Secondhand smoke and incidence of dental caries in deciduous teeth among children in Japan: population based retrospective cohort study
Study question:
Does maternal smoking during pregnancy and exposure of infants to tobacco smoke at age 4 months increase the risk of caries in deciduous teeth?
Methods:
Population based retrospective cohort study of 76 920 children born between 2004 and 2010 in Kobe City, Japan who received municipal health check-ups at birth, 4, 9, and 18 months, and 3 years and had information on household smoking status at age 4 months and records of dental examinations at age 18 months and 3 years. Smoking during pregnancy and exposure of infants to secondhand smoke at age 4 months was assessed by standardised parent reported questionnaires. The main outcome measure was the incidence of caries in deciduous teeth, defined as at least one decayed, missing, or filled tooth assessed by qualified dentists without radiographs. Cox regression was used to estimate hazard ratios of exposure to secondhand smoke compared with having no smoker in the family after propensity score adjustment for clinical and lifestyle characteristics.
Study answer and limitations:
Prevalence of household smoking among the 76 920 children was 55.3% (n=42 525), and 6.8% (n=5268) had evidence of exposure to tobacco smoke. A total of 12 729 incidents of dental caries were observed and most were decayed teeth (3 year follow-up rate 91.9%). The risk of caries at age 3 years was 14.0% (no smoker in family), 20.0% (smoking in household but without evidence of exposure to tobacco smoke), and 27.6% (exposure to tobacco smoke). The propensity score adjusted hazard ratios of the two exposure groups compared with having no smoker in the family were 1.46 (95% confidence interval 1.40 to 1.52) and 2.14 (1.99 to 2.29), respectively. The propensity score adjusted hazard ratio between maternal smoking during pregnancy and having no smoker in the family was 1.10 (0.97 to 1.25).
What this study adds:
Exposure to tobacco smoke at 4 months of age was associated with an approximately twofold increased risk of caries, and the risk of caries was also increased among those exposed to household smoking, by 1.5-fold, whereas the effect of maternal smoking during pregnancy was not statistically significant.
Funding, competing interests, data sharing: This study was supported by a grant in aid for scientific research 26860415. The authors have no competing interests or additional data to share.
Authors:
Shiro Tanaka; Maki Shinzawa; Hironobu Tokumasu; Kahori Seto; Sachiko Tanaka; Koji Kawakami
Authors‘ workplace:
Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan
Published in:
BMJ 2015, 351:h5397
Category:
Research
doi:
https://doi.org/10.1136/bmj.h5397
© BMJ Publishing Group Ltd 2015
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bmj.h5397).
Overview
Study question:
Does maternal smoking during pregnancy and exposure of infants to tobacco smoke at age 4 months increase the risk of caries in deciduous teeth?
Methods:
Population based retrospective cohort study of 76 920 children born between 2004 and 2010 in Kobe City, Japan who received municipal health check-ups at birth, 4, 9, and 18 months, and 3 years and had information on household smoking status at age 4 months and records of dental examinations at age 18 months and 3 years. Smoking during pregnancy and exposure of infants to secondhand smoke at age 4 months was assessed by standardised parent reported questionnaires. The main outcome measure was the incidence of caries in deciduous teeth, defined as at least one decayed, missing, or filled tooth assessed by qualified dentists without radiographs. Cox regression was used to estimate hazard ratios of exposure to secondhand smoke compared with having no smoker in the family after propensity score adjustment for clinical and lifestyle characteristics.
Study answer and limitations:
Prevalence of household smoking among the 76 920 children was 55.3% (n=42 525), and 6.8% (n=5268) had evidence of exposure to tobacco smoke. A total of 12 729 incidents of dental caries were observed and most were decayed teeth (3 year follow-up rate 91.9%). The risk of caries at age 3 years was 14.0% (no smoker in family), 20.0% (smoking in household but without evidence of exposure to tobacco smoke), and 27.6% (exposure to tobacco smoke). The propensity score adjusted hazard ratios of the two exposure groups compared with having no smoker in the family were 1.46 (95% confidence interval 1.40 to 1.52) and 2.14 (1.99 to 2.29), respectively. The propensity score adjusted hazard ratio between maternal smoking during pregnancy and having no smoker in the family was 1.10 (0.97 to 1.25).
What this study adds:
Exposure to tobacco smoke at 4 months of age was associated with an approximately twofold increased risk of caries, and the risk of caries was also increased among those exposed to household smoking, by 1.5-fold, whereas the effect of maternal smoking during pregnancy was not statistically significant.
Funding, competing interests, data sharing: This study was supported by a grant in aid for scientific research 26860415. The authors have no competing interests or additional data to share.
Introduction
Dental caries is a continuing problem worldwide. Among all causes of disability adjusted life years evaluated in the Global Burden of Disease 2010 Study, the global prevalence of untreated caries was the highest, with no decreasing trends between 1990 and 2010, and its global burden is ranked 80th.1 In developed countries, the prevalence of caries in deciduous teeth remains high (20.5% in children aged 2 to 5 years in the United States2 and 25.0% in children aged 3 years in Japan),3 and established measures for caries prevention in young children is limited to sugar restriction, oral fluoride supplementation, and fluoride varnish.4
The cause of caries involves various physical, biological, environmental, and lifestyle factors—for example, cariogenic bacteria, inadequate salivary flow, insufficient exposure to fluoride, and poor oral hygiene,5 and the crucial event in the clinical course is the initial acquisition of Streptococcus mutans. However, the efficacy of caries prevention by chlorhexidine, which effectively eliminates S mutans, is inconclusive. Randomised controlled trials in adults and school children have shown that chlorhexidine is not effective, and the American Dental Association does not recommend its use.6 However, a two year randomised controlled trial of 334 preschool children aged 4 and 5 years found a small but significant reduction of dental caries in deciduous teeth with chorhexidine use.7 S mutans is usually transmitted from mothers and possibly from cross infection among children in nursery environments.8 The risk of acquisition is particularly high from 19 to 31 months of age, referred to as a window of infectivity.9 Therefore the effects of preventing or delaying the acquisition of S mutans before or during the window of infectivity remain unknown.
Secondhand smoke may directly influence teeth and microorganisms.10 The adverse effects of secondhand smoke include inflammation of the oral membrane and impaired salivary gland function11 and a decrease in serum vitamin C levels12 as well as immune dysfunction. Children exposed to passive smoking also have lower salivary IgA levels and higher levels of sialic acid with higher activity.12 Sialic acid enhances agglutination of S mutans, leading to the formation of dental plaque and caries.13 In addition to the direct effects of secondhand smoke, inhibition of the morphology and mineralisation of dental hard tissue in the offspring of rats exposed to passive smoking was also reported.14 The global prevalence of those exposed to secondhand smoke is estimated to be 40% of children and more than 30% of non-smokers.15 Cross sectional studies have suggested associations between secondhand smoke and caries in deciduous and permanent teeth,10 16 17 18 but data from cohort studies are limited to the registry of 18 142 teenagers in Sweden.19 In that study, maternal smoking during early pregnancy and exposure to secondhand smoke from mothers were linked to an increased risk of increments in caries during the ages of 13 to 19 years, whereas these associations may be confounded by unmeasured lifestyle factors such as tooth brushing.20 Hence it is still uncertain whether a reduction in the prevalence of exposure to secondhand smoke among children would contribute to caries prevention. We investigated maternal smoking status during pregnancy and before the window of infectivity as risk factors for the incidence of caries in deciduous teeth in a cohort of 76 920 Japanese children, taking lifestyle factors of the children into consideration.
Methods
Settings and study design
The Kobe Offspring Study was designed as a population based retrospective cohort study using records of municipal health check-ups in Kobe City, Japan. In Japan, health check-ups are mandatory for women of childbearing potential and children up to 3 years old according to the Maternal and Child Health Act.21 We had access to deidentified data on health check-ups from 31 March 2004 to 1 April 2014 after approval by the Planning and Coordination Bureau of Kobe.
Kobe City is the sixth largest city in Japan, with a population of about 1.5 million, and is the capital city of Hyogo Prefecture on the southern side of the main island of Japan. According to vital statistics for 2013 there were 90 216 births in Kobe between 2004 and 2010 (see supplementary figure 1). All women of childbearing age and children from pregnancy to 3 years of age residing in Kobe City participated in the health check-up programme. We included children who were born between 2004 and 2010 with available information on associated smoking at age 4 months and records of dental examinations at 18 months and 3 years. In the study protocol, we estimated the cohort size based on the annual number of participants, but the sample size calculation based on statistical considerations was not relevant owing to the retrospective design of the study.
Patient involvement
There was no direct patient involvement in this study. The datasets used for analysis did not include names and identity numbers of citizens.
Measurements
The health check-up programme in Kobe City consisted of completing a standardised pregnancy notification form, neonatal health check-ups, and advice provided during home visits and health check-ups of infants at ages 4, 9, and 18 months and 3 years at healthcare centres of ward offices or designated clinics. Personal and physical data on pregnancy provided by the mother included maternal age at birth, planned and actual date of delivery, height, body weight, occupation, birth order and gestational age of the infant, and multiple births. Personal, physical, and laboratory data from the infant’s birth to 3 years of age included gestational age at birth; abnormalities during pregnancy and at delivery; body weight; height; head and chest circumference; physical, neurological, ophthalmological, and dental examinations; hearing tests; urinary protein level; and occult blood by a dipstick test.
Information on lifestyle factors was based exclusively on information from standardised parent reported questionnaires, which mothers were required to fill out at every health check-up. Exposure to secondhand smoke from pregnancy to 3 years of age was assessed as: maternal smoking during pregnancy (never, former, or current smoker), daily number of cigarettes smoked during pregnancy, presence of smokers in the household during pregnancy, smoking status of parents and family members when the infant was 4 months of age (non-smoker, smoking away from child, or smoking in front of child), and presence of smokers in the family at 9 months, 18 months, and 3 years. Information on third hand smoke was not available. In the current analysis we defined household smoking as smoking by family members in the household when the infant was 4 months old, and we defined exposure to tobacco smoke as smoking by family members in front of the infant at age 4 months. Other lifestyle factors included the number of family members in the household; people involved in parenting and childcare; use of a babysitter or nursery; mental status of the mother, assessed by a picture face scale with five levels from a smile to a tearful face; frequency of alcohol consumption during pregnancy; sleeping hours or sleep duration of the child; dietary habits of the child, such as breast feeding and bottle feeding and frequency of eating sweets and drinking juice; and oral care, such as tooth brushing alone or by parents.
Assessment of dental caries
Qualified dentists assessed the oral conditions of the children at 18 months and 3 years of age through visual examination and not radiography. They classified each tooth into one of seven types: normal, decayed, missing, filled, treated by diammine silver fluoride, observation required, or treated by a dental sealant. We counted teeth treated by diammine silver fluoride as well as decayed teeth as decayed. Incidence of dental caries was defined as the occurrence of at least one decayed, missing, or filled tooth. Other records of dental examinations included the caries activity test (0 to 4 points, 4 points indicating most active), presence of plaque, abnormal conditions of soft tissues and occlusion, and treatment with fluoride varnish.
Statistical analysis
The primary outcome was time to the first incidence of caries in deciduous teeth. Secondary outcomes were the first incidence of caries in mandibular or maxillary anterior teeth or molars and numbers of decayed, missing, or filled teeth at 18 months and 3 years, using the DMF (decayed, missing, filled) index. We used the difference between birth date and the first date of assessment when dental caries was diagnosed as failure time, and the difference between birth date and the last date of assessment (18 months if assessment at 3 years was not done) as censored time. The Kaplan-Meier method was used to estimate the risks of caries at 3 years of age. We expressed the effects of secondhand smoke on the incidence of caries as hazard ratios with 95% confidence intervals, estimated by Cox regression adjusted for a linear term of the propensity score. The proportional hazards assumption was confirmed with log-negative log graphs. We compared the numbers of decayed, missing, or filled teeth using mixed models adjusted for a linear term of the propensity score. For each infant we calculated the propensity score, defined as the conditional probability of a child being exposed to secondhand smoke at 4 months of age given several confounders (see box), using logistic regression and single mean imputation for missing covariates.
Sensitivity analyses
We performed four sensitivity analyses: Cox regression analysis restricted to first born singletons, which accounts for the effects of clustering of children within the same family; Cox regression analysis excluding children with a propensity score below the first centile and above the 99th centile, which ensures strict overlap of propensity scores of different groups; and Cox regression analysis further adjusting for the covariates of number of teeth at 9 months, fluoride varnish treatment at 18 months and 3 years, tooth brushing alone at 18 months and 3 years, tooth brushing by parents at 18 months and 3 years, bottle feeding at 4 months and 9 months, baby food intake at 9 months, age at start of baby food, frequency of eating sweets at 18 months and 3 years, eating sweets irregularly at 18 months and 3 years, and drinking juice every day at 18 months and 3 years, which adjusts for post-exposure covariates as potential confounders; and exponential regression analysis handling the time to event data as interval censored, which accounts for the fact that time to events were not known exactly.
All reported probability values were two sided, and we considered P<0.05 to be statistically significant. An academic statistician conducted all analyses using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
The database of the health check-up programme in Kobe City consisted of records of 145 318 participants in the health check-up programme in Kobe City between 2004 and 2014. We initially identified 82 543 infants born between 2004 and 2010 who received a health check-up at 4 months of age. Information about exposure to smoking at 4 months was available for 82 409 (99.8%) children and the records of a dental examination were available for 76 920 (93.2%) of these children (see supplementary figure 1). Thus the analysis population used for time to event analysis consisted of the 76 920 children. Background characteristics differed significantly for mother’s age, smoking and alcohol consumption, gestational week, and birth weight between those included and excluded in this analysis. The differences were, however, generally small (see supplementary table 1).
Tables 1 and 2 describe the baseline characteristics and lifestyles of the 76 920 children, categorised into three groups according to details of family smoking at age 4 months: family members did not smoke, family members smoked away from the infant; and infant was exposed to secondhand smoke. Prevalence of smoking in the household (family members who smoked when the infant was 4 months old) was 55.3% (42 525/76 920), and most smokers were the fathers (see supplementary table 2). Among them, 5268 (6.8%) children had evidence of exposure to tobacco smoke—that is, at least one family member smoked in their presence. Prevalence of household smoking at age 3 years in the three groups was 4.9%, 68.4%, and 76.2%, respectively (see supplementary table 2). The mothers of children who were exposed to smoking tended to be younger, and around 25% of those whose infants were exposed to secondhand smoke during pregnancy (table 1). Abnormalities at delivery, gestational age, and birth weight did not differ significantly across the three groups (table 1). More than 99% of children received fluoride varnish at 18 months. Four month old children with family members who smoked had their teeth brushed less frequently by themselves or by parents. The frequency of eating sweets was similar across the three groups, but exposure to smoke was associated with higher proportions of bottle feeding, drinking juice every day, and use of a babysitter or nursery (table 2).
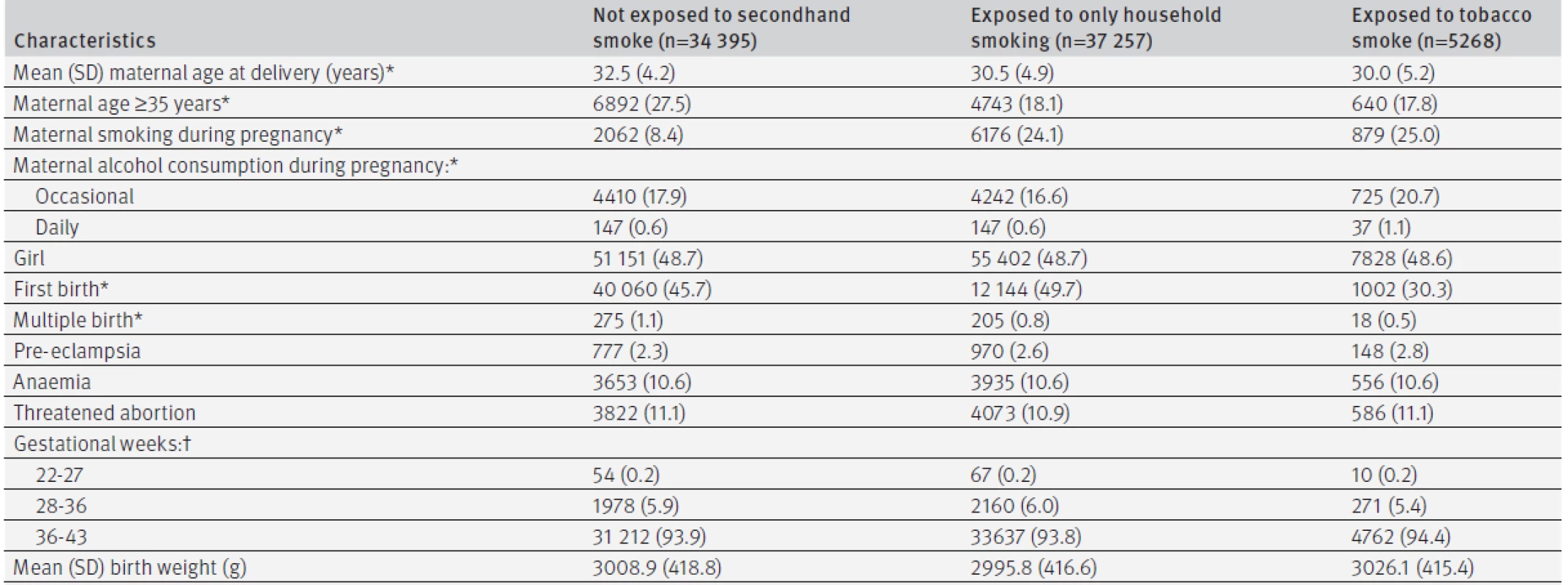
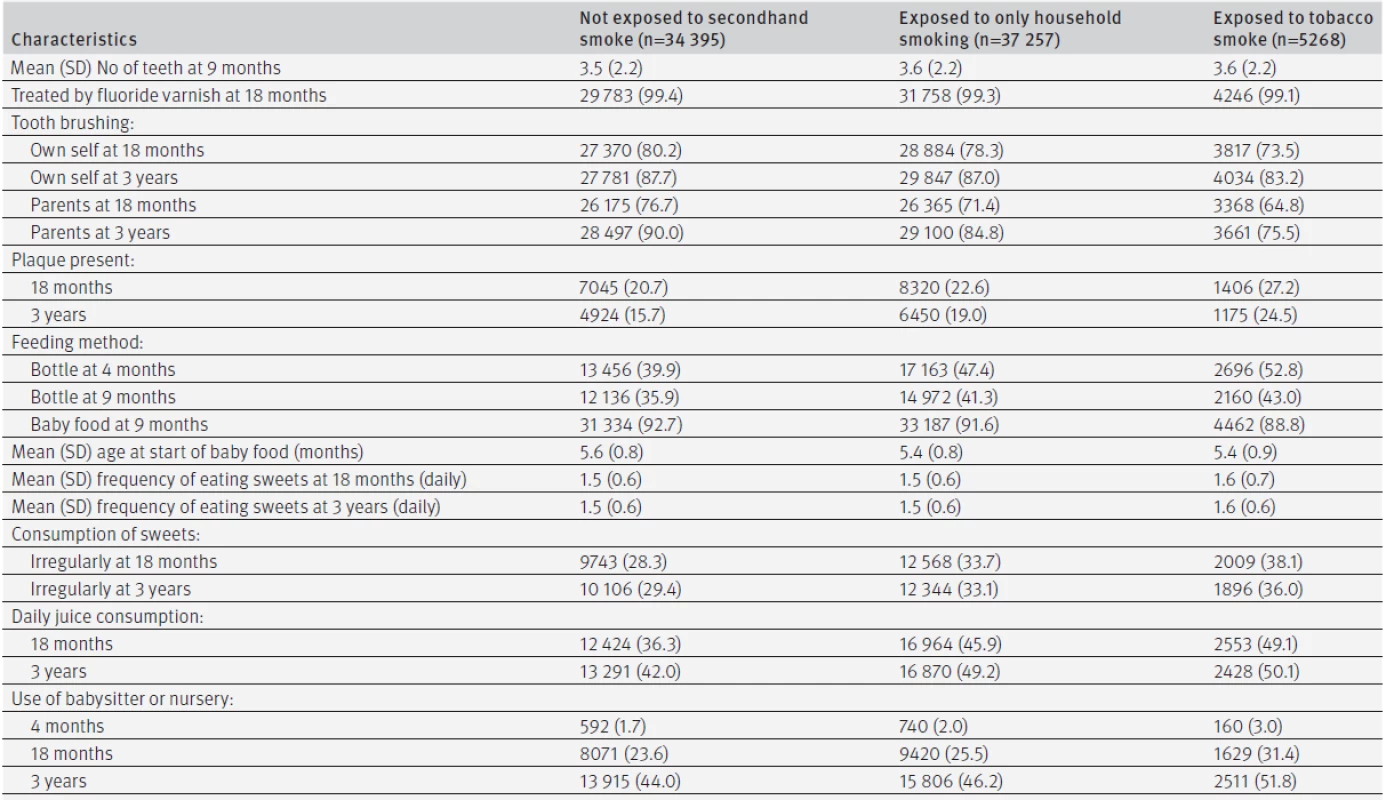
Of the 76 920 children, 70 711 (91.9%) attended a dental examination at 3 years of age. There were significant differences in mother’s age, child’s sex, first born status, and maternal anaemia at delivery between those who were followed for three years and those who were not, including smoking status at four months (see supplementary table 3). Overall, 12 729 cases of dental caries were observed, with 12 579 related to decayed teeth. The mean DMF index (the numbers of decayed, missing, or filled teeth) was 0.06 (2.5 centile: 0, median: 0, 97.5 centile: 0) at age 18 months and 0.61 (2.5 centile: 0, median: 0, 97.5 centile: 6) at age 3 years. Unadjusted three year risks of caries calculated by the Kaplan-Meier method were 18.0% in total and 14.0% for infants in households where no family members smoked, 20.0% when family members smoked away from infants, and 27.6% when infants were exposed to tobacco smoke at age 4 months (table 3). The propensity score adjusted hazard ratios of having family members who smoked away from or in front of children compared with having no smoker in the family were 1.46 (95% confidence interval 1.40 to 1.52, P<0.01) and 2.14 (1.99 to 2.29, P<0.01), respectively. Similar associations were observed for different sites (mandibular or maxillary, anterior teeth or molars). Sensitivity analysis indicated that these associations were robust against the influence of behaviour patterns from the age of 4 months to 3 years. Supplementary table 4 provides propensity score adjusted risk ratios for caries at 18 months and 3 years (that is, analysis as binary outcomes). Children with family members who smoked had significantly more decayed, missing, or filled teeth than those with no smokers in the family. The mean DMF index at 18 months was 0.03 (2.5 centile: 0, median: 0, 97.5 centile: 0) with no family members who smoked, 0.07 (2.5 centile: 0, median: 0, 97.5 centile: 0, P<0.01) with family members who smoked away from infants, and 0.11 (2.5 centile: 0, median: 0, 97.5 centile: 2, P<0.01) with infants exposed to tobacco smoke at age 4 months. The mean DMF index at 3 years in the three groups was 0.44 (2.5 centile: 0, median: 0, 97.5 centile: 5), 0.72 (2.5 centile: 0, median: 0, 97.5 centile: 7, P<0.01), and 1.07 (2.5 centile: 0, median: 0, 97.5 centile: 9, P<0.01), respectively.
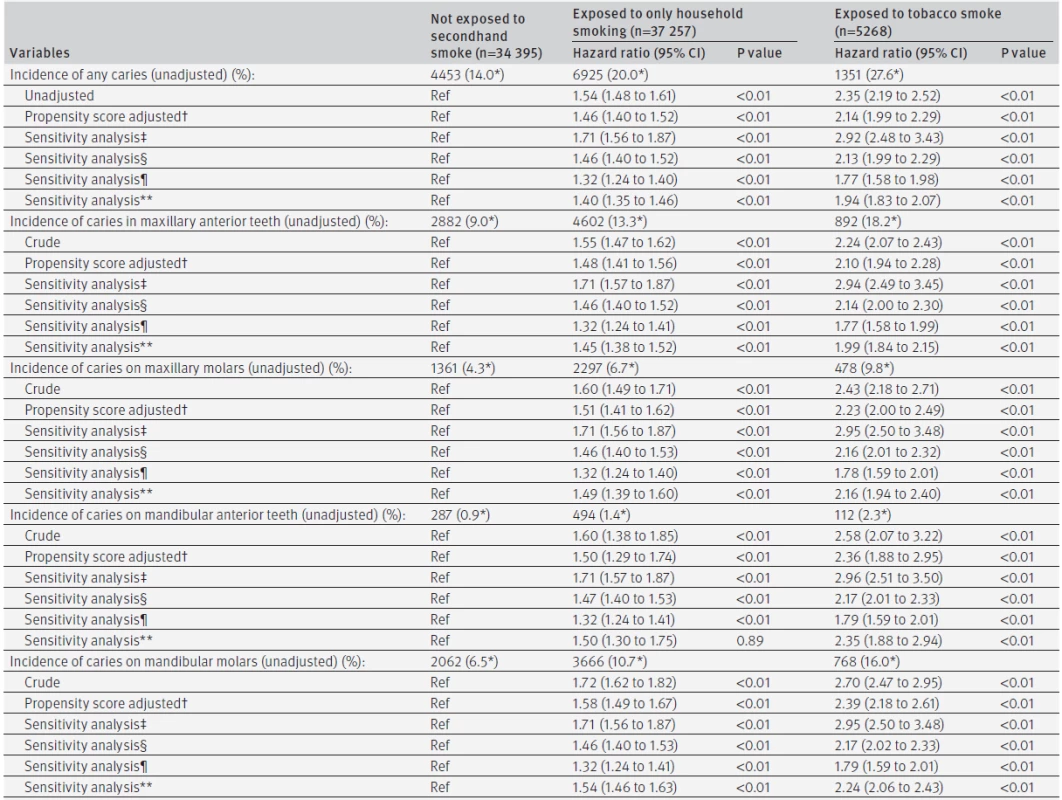
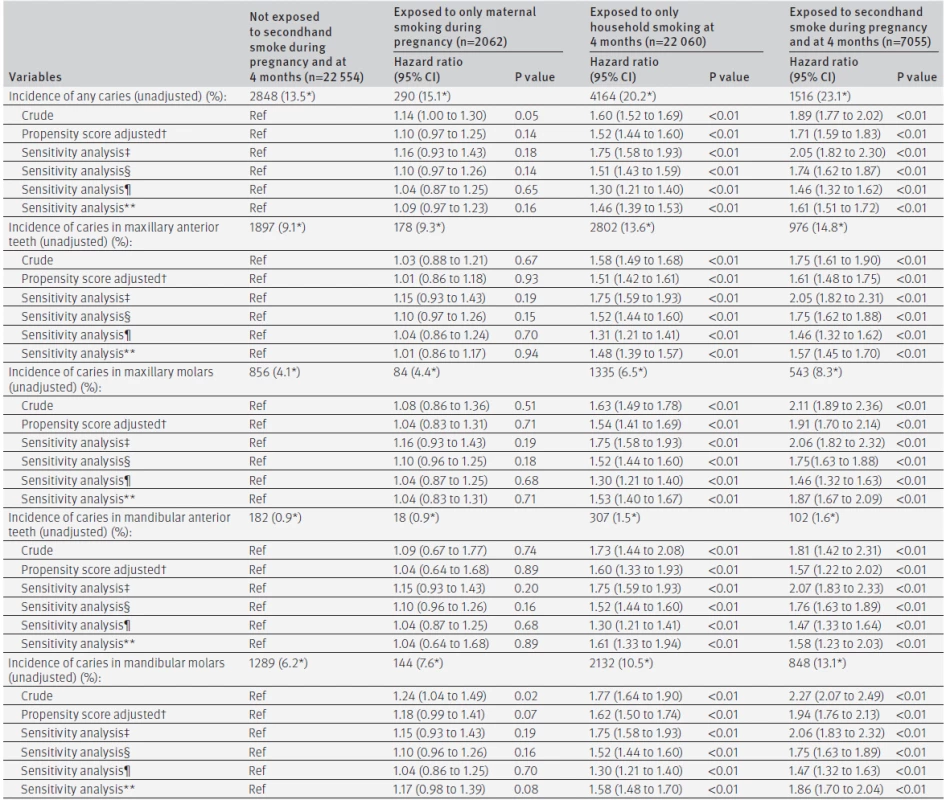
Table 4 shows associations between maternal smoking during pregnancy and incidence of caries. The crude risk of caries among children exposed to maternal smoking during pregnancy was higher than that of those who were not exposed (crude hazard ratio 1.14, 95% confidence interval 1.00 to 1.30, P=0.05), but this association was weakened in the propensity score adjusted analysis (adjusted hazard ratio 1.10, 95% confidence interval 0.97 to 1.25, P=0.14). The mean DMF index at 18 months was 0.04 (2.5 centile: 0, median: 0, 97.5 centile: 0) for infants exposed to secondhand smoke, 0.04 (2.5 centile: 0, median: 0, 97.5 centile: 0, P=0.59) for infants exposed to only maternal smoking during pregnancy, 0.07 (2.5 centile: 0, median: 0, 97.5 centile: 0, P<0.01) for infants exposed to only household smoking at 4 months, and 0.7 (2.5 centile: 0, median: 0, 97.5 centile: 0, P<0.01) for infants exposed to secondhand smoke during pregnancy and at 4 months. The mean DMF index at 3 years was 0.42 (2.5 centile: 0, median: 0, 97.5 centile: 5), 0.46 (2.5 centile: 0, median: 0, 97.5 centile: 8, P=0.74), 0.72 (2.5 centile: 0, median: 0, 97.5 centile: 7, P<0.01), and 0.84 (2.5 centile: 0, median: 0, 97.5 centile: 6, P<0.01), respectively.
Discussion
In this population based retrospective cohort study of 76 920 Japanese children, exposure to tobacco smoke was associated with an approximately twofold increased risk of caries in deciduous teeth. The risk of caries was also increased, by 1.5-fold, among infants exposed to smoking in the household, whereas the effect of maternal smoking during pregnancy was only 1.1-fold. Differences in behaviour patterns were apparent between those exposed to and not exposed to smoking, such as lack of tooth brushing and irregular consumption of sweets. We confirmed our findings through sensitivity analysis using information about behaviour patterns during the ages of 4 months to 3 years, but we cannot completely exclude the possibility of bias due to residual confounding.
Secondhand smoke was operationally defined in previous studies as exposure to smoking by one or both parents or family members, maternal smoking during pregnancy, or high serum cotinine levels. We used three definitions for secondhand smoke—maternal smoking during pregnancy, smoking in the household when the infant was aged 4 months, and exposure to tobacco smoke at age 4 months. Kobe City published guidelines for prevention of secondhand smoke in 2004 and recommended separation of smoking areas at home as well as in the workplaces. In this study, fewer infants at age 4 months were exposed to tobacco smoke than those exposed to smoking in the household, possibly reflecting the wide spread separation of smoking areas at home, but the effects on the risk of caries were significant even for smoking in the household. These findings are consistent with past cross sectional studies in which 10 out of 11 studies found significant positive associations between secondhand smoke and caries of deciduous teeth.10 On the other hand, only a few studies22 23 24 have examined the effects of maternal smoking during pregnancy. Two studies from the National Health and Nutrition Examination Survey reported that the incidence density ratios of maternal smoking during pregnancy were 1.54 (P=0.02)22 and 3.85 (P=0.054)23 among children aged 2 to 5 or 6 years, whereas the prevalence ratio of caries between 3 year old Japanese children with and without exposure to maternal smoking was 1.78 (P<0.05).24 These results are opposite to our findings. However, it is notable that in the National Health and Nutrition Examination Survey (NHNES) the effects of maternal smoking and household smoking may be confounded22 23 because exposure to maternal smoking during pregnancy would be correlated with household smoking after childbirth, which was not handled separately in the NHNES analysis. Other differences in design include availability of data on oral care and dietary habits, which could be important confounders,20 and cross sectional or cohort design. Taken together, further research is needed for a definitive conclusion, although our findings suggest that the effects of maternal smoking during pregnancy are weaker than those of exposure to secondhand smoke after childbirth.
The estimated hazard ratios of exposure to tobacco, around 1.5-fold to twofold higher, are small but may be important from a public health viewpoint. The three year risk of caries in this cohort was 18.0%. This estimate is slightly lower than the averages in the United States2 and Japan,3 and the high utilisation of fluoride varnish, tooth brushing, and dental examinations may have contributed to the reduction in risk of caries. However, more than half of the children in this cohort had family members who smoked, and most smokers were their fathers. These results can be considered representative of children in large cities in Japan, given the high participation rate in this study. Indeed, exposure to secondhand smoke is widespread among children worldwide, at a rate of 40%, which is higher than any other age categories.15 The associations between secondhand smoke and risk of caries would support extending public health and clinical interventions to reduce secondhand smoke. For example, education on the harm of secondhand smoke might increase if dentists became aware of the risk of caries due to secondhand smoke as well as tobacco consumption of their clients. However, further investigation is necessary to conclude whether a smoking prevention programme would reduce the risks of caries, since the size of effects of secondhand smoke was not large. Propensity score analysis allowed adjustment for confounders in this study, but residual bias due to unmeasured confounders, although potentially small, cannot be ruled out.
Limitations of this study
These findings must be interpreted in the context of study limitations. Firstly, information on smoking status was obtained by questionnaires completed by mothers, and biomarkers such as serum cotinine levels were not available in this study. In particular, the prevalence of maternal smoking during pregnancy may be underreported. It is also difficult in an epidemiological study to separate the effects of secondhand smoke from those of third hand smoke—the residual contamination from tobacco smoke that remains on a variety of indoor surfaces. Secondly, oral conditions were not necessarily assessed by paediatric dentistry. Thirdly, as we carried out an observational study rather than a randomised trial, it is impossible to establish causality. In addition to the possibility of unmeasured confounders, we cannot entirely exclude the potential of bias owing to missing covariates. We calculated the propensity score with the use of single imputation, but multiple imputation outperforms single imputation theoretically. However, we expect that it would not make much difference in this situation. Fourthly, the portion of children exposed to smoke only during pregnancy was relatively small and therefore the non-significant results for maternal smoking may be due to low statistical power to detect a small effect. Finally, given the substantial variability in the prevalence of caries, exposure to secondhand smoke, and lifestyle across countries, our results may not be generally applicable to populations with different environmental and lifestyle factors. For example, fluoridation of water in the community has not been carried out in Japan since 1972, although fluoride varnish (table 2) and fluoride toothpaste is common. Furthermore, sugar intake for each person also varies across countries (for example, 48 g/day in Japan, 84 g/day in the US, and 107 g/day in Britain in 2011).25
Conclusion
Exposure to secondhand smoke at 4 months of age, which is experienced by half of all children of that age in Kobe City, Japan, is associated with an increased risk of caries in deciduous teeth. Although these findings cannot establish causality, they support extending public health and clinical interventions to reduce secondhand smoke.
We thank the Child and Family Bureau and Public Health and Welfare Bureau of Kobe City for providing the health check-up data and advice; C Wilunda and C Hongyan (Kyoto University) for their advice; and K Fujii (Kyoto University) for secretarial assistance.
Contributors
ShT performed statistical analysis and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MS, HT, and KK contributed to the design and conduct of the study. SK and SaT contributed to the writing of the manuscript. KK is the principal investigator and the guarantor of the study. The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Funding
This study was supported by a grant in aid for scientific research 26860415.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
This study was exempt from obtaining individual informed consent based on the Ethical Guidelines for Epidemiological Research by Ministry of Health, Labour, and Welfare. The study protocol was approved by the Ethics Committee, Kyoto University Graduate School and Faulty of Medicine (E2045). We managed the data based on the Act of Personal Information Protection in Kobe City and take responsibility for their integrity.
Data sharing
No additional data available.
Transparency
The lead author (KK) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Accepted 22 September 2015
Correspondence to:
K Kawakami
kawakami.koji.4e@kyoto-u.ac.jp
Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University, Yoshida-Konoe-cho, Sakyo-ku, Kyoto 606-8501, Japan
Sources
1 Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res 2013;92 : 592-7.
2 Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 2007;(248):1-92.
3 Ministry of Health, Labour and Welfare. Survey of dental diseases 2011. www.mhlw.go.jp/toukei/list/62-23.html.
4 Chou R, Cantor A, Zakher B, Mitchell JP, Pappas M. Preventing dental caries in children <5 years: systematic review updating USPSTF recommendation. Pediatrics 2013;132 : 332-50.
5 Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369 : 51-9.
6 Rethman MP, Beltrán-Aguilar ED, Billings RJ, et al.; American Dental Association Council on Scientific Affairs Expert Panel on Nonfluoride Caries-Preventive Agents. Nonfluoride caries-preventive agents: executive summary of evidence-based clinical recommendations. J Am Dent Assoc 2011;142 : 1065-71.
7 Du MQ, Tai BJ, Jiang H, Lo EC, Fan MW, Bian Z. A two-year randomized clinical trial of chlorhexidine varnish on dental caries in Chinese preschool children. J Dent Res 2006;85 : 557-9.
8 Alves AC, Nogueira RD, Stipp RN, et al. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol 2009;58(Pt 4):476-81.
9 Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 1993;72 : 37-45.
10 Hanioka T, Ojima M, Tanaka K, Yamamoto M. Does secondhand smoke affect the development of dental caries in children? A systematic review. Int J Environ Res Public Health 2011;8 : 1503-19.
11 Strauss RS. Environmental tobacco smoke and serum vitamin C levels in children. Pediatrics 2001;107 : 540-2.
12 Avşar A, Darka O, Bodrumlu EH, Bek Y. Evaluation of the relationship between passive smoking and salivary electrolytes, protein, secretory IgA, sialic acid and amylase in young children. Arch Oral Biol 2009;54 : 457-63.
13 Dong Q, Wu H, Dong G, Lou B, Yang L, Zhang L. The morphology and mineralization of dental hard tissue in the offspring of passive smoking rats. Arch Oral Biol 2011;56 : 1005-13.
14 Levine MJ, Hertzberg MC, Levine MS, Ellison AS, Stinson MW, Li HC. Specificity of salivary bacterial interactions: role of terminal sialic acid residues in the interactions of salivary glycoproteins with streptococcus sanguis and streptococcus mutans. Infect Immun 1978;19 : 107-15.
15 Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377 : 139-46.
16 Aligne CA, Moss ME, Auinger P, Weitzman M. Association of pediatric dental caries with Second-hand smoking. JAMA 2003;289 : 1258-64.
17 Majorana A, Cagetti MG, Bardellini E, et al. Feeding and smoking habits as cumulative risk factors for early childhood caries in toddlers, after adjustment for several behavioral determinants: a retrospective study. BMC Pediatr 2014;14 : 45.
18 Nakayama Y, Mori M. Association of environmental tobacco smoke and snacking habits with the risk of early childhood caries among 3-year-old Japanese children. J Public Health Dent 2015;75 : 157-62.
19 Julihn A, Ekbom A, Modéer T. Maternal overweight and smoking: prenatal risk factors for caries development in offspring during the teenage period. Eur J Epidemiol 2009;24 : 753-62.
20 Alm A, Wendt LK, Koch G, Birkhed D. Oral hygiene and parent-related factors during early childhood in relation to approximal caries at 15 years of age. Caries Res 2008;42 : 28-36.
21 Ministry of Health, Labour and Welfare. The Maternal and Child Health Act 2014. http://law.e-gov.go.jp/htmldata/S40/S40HO141.html.
22 Iida H, Auinger P, Billings RJ, Weitzman M. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics 2007;120:e944-52.
23 Shulman JD. Is there an association between low birth weight and caries in the primary dentition? Caries Res 2005;39 : 161-7.
24 Tanaka K, Miyake Y, Sasaki S. The effect of maternal smoking during pregnancy and postnatal household smoking on dental caries in young children. J Pediatr 2009;155 : 410-5.
25 Food and Agriculture Organization of the United Nation. FAOSTAT 2011. http://faostat.fao.org/site/609/default.aspx#ancor.
Labels
Dental medicineArticle was published in
BMJ

2015 Issue h5397
- What Effect Can Be Expected from Limosilactobacillus reuteri in Mucositis and Peri-Implantitis?
- The Importance of Limosilactobacillus reuteri in Administration to Diabetics with Gingivitis
Most read in this issue


