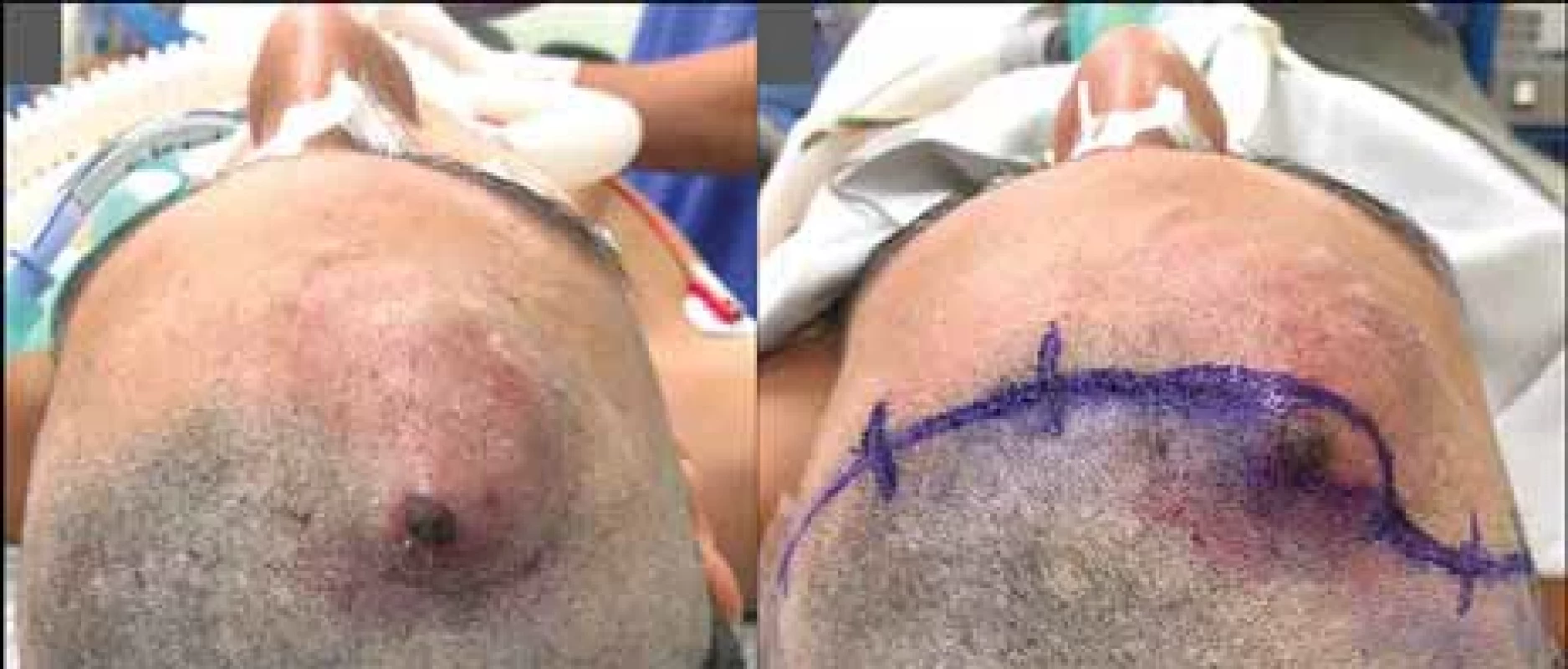
At the end of the surgery, the area of necrosis was resected, making it possible to perform primary closure without tension (Fig. 4).
The patient was discharged 2 days after the procedure, with the total hospital stay lasting for 9 days. After 2 months of follow-up, the patient was satisfied with the aesthetic results, and the control arteriography showed no lesion (Fig. 5).
Discussion
SAVM are rare and challenging lesions and are often treated by a multidisciplinary team.
It is more likely to occur in men as compared to women with the ratio of affected males and females being 2.5 : 1 and it can occur at any age with the median age of diagnosis being 25 years [6]. The male to female ratio observed in our study was 2.5 : 1.
The various possible aetiologies for occurrence of SAVM are congenital or spontaneous, traumatic, or iatrogenic [6]. In our study, the most frequent aetiologies were spontaneous, in 57.1% of cases, followed by traumatic observed in 28.6% of cases.
The pathophysiology is poorly understood, but congenital or spontaneous fistulas are believed to arise from persistent embryologic communications, vascular hamartomas, or spontaneous fistulas at cross-sectional sites. Traumatic fistulas can form due to simultaneous lesions in arteries and veins, at nearby sites, or due to angiogenesis and anomalous endothelial proliferation with pathological communications. Iatrogenic fistulas are less common and can arise after craniotomies, temporomandibular arthroscopies, or even hair transplants [3,6].
The regions where the SAVM are commonly observed are the frontal region, followed by the temporal and occipital regions [6,11]. Similarly, parietal and frontal lesions were the most common areas of occurrence of the aneurysms, observed in our institution, accounting for more than half (57.4%) of the cases.
Most lesions (79.3%) are nourished by the superficial temporal artery, possibly since its location makes it more susceptible to trauma [6]. Other vessels, such as the occipital artery, have also been observed to provide nourishment to the lesions in a few case reports [12].
In all cases reviewed by us, lesions were observed to receive nourishment from two or more vessels. There is no mention of the involvement of more than one vessel in literature in most series, and multiple sources of nourishment is an important factor to be considered when approaching these lesions, both endovascularly and surgically.
Clinically, the lesions present as pulsatile masses in most cases [3]. Headache and tinnitus may be the symptoms associated with the lesion in some patients [6]. However, more serious complications, such as recurrent bleeding, skin necrosis, theft of intracranial flow, ischemia, and even heart failure may be observed [7]. The discomfort of presence of a pulsatile mass from aesthetic point of view was the most frequent complaint among our patients (86%); however, two patients had bleeding and one suffered from headaches before surgery.
The differential diagnoses include other low-flow vascular malformations such as haemangiomas, hamartomas, and sinus pericranii [3,6].
A recent systematic review of the literature suggests that surgical treatment preceded by embolization is the preferred approach for treatment [6]. A higher rate of complications is associated with pure endovascular treatment (55.8%), as compared to surgical treatment alone (9.9%) and combined treatment (0%) [6]. Preoperative embolization is believed to reduce bleeding as well as surgical time and is the technique chosen in the treatment of our subjects [13]. However, some authors still prefer the pure surgical approach [3,7,8].
Surgery can also give rise to complications, such as necrosis, infections, bleeding, and even recurrence in the event of incomplete resection [6]. In our study group, post-embolization scalp necrosis was relatively frequent and was observed in 50% of patients who underwent endovascular treatment. However, we emphasize that necrosis was restricted to the margins of the lesion resection, and a primary or secondary reconstruction, together with plastic surgery, was possible in all cases.
The surgical technique, in general, involves identification and coagulation or ligation of the nourishing vessels, circumferentially, followed by identification of the periosteum [3]. Although the lesion is predominantly located in the subcutaneous layer [6], in several cases, histological involvement of the pericranium with the presence of arterialized vessels has been observed [3]. Resection of the pericranium underlying the lesion as a way to reduce recurrence is the preferred approach of several authors [3,4,6,7]. However, some authors have also reported good outcomes following preservation of the pericranium when there is no obvious involvement other than some local thickening, which is usually the case [11,13]. As per our experience, the pericranium is evaluated macroscopically after resection. We found that, in most cases, due to its involvement, the pericranium is generally resected along with the lesion.
The reconstruction of the defect after resection of the lesion may involve simple closure, or it may involve rotation of the vascularized flap and skin graft, which is subject to complications at the donor site, risk of necrosis and cosmetic defect. Staged reconstruction, with preoperative placement of tissue expanders, is an option when more extensive skin defects are expected [14].
Skin grafting is an effective way of closing the surgical wound if the pericranium is kept intact. In the case of a partial loss of the pericranium, it is not possible to perform skin grafting effectively, due to the lack of adequate blood supply bed that supports its integration into the areas of bone.
In this situation, a 2-stage acellular dermal matrix consisting of animal collagen can be used (delayed skin grafting). There is no possibility of rejection because it is acellular. The dermal matrix usually takes around three weeks for integration, but in cases associated with negative pressure therapy (NPT), this time usually drops to 2 weeks.
Following integration of the matrix, the silicone surface layer is removed and the matrix is covered by thin skin grafting (0.2 mm thick) using an electric dermatome. In these cases, the “bridge effect” is observed, where the blood circulation now permeates through the entire surface of the matrix, thus covering the avascular area of partial loss of the pericranium, as if it were a bridge of blood circulation induced by the matrix.
The disadvantage of skin grafting is the frequent association of alopecia, the prevalence of which increases with increasing size and subsequent scalp involvement of the tumour. However, areas of alopecia can be partially or completely removed in stages by tissue expansion from the adjacent intact scalp.
The recurrence rate is generally low, ranging from 0% in mixed treatment, 6.3% in pure surgical treatment, and 8.3% in endovascular treatment [6]. However, late recurrences, as late as 18 years, have been described [13].
Almost none of the patients included in our review, and who underwent complete resection had recurrence. The only patient with a small residual lesion, following embolization, is on a long-term follow-up, without new complications or symptoms.
Conclusion
SAVM are uncommon lesions. Their diagnosis is based on an adequate clinical history which enables detection of the risk factors associated with the lesion, as well as an arteriography, which can delimit its anatomy and the vessels involved in the malformations.
Surgical resection preceded by embolization is the most effective treatment for this condition. Complete resection of the lesions prevents associated complications, such as bleeding and recurrence.
Scalp necrosis is a frequent complication in the treatment of these lesions, and a multidisciplinary approach along with reconstructive plastic surgery should always be considered.
Roles of authors
All authors contributed to the work equally.
Funding: The authors have no funding sources to declare in the present study.
Disclosures: The authors have no disclosures or conflicts of interest to declare. No portion of the paper has been presented previously but not published elsewhere. All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Sources
1. Albuquerque Sousa LH., Maranha Gatto LA., Demartini Z. Jr, et al. Scalp cirsoid aneurysm: an updated systematic literature review and an illustrative case report. World Neurosurg. 2018, 119 : 416–427.
2. Elkin DC. Cirsoid aneurism of the scalp: with the report of an advanced case. Ann Surg. 1924, 80 (3): 332–340.
3. Fisher-Jeffes ND., Domingo Z., Madden M., et al. Arteriovenous malformations of the scalp. Neurosurgery. 1995, 36 (4): 656–660.
4. Gurkanlar D., Gonul M., Solmaz I., et al. Cirsoid aneurysms of the scalp. Neurosurg Rev. 2006, 29 (3): 208–212.
5. Coleman CC., Hoopes JE. Congenital arteriovenous anomalies of the head and neck. Plast Reconstr Surg. 1971, 47 (4): 354–364.
6. Sofela A., Osunronbi T., Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020, 86 (2): E98–E107.
7. Karki M., Roka YB. Surgical excision of cirsoid aneurysm of the scalp: case series and review of the literature. World Neurosurg. 2021, 155: e600–e604.
8. AlFawaz AA., AlShatti HJ., Safar AH. Surgical management of scalp cirsoid aneurysms: Kuwait experience (case series). Ann Med Surg (Lord). 2022, 76 : 103479.
9. Karsy M., Raheja A., Guan J., et al. Scalp arteriovenous malformation with concomitant, flow-dependent malformation and aneurysm. World Neurosurg. 2016, 90 : 708.e5–708.e9.
10. Costa ND., Engstrom EM., Siqueira SA. Public policy and the institutional role of the Ministry of health in Brazil. Cien Saude Colet. 2017, 22 (5): 1394.
11. Muthukumar N., Rajagopal V., Manoharan AV., et al. Surgical management of cirsoid aneurysms. Acta Neurochir (Wien). 2002, 144 (4): 349–356.
12. Punia P., Shah SB., Malankar TE., et al. Cirsoid aneurysm of occipital artery-rare of the rarest. J Maxillofac Oral Surg. 2022, 21 (3): 995–997.
13. Elkiran YM., Abdelgawwad MS., Abdelmaksoud MA., et al. surgical management of cirsoid aneurysms of the scalp: ten years’ experience. World Neurosurg. 2021, 150: e756–e764.
14. Ung TH, Delcont MR, Colakoglu S, et al. Reconstruction of complex scalp defect after cirsoid aneurysm resection: a multidisciplinary approach. World Neurosurg. 2020, 143 : 190–196.
Rodrigo Inácio Pongeluppi, MD
Division of Neurosurgery, University Hospital, Medical School of Ribeirão Preto, University of São Paulo
716 Bernardino de Campos Street, Ribeirão Preto
São Paulo State
Brazil, ZIP code: 14.015-130
e-mail: rodrigopongeluppi@gmail.com
Submitted: 3. 6. 2023
Accepted: 27. 12. 2023
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2023 Issue 3-4
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
