Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels
Background:
Many HIV-1-infected patients on suppressive antiretroviral therapy (ART) have transiently elevated HIV RNA levels. The clinical significance of these viral blips is uncertain. We have determined the incidence of blips and investigated important associations in the Swedish HIV-cohort.
Methods:
HIV-1-infected ART naïve adults who commenced ART 2007–2013 were retrospectively included. Viral blips were defined as a transient viral load between 50 and 500 copies/mL Subjects not suppressed after six months on ART were excluded.
Results:
Viral blips were found in 76/735 included subjects (10.3 %) and in 90/4449 samples (2.0 %). Median blip viral load was 76 copies/mL (range 56–138). Median follow-up time was 170 weeks (range 97–240). Baseline viral load was higher in subjects with viral blips (median log10 4.85 copies/mL) compared with subjects without blips (median log10 4.55 copies/mL) (p < 0.01). There was a significant association between viral blips and risk for subsequent virological failure (p < 0.001).
Conclusions:
The Swedish national HIV-cohort has a low incidence of viral blips (10 %). Blips were associated with high baseline viral load and an increased risk of subsequent virological failure.
Keywords:
Viral blip, Transient viremia, HIV-1, Antiretroviral therapy
Authors:
Erik Sörstedt 1*; Staffan Nilsson 2; Anders Blaxhult 3; Magnus Gisslén 1; Leo Flamholc 4; Anders Sönnerborg 5,6; Aylin Yilmaz 1
Authors‘ workplace:
Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, 41 90 Gothenburg, Sweden.
1; Department of Mathematical Sciences, Chalmers University of Technology, 12 8 Gothenburg, Sweden.
2; Department of Infectious Diseases, Venhälsan-Södersjukhuset, 118 83 Stockholm, Sweden.
3; Department of Infectious Diseases, Malmö University Hospital, 20 02 Malmö, Sweden.
4; Department of Infectious Diseases, Karolinska Institute, Karolinska University Hospital, 141 8 Stockholm, Sweden.
5; Department of Clinical Microbiology, Karolinska Institute, Karolinska University Hospital, 141 86 Stockholm, Sweden.
6
Published in:
BMC Infectious diseases 2016, 16:305
Category:
Research article
doi:
https://doi.org/10.1186/s12879-016-1628-6
© 2016 The Author(s).
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The electronic version of this article is the complete one and can be found online at: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1628-6
Overview
Background:
Many HIV-1-infected patients on suppressive antiretroviral therapy (ART) have transiently elevated HIV RNA levels. The clinical significance of these viral blips is uncertain. We have determined the incidence of blips and investigated important associations in the Swedish HIV-cohort.
Methods:
HIV-1-infected ART naïve adults who commenced ART 2007–2013 were retrospectively included. Viral blips were defined as a transient viral load between 50 and 500 copies/mL Subjects not suppressed after six months on ART were excluded.
Results:
Viral blips were found in 76/735 included subjects (10.3 %) and in 90/4449 samples (2.0 %). Median blip viral load was 76 copies/mL (range 56–138). Median follow-up time was 170 weeks (range 97–240). Baseline viral load was higher in subjects with viral blips (median log10 4.85 copies/mL) compared with subjects without blips (median log10 4.55 copies/mL) (p < 0.01). There was a significant association between viral blips and risk for subsequent virological failure (p < 0.001).
Conclusions:
The Swedish national HIV-cohort has a low incidence of viral blips (10 %). Blips were associated with high baseline viral load and an increased risk of subsequent virological failure.
Keywords:
Viral blip, Transient viremia, HIV-1, Antiretroviral therapy
Background
Most patients on combination antiretroviral therapy (cART) reach the goal of therapy, HIV RNA < 50 copies/mL blood, within three to six months after initiation of cART [1, 2]. When quantified with more sensitive methods, HIV can usually be detected in low concentrations in almost all adherent patients on effective therapy. The level of this so-called residual viremia has been demonstrated to be between 1 and 10 copies/mL [3, 4, 5].
Even though the plasma viral load is suppressed to < 50 copies/mL in a majority of treated patients, it may increase to detectable levels from time to time, usually to a maximum of 500 copies/mL, before decreasing to < 50 copies/mL again, so called viral blips [6]. There may be several reasons for these viral blips, including technical errors or an influence of the type of blood collection tube used [7]. Other explanations could be stochastic variations [8], conditions that temporarily could lead to increased HIV replication such as infections [9] or vaccinations [10, 11, 12], and low drug concentrations in blood because of poor adherence or poor absorption of antiretroviral drugs. Prior studies have reported blip incidence between 13 and 40 % [13, 14, 15, 16]. The large differences are due to different blip definitions and viral load assays.
Possible consequences of viral blips are not clear. Some studies have found a correlation between viral blips and subsequent virological failure [17, 18, 19, 20], whereas others have not [8, 13, 14, 21, 22, 23, 24, 25, 26]. The aim of this study was to analyse the occurrence of viral blips in Swedish HIV-1 infected individuals with modern cART, and to find out whether any predictive factors could be identified between viral blips and variables such as nadir CD4+ T-cell count, pre-treatment viral load, choice of cART, and some co-morbidities.
Methods
As part of clinical care, > 99 % of HIV-1-infected individuals living in Sweden are followed from diagnosis and onwards using a clinical decision support tool, called InfCare HIV. Clinical data from InfCare HIV is transferred in real time to a research database. All demographic data, all HIV RNA levels, all CD4+ T-cell counts, all ART regimens the patient has ever been on, are registered in the database. In this retrospective study, we included subjects matching our inclusion criteria. Participants had to be HIV-1-infected adults (≥18 years old) receiving their first line of cART and they had to have been on treatment for at least six months with at least one HIV RNA value < 50 copies/mL to secure treatment response before data was collected. The included patients were from five of Sweden’s largest HIV-clinics (the Department of Infectious Diseases at Karolinska University Hospital, Stockholm, Venhälsan at Södersjukhuset, Stockholm, the Departments of Infectious Diseases and Dermatology at Sahlgrenska University Hospital, and the Department of Infectious Diseases at Malmö University Hospital). These five clinics account for approximately two thirds of all HIV-1-infected patients in Sweden. Subjects were followed from August 2007 to December 2013 in two clinics (596 patients), and from June 2009 to December 2013 in the remaining three clinics (139 patients). The different starting dates for inclusion was because the ethylene diamine tetraacetic acid (EDTA) test tubes and the highly sensitive COBAS TaqMan HIV-1 technique (CAP/CTM2; Roche, Molecular Systems, Branchburg, NJ, USA), with a lower detection limit of 40 and 20 copies/mL (version 1 and 2), respectively, were introduced at different time-points at the different clinics. All patients at these clinics are followed according to the Swedish guidelines for treatment of HIV-1 infection. After initiation of cART, plasma HIV RNA is followed after 3 and 6 months, and after that every third to sixth months depending on the patient and the clinician.
Viral blips were defined as transient plasma HIV RNA levels between 50 and 500 copies/mL preceded and followed by HIV RNA < 50 copies/mL. Two values within six weeks apart were interpreted as one isolated blip where the highest HIV RNA level was noted. Patients were excluded if they had a viral failure, defined as a single HIV RNA measurement > 500 copies/mL or two consecutive samples ≥ 50 copies/mL drawn more than six weeks apart. Treatment interruptions of shorter than one month caused a pause in data collection for six months from the reinitiation of ART. If the interruption was longer than one month no more data was analysed after the interruption. If the last registered sample was 50–499 copies/mL this data was excluded to avoid confusion between possible blips and viral failure.
Samples with HIV RNA levels between 20 and 50 copies/mL were registered separately unless they were taken within six weeks from a blip and thus interpreted as part of the transient viremia. Demographic and medical data were collected, and included country of origin, age, gender, source of transmission, ethnic background, date of diagnosis and start of treatment, treating clinic, baseline viral load (VL) sampled within one month before treatment initiation, CD4 nadir, baseline primary drug-resistance mutations, choice of cART, co-infections with hepatitis B and/or C, and the presence or not of AIDS-defining conditions. Treatment regimen was registered for each blood sample during the study period.
Statistical analysis
Mann-Whitney and chi-square tests were used for group comparisons of continuous and categorical data, respectively. Descriptive continuous data are presented as median and interquartile range (IQR) while categorical variables are listed as numbers and percentages unless otherwise stated. Statistical analyses were performed using SPSS (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
To deal with the repeated measurements we primarily used Generalized Estimation Equations (GEE) with a binary logistic link for both univariable and multivariable analysis. The association between blips and HIV RNA measurement in the range between 20 and 50 copies/mL, sometimes referred to as low-level viremia was evaluated by stratifying the subjects for the number of samples below 50 copies/ml and then randomly shuffling the blip indicator within each strata to achieve an empirical distribution from which the significance could be obtained. A similar procedure was used to evaluate the association between failure and blips.
Results
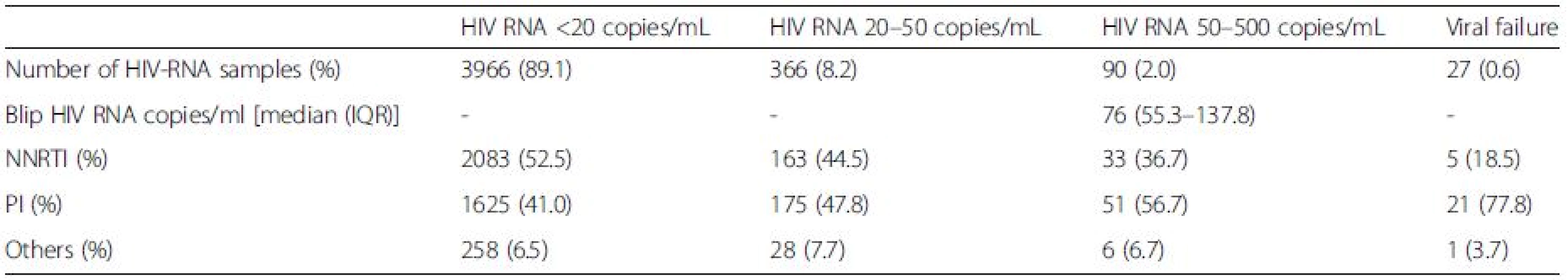
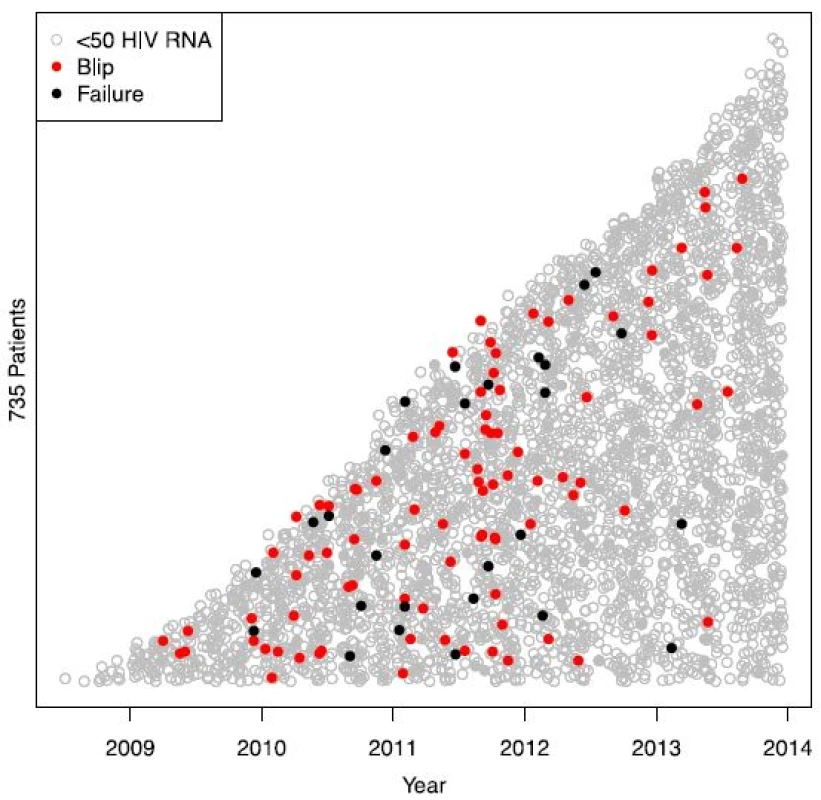
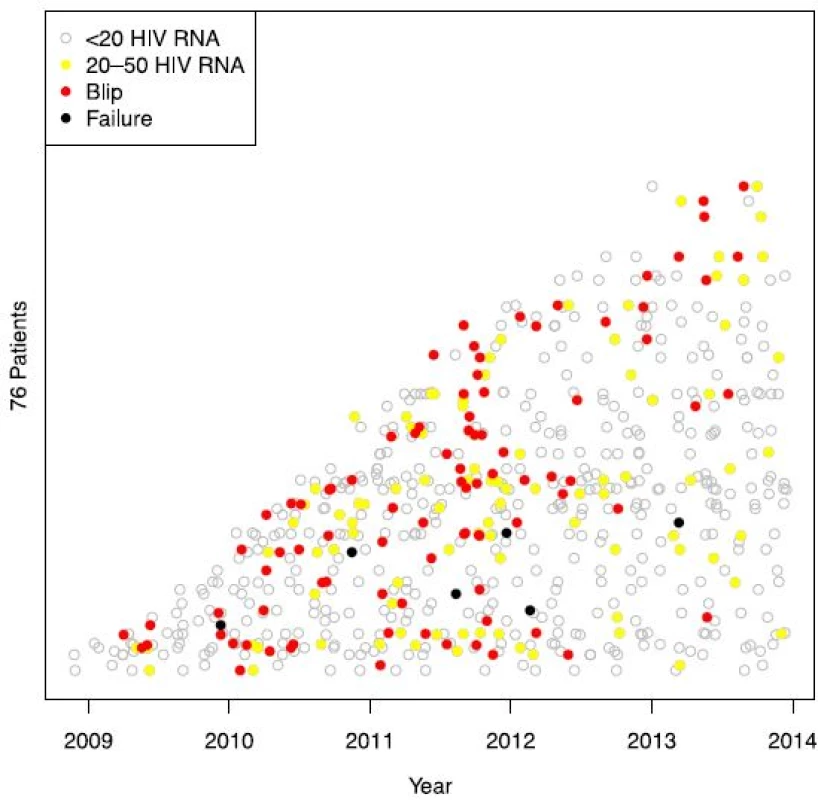
In total, 4449 blood samples from 735 HIV-1-infected individuals were included (515 men and 224 women, ages ranging from 22 to 81 years) (Fig. 1). Median follow-up time was 170 weeks (range 97–240). Both mean and median number of registered blood samples per patient was 6 (range 1–22). Seventy-six out of the 735 patients had at least one episode with transient viremia (Fig. 2), resulting in an incidence of 10.3 %. The majority of subjects (63/76) had one blip, twelve subjects had two blips, and one had three blips. HIV RNA levels during the blips ranged from 50 to 443 copies/mL (median 76). Patient characteristics are shown in Table 1.
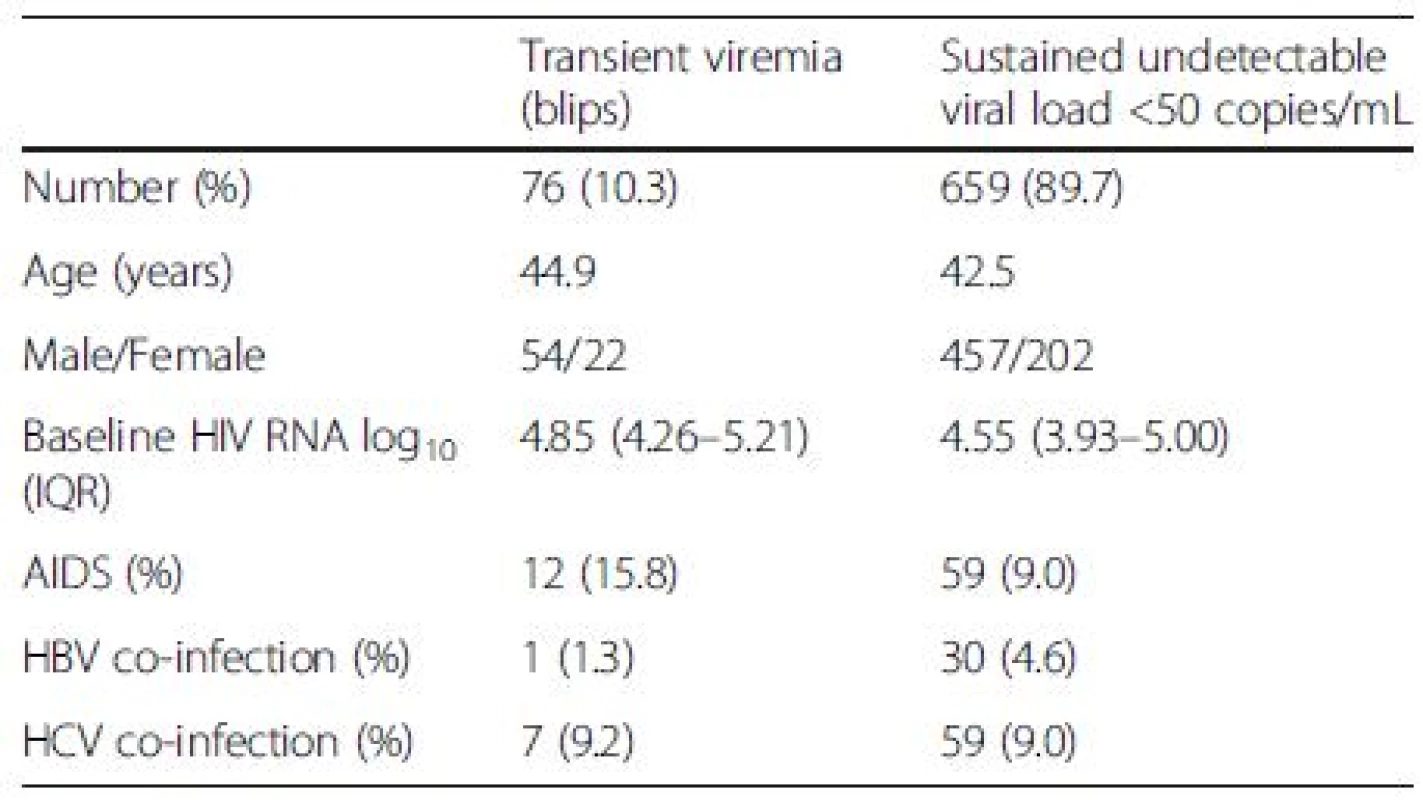
Baseline plasma HIV RNA levels were significantly higher in patients with viral blips than in patients with sustained viral suppression, 4.85 log10 and 4.55 log10 copies/mL, respectively (p < 0.01) (Fig. 3).
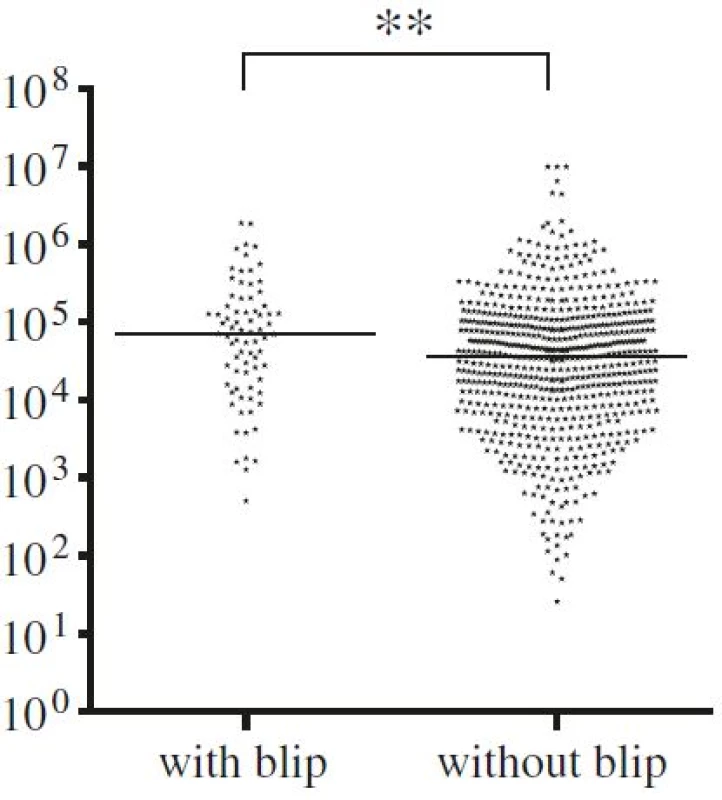
Among the 659 patients who were suppressed during the entire study period, 481 patients (73 %) had a continuous viral load under the detection threshold (HIV RNA <40/20 copies/mL depending on assay version) after the initial six months. Two hundred and thirty-four patients (32 %, 366 samples) had at least one plasma HIV RNA measurement between 20 and 50 copies/mL. A significantly larger percentage of these samples were found in the group with viral blips, 111 out of 603 (18.4 %), compared with 255 out of 3453 (7.4 %) samples collected from suppressed patients (p < 0.0001). Twenty-seven patients developed viral failure, 55.6 % due to consecutive samples > 50 copies/mL more then 6 weeks apart and 44.6 % due to a single HIV RNA > 500 copies/mL. The median HIV RNA among the last group was 1740 (range 662–12900) copies/mL. A significant correlation was observed between viral failure and viral blips (p = 0.005). The 27 patients with HIV RNA > 500 copies/mL or 2 consecutive samples 50–500 copies/mL did not have a significant higher baseline viral load. Twenty-six of the 27 patients with viral failure were subsequently re-suppressed, in 77 % without treatment alteration. One patient was lost to follow-up after leaving the country.
As expected, subjects with transient plasma viremia had been sampled more frequently, in median nine times compared with five times in suppressed subjects (p < 0.0001).
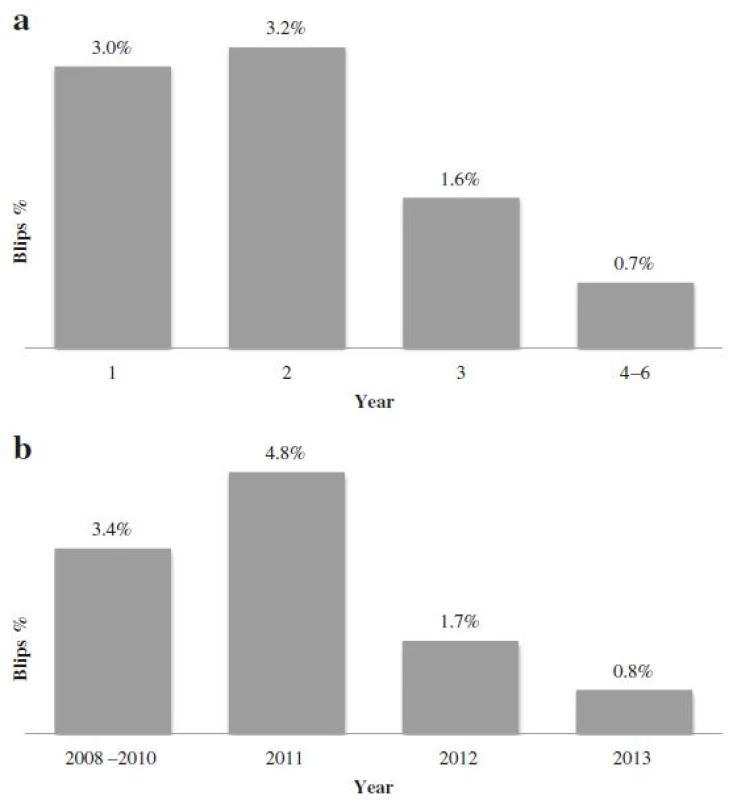
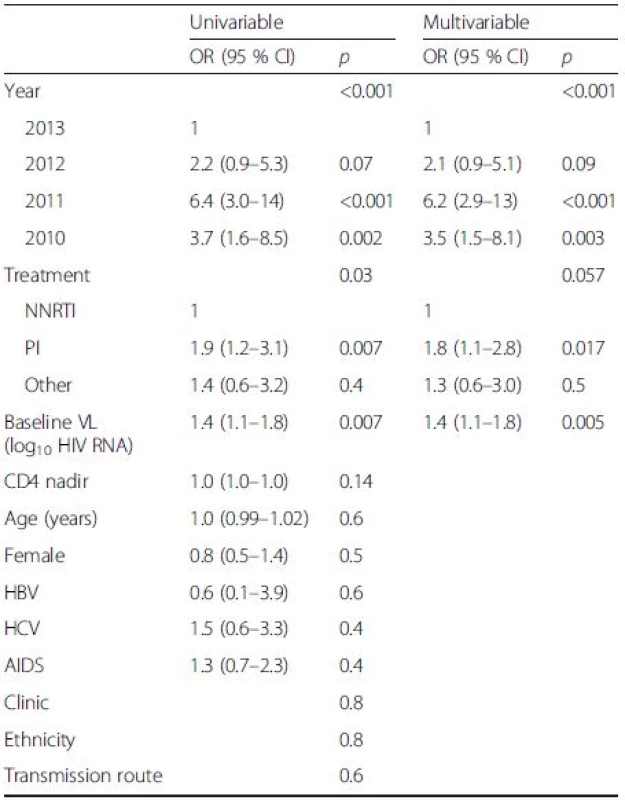
Transient viremia with HIV RNA between 50 and 500 copies/mL was seen in 90 out of 4449 samples (2.0 %). There was a significant difference in blip incidence when comparing different years after ART initiation, with the highest incidence during the second year of observation (Fig. 4a). There was, however, an even more significant difference when comparing calendar years with a peak in 2011 (Fig. 4b). Calendar year and year after initiation are by necessity confounded and when both are entered as predictors in the statistical model only calendar year remains significant (p = 0.0001).
There was no significant difference between subjects with and without blips with respect to sex, AIDS-defining diagnoses, co-infection with hepatitis B and/or C viruses, number of primary drug resistance-associated mutations or where they were treated.
Fifty-one per cent of the whole study population was on a regimen containing a non-nucleoside reverse transcriptase inhibitor (NNRTI) and 42 % on a regimen containing a ritonavir-boosted protease inhibitor (PI/r) (Table 2). Only 0.5 % of the samples were registered while the patients were on a dual NNRTI-PI/r-therapy. As dual NRTI backbone, TDF/FTC was used in 66 % of the registered samples and ABC/3TC in the remaining one third.
The percentage of patients with blips on a PI/r-based regimen was significantly higher than for subjects on a NNRTI-based regimen, 2.8 % and 1.4 %, respectively (p = 0.007 in the logistic model). The same pattern was seen in patients with viral failure where the use of PI/r was significantly more common (p = 0.001).
Variables from univariate analyses with p-values < 0.1 were entered in the multivariable analysis. This analysis showed that year of measurement, choice of treatment regimen, and baseline VL were all predictors for viral blips (Table 3).
Discussion
Viral blips are defined as transient episodes of detectable viremia in patients on suppressive cART within a given timeframe. There is no generally accepted definition of the upper limit of a viral blip. We defined viral blips as plasma HIV RNA between 50 and 500 copies/mL within a time period of six weeks. The upper limit of 500 copies/mL was chosen because viral loads above this level have been associated with treatment failure [19, 27]. Some studies have, however, reported an increased risk of viral failure with transient viremia with a magnitude less than 500 copies/mL [6, 26] and others found no correlation at all between viral failure and the magnitude of the blips [23]. The upper limit of viral blips in various studies varies from 400 to 2000 HIV RNA copies/mL. Viral loads higher than the upper limit defining a viral blip have usually been considered viral failures [28]. In clinical practise, it is of course very important to distinguish between harmless viral blips and potentially harmful virological failure, where the latter most likely will lead to a change in ART regimen.
In addition to the level of viremia when determining what is a blip or not, there is also the time aspect. When elevated viral loads are found in two consecutive samples drawn within a short timeframe, they are most likely part of a single blip [29]. To separate viral blips from emerging virological failure we chose an interval of six weeks to be considered as a solitary blip. We hypothesized that this would be an appropriate time for a blip to emerge and subside.
The different definitions of viral blips and use of different quantification assays make comparisons between studies of viral blips difficult. Thus, when viral blips in the range 50–500 copies/mL were quantified with three different methods (Versant HIV-1 RNA 1.0 kPCR (Siemens), Abbot Realtime HIV-1, and COBAS Ampliprep/COBAS TaqMan HIV-1 v 2.0 (Roche) assays), only 19 % of the detected viral blips could be reproduced with any of the other methods [30].
We found an incidence of viral blips of 10 % in our Swedish cohort. Older studies have found an incidence of 33–40 % using assays with higher quantification limits [13, 14]. In a more recent study where the same quantification method as in our study was used, but with the upper limit of a viral blip defined as 200 copies/mL, the incidence of blips was 38.1 % [15]. If the same definition was applied to our material, only 69 patients (9.4 %) would have had registered blips. Another recent study reported blip incidence of 13 % but since they used different viral load assays a direct comparison is not possible [16]. The differences between our and other results are probably in part due to differences in frequency of plasma viral load sampling, which was not presented in the latter study. More frequent sampling increases the probability of detecting a viral blip and patients with viral blips are in addition more likely to be sampled more often, at least in close proximity to a viral blip. It can therefore be helpful to not only analyse the number of patients with blips, but also the proportion of blood samples containing blips [28]. One study using the older reverse transcriptase PCR assay with 50 copies/mL as lower limit of detection found viral blips in 3.6 % of the blood samples [8]. The same assay was used in another study where they separated patients with primary - from chronic infection and found ratios of 6.0 % and 13.3 %, respectively [31]. The lowest incidences of reported viral blips are between 1.47 % in a high - and 1.64 % in a low/middle-income cohort [32]. It is not possible to draw any conclusions from these results, however, since there is no information about the quantification methods that were used.
Baseline VL was significantly higher in subjects with blips (median 4.85 log10 copies/mL) than in suppressed patients (4.55 log10 copies/mL). This is in accordance with findings obtained by older quantification assays [27, 31, 33] and from a recent study where different, not specified, viral load assays were used [16]. The correlation between high pre-treatment viral loads and viral blips is perhaps not unexpected. It is well known that ART is effective in suppressing but not eliminating HIV-infection. Almost all patients who are on cART have residual low-level viremia, approximately 1–10 HIV RNA copies/mL plasma [4, 5, 34, 35, 36], and the level of residual viremia has been demonstrated to correlate with pre-therapy plasma HIV RNA levels [3,37, 38]. Even in patients who have been on suppressive ART for more than ten years, there is an increased risk of viral blips in those with high baseline HIV RNA levels [39].
There is strong evidence that persistent low level viremia in patients on ART is derived from reservoirs of long-lived virus-producing cells that are not affected by currently available drugs that target new cycles of viral replication [35, 40, 41, 42, 43]. Viral blips were significantly more common in patients on a boosted PI-based regimen than in patients on an NNRTI-based regimen. This is in line with several other reports, but the opposite has also been found [27, 38, 44, 45]. Possible explanations for why viral blips could be more common in patients on PIs are tolerability issues, which may affect adherence and pharmacokinetic aspects including penetration to sanctuary compartments. There can also be a selection bias between PI - and NNRTI-based regimens such that clinicians are inclined to choose a PI-based treatment for patients with advanced disease or where low adherence could be expected. The association between PI and viral blips then serves as a confounder where it is really the non adherence and the secondary possibility of viral replication in sanctuary sites that is the relevant factor.
The origin of viral blips is still uncertain but probably multifactoral. As for low-level residual viremia, it has been hypothesised that blips are caused by virus released from long-lived cell populations. Other theories are that viral blips are caused by continuous viral replication in “sanctuary sites” with suboptimal drug concentrations despite systemic effective ART and latent reservoirs with productively infected CD4+ T cells and macrophages with a very low cell turnover speed that occasionally shed virions [34, 46, 47, 48].
One of the most important questions is whether there are any consequences of viral blips, particularly development of subsequent viral failure. We found a significant association between viral blips and viral failure (p = 0.005) in accordance with another study [49]. Most likely the majority of the detected cases with viral failure in this study represents treatment interruptions due to adherence issues, especially when more then two thirds were subsequently normalised without treatment alteration. We believe that consecutive HIV RNA > 50 copies/mL more likely represent an actual treatment failure than a single HIV RNA > 500 copies/mL. In some cases however the HIV RNA dynamics cause suspicion that the consecutive samples are in fact one single peak despite more than 6 weeks apart. Others with several months between the sampling could be due to recurrent blips where infrequent sampling fail to detect re-suppression between the events.
Blip magnitude > 200 HIV RNA copies/mL in adherent patients has been associated with a higher risk of viral rebound [6]. Although the opposite has been shown in older studies [8, 13, 21, 22, 25, 26] this might be regarded as an indication to monitor patients with viral blips more closely. Since this is not a prospective study, no causality between blips and viral failure can be proven.
Subjects with viral blips in this study also had a significantly higher incidence of HIV RNA levels in the range 20–50 copies/mL compared with those without blips. This might reflect that patients with high baseline HIV RNA levels, and a large viral reservoir, are more prone to release virus from this reservoir now and then, in the range 20–50, as well as in the range 50–500 copies/mL. It might, however, also be due to overall infrequent sampling, reflecting that the values between 20 and 50 copies/mL are actually the start or tail of missed blips. This low-level viremia has no clinical importance that we know today, but some experts advocate that HIV RNA levels less than 20 copies/mL should be the new cART virological goal based on a study where a correlation between low level viremia and virological rebound was found.
Patients initiating ART are more closely monitored during the first year on treatment than later on. Patients with unexpected increases in plasma viral loads are sampled more frequently in order to determine the nature and course of the viremia. Mapping the natural course of a viral blip would require frequent, perhaps daily, blood sampling of patients, which for obvious reasons is not done in clinical practice. In a patient with unexpected viremia, a new viral load is usually checked within one to three months depending on the magnitude of the increase. By the time of the subsequent blood sampling, the viral load is often undetectable again. Blood sampling as often as every 2–3 days of patients with blips has been performed in one study with 10 patients [8]. Nine out of ten participants had at least one blip during the 3–4 months the study was conducted. These results are different from other studies but they represent a limited population from a short observation time and it has been suggested that they represent stochastic statistical and biological variations [28].
Our study has some limitations. First of all, it is a retrospective study and sampling has therefore been based on clinical guidelines and decisions. Patients are asked about adherence at every visit to the clinics, but data on adherence has not been documented in a standardized way and antiretroviral drug concentrations have not been measured. Poor adherence has been associated with more frequent viral blips in one study [25], but not in others [8, 16, 50]. It is not unlikely that patients missing some doses could have suboptimal plasma antiretroviral drug concentrations which could lead to subsequent viral replication, but poor adherence is probably not the only explanation for viral blips as noted in in a recent study from a highly adherent population [16].
The highest percentage of viral blips was found during the second year after ART initiation (3.2 %). In the multivariat analysis the strong correlation between viral blips and year on treatment is caused by calendar year, which was unexpected. A prior study demonstrated that switching from COBAS TaqMan v1.0 to v2.0, as was the case in this study, resulted in a increased number of detected HIV RNA levels that the researchers concluded was the result of increased sensitivity due to reduced underquantification [51]. Upgrading to COBAS Taqman v2.0 thus theoretically would increase the number of reported blips and not the opposite.. PCR master mix batches are known to have some inter-variability but since they are regularly switched and compared to a kit independent control they are unlikely the source of decreasing blip incidence. Treatment guidelines did not undergo any radical changes during these years and there has not been a substantial change in our patient population. One contributing factor to the decreasing frequency of viral blips could be the overall improved treatment results that we see in Sweden and in other countries. In a review, Fung et al showed that studies based on clinical data tended to have more frequent sampling during the first part of the trials with the consequence that possible blips are missed later because of limited testing [28].
Conclusions
The Swedish national HIV-cohort has a low incidence of viral blips (10 %). Blips were associated with high baseline viral load and an increased risk of subsequent virological failure. More data is needed to determine the clinical relevance of this phenomenon and further studies would benefit from harmonised definitions.
Abbreviations
AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load
Acknowledgements
We are thankful to all participating patients and would like to acknowledge all laboratory technicians for their contributions.
Funding
This work was supported by the Swedish Research Council [K2011-58P-20931-01, 2012-3476], the Sahlgrenska University Hospital [ALFGBG-430271] and by grants provided by the Stockholm County Council [20130042].
Availability of data and materials
Datasets will be provided in supporting file.
Authors’ contributions
MG, ES, and AY designed the study, analysed the data and drafted the manuscript. SN assisted in data analysis, performed the statistics, and reviewed the manuscript. AB, LF and AS assisted with recruitment, data analysis, and reviewed the manuscript. All authors have read and have approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
The study was approved by the Ethics Committee in Gothenburg (Dnr: 532-11). The study data was obtained from the quality and research register InfCare HIV and anonymized and de-identified prior to analysis. The patients were informed and gave their approval that data entered into InfCare HIV could be used for research.
Received: 3 March 2016
Accepted: 7 June 2016
Published: 21 June 2016
* Correspondence:
Erik Sörstedt
Department of Infectious Diseases
Institute of Biomedicine
Sahlgrenska Academy
University of Gothenburg
413 90 Gothenburg
Sweden
erik.sorstedt@vgregion.se
Sources
1. Rizzardi GP, De Boer RJ, Hoover S, Tambussi G, Chapuis A, Halkic N, Bart PA, Miller V, Staszewski S, Notermans DW, et al. Predicting the duration of antiviral treatment needed to suppress plasma HIV-1 RNA. J Clin Invest. 2000;105(6):777–82.
2. S Jose LW, S Edwards, et al. The impact of baseline viral load (VL) and time to viral suppression on treatment responses to first-line combination antiretroviral therapy (cART). In: 14th European AIDS Conference (EACS 2013). Brussels; 2013.
3. Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, Kovacs JA, Davey RT, Rock-Kress D, Dewar R, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46.
4. Di Mascio M, Dornadula G, Zhang H, Sullivan J, Xu Y, Kulkosky J, Pomerantz RJ, Perelson AS. In a subset of subjects on highly active antiretroviral therapy, human immunodeficiency virus type 1 RNA in plasma decays from 50 to <5 copies per milliliter, with a half-life of 6 months. J Virol. 2003;77(3):2271–5.
5. Dornadula G, Zhang H, VanUitert B, Stern J, Livornese Jr L, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282(17):1627–32.
6. Young J, Rickenbach M, Calmy A, Bernasconi E, Staehelin C, Schmid P, Cavassini M, Battegay M, Gunthard HF, Bucher HC, et al. Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV Cohort Study. BMC Infect Dis. 2015;15(1):382.
7. Stosor V, Palella Jr FJ, Berzins B, Till M, Leake A, Chmiel JS, Murphy RL. Transient viremia in HIV-infected patients and use of plasma preparation tubes. Clin Infect Dis. 2005;41(11):1671–4.
8. Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J Jr., Gallant JE, Quinn TC, Jackson B, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005; 293(7):817–29.
9. Jones LE, Perelson AS. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull Math Biol. 2005;67(6):1227–51.
10. Gunthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, Hwang J, Haubrich R, Havlir D, Richman DD. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181(2):522–31.
11. Jones LE, Perelson AS. Modeling the effects of vaccination on chronically infected HIV-positive patients. J Acquir Immune Defic Syndr. 2002;31(4):369–77.
12. Kolber MA, Gabr AH, De La Rosa A, Glock JA, Jayaweera D, Miller N, Dickinson GM. Genotypic analysis of plasma HIV-1 RNA after influenza vaccination of patients with previously undetectable viral loads. AIDS. 2002; 16(4):537–42.
13. Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, Hirsch MS, Ignacio C, Condra J, Gunthard HF, et al. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA. 2001;286(2):171–9.
14. Sungkanuparph S, Overton ET, Seyfried W, Groger RK, Fraser VJ, Powderly WG. Intermittent episodes of detectable HIV viremia in patients receiving nonnucleoside reverse-transcriptase inhibitor-based or protease inhibitorbased highly active antiretroviral therapy regimens are equivalent in incidence and prognosis. Clin Infect Dis. 2005;41(9):1326–32.
15. Wojewoda CM, Spahlinger T, Harmon ML, Schnellinger B, Li Q, Dejelo C, Schmotzer C, Zhou L. Comparison of Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test version 2.0 (CAP/CTM v2.0) with other real-time PCR assays in HIV-1 monitoring and follow-up of low-level viral loads. J Virol Methods. 2013;187(1):1–5.
16. Farmer A, Wang X, Ganesan A, Deiss RG, Agan BK, O’Bryan TA, Akers K, Okulicz JF. Factors associated with HIV viral load “blips” and the relationship between self-reported adherence and efavirenz blood levels on blip occurrence: a case-control study. AIDS Res Ther. 2016;13 : 16.
17. Greub G, Cozzi-Lepri A, Ledergerber B, Staszewski S, Perrin L, Miller V, Francioli P, Furrer H, Battegay M, Vernazza P, et al. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16(14):1967–9.
18. Easterbrook PJ, Ives N, Waters A, Mullen J, O’Shea S, Peters B, Gazzard BG. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to < 400 copies/ml. AIDS. 2002;16(11):1521–7.
19. Moore AL, Youle M, Lipman M, Cozzi-Lepri A, Lampe F, Madge S, Nesaratnam S, Tyrer M, Cuthbertson Z, Ransom D, et al. Raised viral load in patients with viral suppression on highly active antiretroviral therapy: transient increase or treatment failure? AIDS. 2002;16(4):615–8.
20. Masquelier B, Pereira E, Peytavin G, Descamps D, Reynes J, Verdon R, Fleury H, Garraffo R, Chene G, Raffi F, et al. Intermittent viremia during first-line, protease inhibitors-containing therapy: significance and relationship with drug resistance. J Clin Virol. 2005;33(1):75–8.
21. Mira JA, Macias J, Nogales C, Fernandez-Rivera J, Garcia-Garcia JA, Ramos A, Pineda JA. Transient rebounds of low-level viraemia among HIV-infected patients under HAART are not associated with virological or immunological failure. Antivir Ther. 2002;7(4):251–6.
22. Sklar PA, Ward DJ, Baker RK, Wood KC, Gafoor Z, Alzola CF, Moorman AC, Holmberg SD. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS. 2002;16(15):2035–41.
23. Raboud JM, Rae S, Woods R, Harris M, Montaner JS. Consecutive rebounds in plasma viral load are associated with virological failure at 52 weeks among HIV-infected patients. AIDS. 2002;16(12):1627–32.
24. Martinez V, Marcelin AG, Morini JP, Deleuze J, Krivine A, Gorin I, Yerly S, Perrin L, Peytavin G, Calvez V, et al. HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2005;19(10):1065–9.
25. Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis. 2007;196(12):1773–8.
26. Garcia-Gasco P, Maida I, Blanco F, Barreiro P, Martin-Carbonero L, Vispo E, Gonzalez-Lahoz J, Soriano V. Episodes of low-level viral rebound in HIVinfected patients on antiretroviral therapy: frequency, predictors and outcome. J Antimicrob Chemother. 2008;61(3):699–704.
27. Grennan JT, Loutfy MR, Su D, Harrigan PR, Cooper C, Klein M, Machouf N, Montaner JS, Rourke S, Tsoukas C, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205(8):1230–8.
28. Fung IC, Gambhir M, van Sighem A, de Wolf F, Garnett GP. The clinical interpretation of viral blips in HIV patients receiving antiviral treatment: are we ready to infer poor adherence? J Acquir Immune Defic Syndr. 2012; 60(1):5–11.
29. Di Mascio M, Percus JK, Percus OE, Markowitz M, Ho DD, Perelson AS. Duration of an intermittent episode of viremia. Bull Math Biol. 2005;67(4):885–900.
30. Ruelle J, Debaisieux L, Vancutsem E, De Bel A, Delforge ML, Pierard D, Goubau P. HIV-1 low-level viraemia assessed with 3 commercial real-time PCR assays show high variability. BMC Infect Dis. 2012;12 : 100.
31. Di Mascio M, Markowitz M, Louie M, Hurley A, Hogan C, Simon V, Follmann D, Ho DD, Perelson AS. Dynamics of intermittent viremia during highly active antiretroviral therapy in patients who initiate therapy during chronic versus acute and early human immunodeficiency virus type 1 infection. J Virol. 2004;78(19):10566–73.
32. Kanapathipillai R, McManus H, Kamarulzaman A, Lim PL, Templeton DJ, Law M, Woolley I. The significance of HIV ‘blips’ in resource-limited settings: is it the same? Analysis of the treat Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD). PLoS One. 2014;9(2):e86122.
33. Goujard C, Bonarek M, Meyer L, Bonnet F, Chaix ML, Deveau C, Sinet M, Galimand J, Delfraissy JF, Venet A, et al. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin Infect Dis. 2006;42(5):709–15.
34. Pomerantz RJ. Reservoirs, sanctuaries, and residual disease: the hiding spots of HIV-1. HIV Clin Trials. 2003;4(2):137–43.
35. Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5.
36. Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999; 340(21):1605–13.
37. Maggiolo F, Callegaro A, Cologni G, Bernardini C, Velenti D, Gregis G, Quinzan G, Soavi L, Iannotti N, Malfatto E, et al. Ultrasensitive assessment of residual low-level HIV viremia in HAART-treated patients and risk of virological failure. J Acquir Immune Defic Syndr. 2012;60(5):473–82.
38. Martin-Blondel G, Saune K, Vu Hai V, Marchou B, Delobel P, Izopet J, Cuzin L, Massip P. Factors associated with a strictly undetectable viral load in HIV-1 - infected patients. HIV Med. 2012;13(9):568–73.
39. Erdbeer G, Sabranski M, Sonntag I, Stoehr A, Horst HA, Plettenberg A, Schewe K, Unger S, Stellbrink HJ, Fenske S, et al. Intermittent viraemia and immune reconstitution in patients with more than 10-15 years of antiretroviral therapy: baseline values still matter. J Int AIDS Soc. 2014;17(4 Suppl 3):19689.
40. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94(24): 13193–7.
41. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997; 278(5341):1295–300.
42. Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008; 197(5):714–20.
43. Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, Choi AL, Girling V, Ho T, Li P, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–61.
44. Doyle T, Smith C, Vitiello P, Cambiano V, Johnson M, Owen A, Phillips AN, Geretti AM. Plasma HIV-1 RNA Detection Below 50 Copies/mL and Risk of Virologic Rebound in Patients Receiving Highly Active Antiretroviral Therapy. Clin Infect Dis. 2012;54(5):724-732.
45. Zoufaly A, Kiepe JG, Hertling S, Hufner A, Degen O, Feldt T, Schmiedel S, Kurowski M, van Lunzen J. Immune activation despite suppressive highly active antiretroviral therapy is associated with higher risk of viral blips in HIV-1-infected individuals. HIV Med. 2014;15(8):449–57.
46. Chun TW, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, Fauci AS. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204(1):135–8.
47. Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10(11):525–31.
48. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–8.
49. Hofstra LM, Mudrikova T, Stam AJ, Otto S, Tesselaar K, Nijhuis M, Wensing AM. Residual viremia is preceding viral blips and persistent low-level viremia in treated HIV-1 patients. PLoS One. 2014;9(10):e110749.
50. Miller LG, Golin CE, Liu H, Hays RD, Hua J, Wenger NS, Kaplan AH. No evidence of an association between transient HIV viremia (“Blips”) and lower adherence to the antiretroviral medication regimen. J Infect Dis. 2004;189(8):1487–96.
51. Sizmann D, Glaubitz J, Simon CO, Goedel S, Buergisser P, Drogan D, Hesse M, Kröh M, Simmler P, Dewald M et al. Improved HIV-1 RNA quantitation by COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0 using a novel dualtarget approach. J Clin Virol. 2010;49(1):41–6.
Labels
Medical virologyArticle was published in
BMC Infectious diseases

2016 Issue 305
Most read in this issue