In vitro Evaluation of the Permeation of Cytotoxic Drugs through Reconstructed Human Epidermis and Oral Epithelium
In vitro hodnocení prostupnosti cytotoxických léčiv přes rekonstruovanou lidskou epidermis a ústní epitel
Východiska:
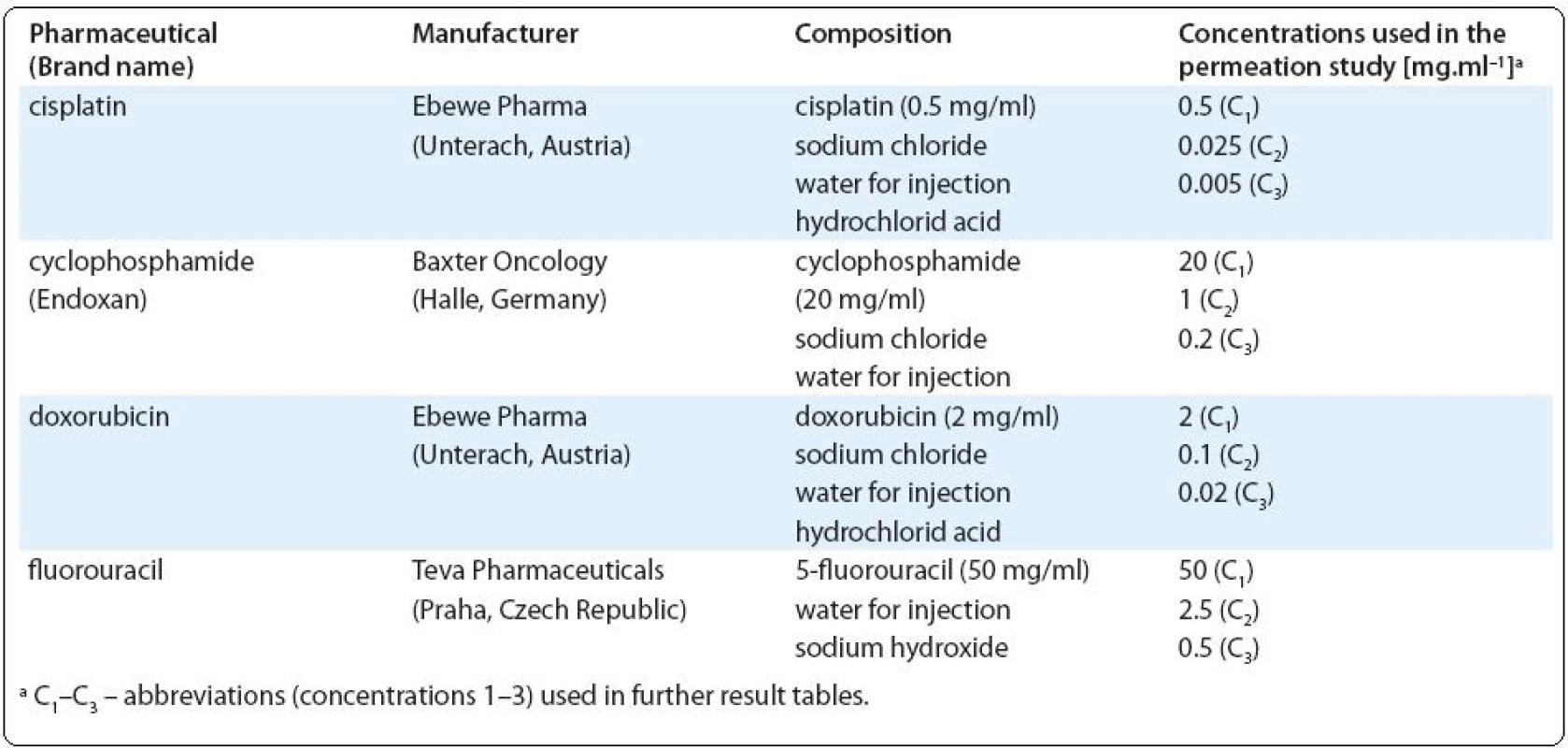
Ačkoli je známo, že profesní expozice cytotoxickým léčivům může mít za následek negativní ovlivnění zdravotního stavu zdravotnického personálu, způsob příjmu těchto látek dosud nebyl dostatečně objasněn. Hlavním cílem této studie bylo stanovit prostupnost čtyř často užívaných cytostatik (cisplatiny, cyklofosfamidu, doxorubicinu a fluorouracilu) přes epidermis a orální epitel.
Materiál a metody:
Experimenty byly provedeny s rekonstruovanými modely uvedených tkání a za podmínek napodobujících reálné expoziční situace (doba trvání 6 hod., tři koncentrace odpovídající manipulovaným roztokům). Množství léčiv, které prostoupilo zkoušenými tkáněmi do receptorového media, bylo stanovováno pomocí ultra účinné kapalinové chromatografie s fotospektrometrickou detekcí.
Výsledky:
Nejvyšší propustnost epidermu (P = 0,2 × 10–3– 1,5 × 10–3 cm.h–1) byla sledována u tří nejvíce polárních léčiv (cisplatina, cyklofosfamid, fluorouracil). Propustnost epidermu pro více hydrofobní doxorubicin byla zřetelně nižší (Pmax = 0,03 × 10–3 cm.h–1). Pro ústní epitel byla dle očekávání zjištěna mnohem vyšší propustnost než u epidermu s maximálními hodnotami naměřenými u cisplatiny a fluorouracilu (P = 180 × 10–3 cm.h–1). Histologické vyšetření exponovaných tkání objevilo především u orálního epitelu četné cytotoxické efekty.
Závěr:
Ačkoliv u epidermu krytého keratinozní vrstvou (stratum corneum) byla zjištěna relativně nízká propustnost a citlivost k toxickému působení, absorpci cytostatik nelze vyloučit ani u jednoho typu hodnocených tkání. Získané výsledky představují výchozí informace pro další práce zabývající se modelováním profesních expozic a hodnocením zdravotních rizik.
Klíčová slova:
absorpce – in vitro – epidermis – ústní sliznice – cytostatika – profesní expozice
Tato práce byla podpořena výzkumnými projekty MŠMT ČR „CYTO“ (projekt č. 2B06171) a INCHEMBIOL (VZ0021622412). Na AAS analýzách cisplatiny spolupracoval výzkumný tým s pracovníky společnosti Pliva-Lachema.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Authors:
P. Odraska 1,2; E. Mazurova 2; L. Dolezalova 1; L. Bláha 1,2
Authors‘ workplace:
Masaryk Memorial Cancer Institute, Hospital Pharmacy, Brno, Czech Republic
1; Faculty of Science, Masaryk University, RECETOX (Research Centre for Environmental Chemistry and Ecotoxicology), Brno, Czech Republic
2
Published in:
Klin Onkol 2011; 24(3): 195-202
Category:
Original Articles
Overview
Backgrounds:
Occupational exposure to antineoplastic agents may represent a risk to health care workers, although the relevance of different exposure routes is not fully understood. The objectives of this study were to determine in vitro permeation of four widely used cytotoxic drugs (cisplatin, cyclophosphamide, doxorubicin, and fluorouracil) through two reconstituted tissue models representing human skin epidermis and oral mucosa.
Materials and Methods:
Experiments were conducted with reconstructed models of human epidermis and oral epithelium, cultured in a chemically-defined medium under conditions simulating possible exposure scenarios (6 h duration, three concentrations corresponding to commonly used application doses). The amounts of drugs permeated through the tissues into the receptor media were determined using ultra performance liquid chromatography with photospectrometric detection.
Results:
The highest epidermis permeations (P = 0.2 × 10–3 – 1.5 × 10–3 cm.h–1) were observed with three polar drugs (cisplatin, cyclophosphamide and fluorouracil), while permeation by more hydrophobic doxorubicin was minor (Pmax = 0.03 × 10–3 cm.h–1). As expected, more pronounced tissue permeation was observed with the reconstructed oral epithelium having the maximum permeability coefficient (P = 180 × 10–3cm.h–1) for cisplatin and fluorouracil. Histological evaluation of the exposed tissues revealed cytotoxic effects at higher doses, especially for oral epithelium.
Conclusion:
Although the skin epidermis with keratinised stratum corneum provided relatively good protection, uptake (of at least some investigated drugs) via both types of tissue should not be underestimated. Our results provide basic experimental data on the skin and oral epithelia permeation for further modelling of exposure and health risk assessment.
Key words:
absorption – in vitro – epidermis – oral mucosa – antineoplastic agents – occupational exposure
Introduction
Health risks resulting from the occupational exposures to antineoplastic drugs with mutagenic, carcinogenic and teratogenic potencies were discussed and documented in numerous studies [1–7]. Correspondingly, detectable concentrations of cytotoxic drugs were reported from hospital samples including air [8–10], various working surfaces and floors [8,11–15] as well as other materials such as cloths, linens, external packages of the drugs etc. [15–17]. All these types of contamination may represent an exposure for health care workers including pharmacists, physicians, nurses and sanitary staff [10,13,18] which was repeatedly confirmed by biological monitoring of some cytotoxic drugs such as cyclophosphamide and fluorouracil and/or their metabolites in urine of hospital workers [9,19–22].
Respiration of the contaminated air is one of the exposure routes but sampling and analyses of the air may be complicated and require highly sensitive analytical methods. Available studies reported that air contamination by cytotoxic drugs is not common, although relatively high concentrations may be detected (concentration of airborne cyclophosphamide in the drug preparation areas reached up to 10–13 µg. m–3 [8,10]).
Another major exposure route stems from the surface contamination. Compounds may be transferred to workers and taken up directly via skin or indirectly via secondarily contaminated food and other unintentional hand-to-mouth contact [23]. Dermal exposure can not be ruled out even when the workers use personal protective equipment like gowns and gloves, since their resistance to permeation of cytotoxic drugs was shown to be limited [24–26]. Several studies highlighted the dermal contact as the main exposure route for cytotoxic drugs [10,13,21,27].
Interestingly, there is only scarce information on the potencies of cytotoxic drugs to permeate skin, which is an important barrier for foreign chemical agents [28]. Nowadays, percutaneous permeation assays may help understand efficiency of topical administration and similar assays were also used in the studies of toxic chemicals [29–31].
The permeation of the skin and epithelial tissues can be studied by different approaches including in vivo experiments with laboratory animals [32] or freshly excised skin [33]. Assays with reconstructed tissues cultured in vitro have also been used [34]. Reconstructed tissues consist of normal human cells cultured on an inert polycarbonate filter at the air-liquid interface with the chemically defined medium [35], and they were used in the tissue corrosion and irritation tests as so as in the tissue permeation studies [34,36].
In the present study we report results of our experiments with four widely used cytotoxic drugs (cisplatin, cyclophosphamide, doxorubicin and 5-fluorouracil) that focused on the permeation through two types of reconstructed human tissues: reconstructed human epidermis (RHE) and human oral epithelium (HOE). Besides the primary characterization of cytotoxicity using human HaCaT keratinocytes, we have characterized the permeation kinetics up to 6 hours at three different concentrations selected with respect to the drug concentrations prepared and used in therapeutic regimens. Our study brings new insights into the toxicokinetics of the cytotoxic drugs and the results may further be used in modelling of the internal exposure doses, which is the critical step for the comprehensive risk assessment of hazardous drugs.
Materials and Methods
Chemicals. Experiments were performed with the brand name drugs provided by the local hospital pharmacy, characterization of the drug preparations is presented in Tab. 1. General reagents used for the chromatography were of analytical grade. Acetonitrile of the ULC-MS grade was used (Biosolve B.V., Valkenswaard, Netherlands). Ultrapure water was obtained from the Milli-Q system (Millipore, Bedford, Mass., USA). Phosphoric acid and potassium phosphate monobasic were of HPLC grade and were purchased from Sigma-Aldrich. Denatured ethanol with hematoxylin and eosin for histological analyses were purchased from Carl Roth GmbH & Co (Karlsruhe, Germany). Paraffin wax was from EMS (Fort Washington, PA, USA).
Cytotoxicity study. Prior to the tissue permeation studies, we investigated cytotoxic effects of individual drugs to the human keratinocyte cell line HaCaT using the neutral red uptake assay as described in [37]. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal calf serum (Mycoplex, PAA, Austria) at 37 °C in a humidified atmosphere of 5% CO2. For experiments, cells were seeded into the 96-well microplates (10,000 cells per well), incubated overnight and then exposed in three replicates to the dilutions of tested drugs for 3, 6 and 24 h. Neutral red (0.5 mg/mL culture media) was then added to each well and the microplate was incubated for 1h. Medium was removed and cells lysed with 1% acetic acid in 50% ethanol and absorbance at 570 nm was measured (only viable cells accumulated neutral red).
Tissue models. RHE and HOE were provided by SkinEthic Laboratories (Nice, France). Upon receipt of the tissues (grown in the 24 well plate format), culture inserts were removed from the nutrient gel and transferred under aseptic conditions into a new sterile 24 well plate (Corning Inc., Corning, N.Y., USA) containing 1.5 ml of a maintenance medium provided by the manufacturer [38]. Tissues were then incubated at 37 °C in a humidified atmosphere of 5% CO2. After 48 h, the maintenance medium at the bottom of the reconstituted tissues was replaced by an assay (receptor) medium (phosphate buffered saline, PBS, 1.4 ml, pH 7.3) and the permeation tests were conducted.
Permeation studies. Drugs were applied in the form of the original solution as used during drug preparation and in two lower concentrations (20-fold and 100-fold diluted solutions prepared in the PBS; for actual concentrations see Tab. 1). In order to ensure stable experimental conditions, the drugs were applied in infinite doses (100 μl per 0.33 cm2 of tissue surface) and the experiments were performed at 37 °C in a humidified atmosphere of 5% CO2. At selected time points (15, 30, 60, 120, 240, 360 min) aliquots (100 μl) of the receptor medium were collected and analyzed for the studied compounds by the ultra-performance liquid chromatography (UPLC) or atomic absorption spectrometry (AAS).
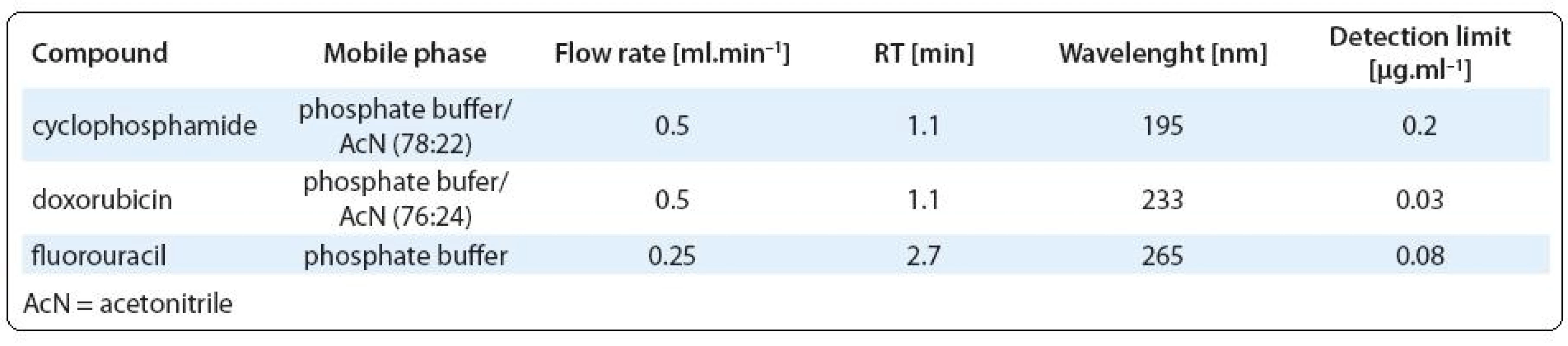
Chemical analyses. Determination of 5-fluorouracil, cyclophosphamide and doxorubicin was performed using Acquity UPLC system (Waters, Milford, MA) equipped with photodiode array detector and C18-reverse-phase column (BEH C18, 1.7 µm, 2.1 × 50 mm). Separation of each analyte was realised isocratically with mobile phase consisted of 7 mM phosphate buffer (pH = 4) and acetonitrile. The column temperature was 40 °C and the injection volume was 10 µl. Compounds were identified according to their retention time and quantifications were based on external standard calibrations. Chromatographic conditions for each analyte (mobile phase composition, flow rate, wavelength and analytical detection limits) are shown in Tab. 2. The limits of detection (LOD) and quantification (LOQ) were determined as the three-fold and ten-fold standard deviation, respectively, of the concentrations measured in blanks of diluted receptor medium (PBS). Cisplatin was determined by electrothermal atomic absorption spectrometry (Perkin-Elmer 3030/HGA 500) at 265.9 nm (bandwith 0.7 nm). Prior to analyses, samples were diluted 1:4 (v/v) with MilliQ water. Samples (25 µl) were injected in pyrolytic graphite furnace. The LOD for analysis of cisplatin was 0.015 µg. ml–1.
Histology. Reconstructed tissues (oral epithelium and skin epidermis) were histologically investigated in all experimental treatments. The samples were fixed in 2.5% glutardialdehyde dissolved in 0.1 M cacodylate buffer (pH 7.2). After wash, the samples were dehydrated in a graded series of ethanol (solutions of 70% – 80% – 90% and 96% ethanol) and embedded in paraffin wax. The sections of 4-micrometer thickness were cut with rotary microtome HM 360 (Zeiss, Germany). The sections were stained with haematoxylin and eosin following the staining protocol described in [39]. The histopathological changes were examined under the light microscope (Zeiss, Axioscope 2, Oberkochen, Germany) and evaluated (the thickness of proliferating layer, the occurrence of lysed and necrotic cells or disintegrated nuclei).
Data analyses. Repeated sampling of the receptor medium during the experiment resulted in the decrease of analyte mass permeated to the receiver well. Therefore, the values derived from the UPLC or AAS were corrected using the equation Mt(n) = VrCn + Vs∑Cm [40], where Mt(n) is the current cumulative mass of the drug transported across the tissue at the time t, Cn represents the current concentration in the receiver medium and ∑Cm denotes the summed total of the previous measured concentrations [ m = 1 to (n–1)]; Vr is the volume of the receptor medium and Vs corresponds to the volume of the sample removed for analysis. After the data correction, the amounts permeated were plotted as a function of time and the maximal permeation rates Jmax (µg. cm–2.h–1) were determined from Fick’s law of diffusion: Jmax = dQr/Adt, where Jmax is diffusive flux calculated from the slope of the steepest part of permeation plott, dQr is the change in quantity of the drug passing through the tissue, A is the surface area and dt is the change in time. The permeability coefficients were calculated according to [41]: P = J/Ci, where J is a flux calculated from the linear portion of the profile and Ci is the concentration of drug in the donor solution. Due to technical limitations, permeation experiments were performed in two replicates. The results of both individual treatments are presented to demonstrate variability. For cytotoxicity studies with HaCaT cells, three replicate experiments were conducted and the results are expressed as mean ± standard error.
Results
Prior to the tissue permeation studies, we investigated cytotoxic effects of the studied compounds towards the human HaCaT keratinocyte cell line in vitro. No toxic effects were observed within the first 3h (data not shown). Decrease in cell viability was observed at the highest tested concentrations of doxorubicin and fluorouracil after 6 hours (Fig. 1A). More pronounced dose-dependent effects were observed after 24h (Fig. 1B) for most compounds with the exception of cyclophosphamide, which is activated by biotransformation enzymes. Based on the 6h experiments, which are close to the real exposure scenarios, three application concentrations (Tab. 1) were selected for detailed permeation studies.

Three compounds (cyclophosphamide, cisplatin and fluorouracil) showed relatively high tissue permeation while the permeation by doxorubicin was rarely observed. Calculated permeation rates and permeability coefficients are in Tab. 3. The cumulative amounts of studied agents, which permeated through the studied tissues are presented in Fig. 2A (RHE) and Fig. 2B (HOE).
![Fig 2. Permeation of antineoplastic drugs through reconstituted human epidermis (panel A) and oral epithelium (panel B). Presented are percentage [%] of the application dose determined in the receptor solution after 6 hours of the test duration (a,b – results of the two replicated experiments).](https://pl-master.mdcdn.cz/media/image/51dfd80420d6d4a2aff0cf4baccd33ec.jpeg?version=1537790313)
Although preliminary in vitro studies with HaCaT cells showed minor toxic effects, histology investigations (Fig. 4) revealed that higher doses of selected drugs (cyclophosphamide, fluorouracil and doxorubicin) caused cytotoxicity in the studied tissues. Cisplatin (used in lower doses in comparison with other agents) had generally lower cytotoxic effects.

Kinetics of the permeation depended on the tissue type. RHE model revealed short lag times after which the low linear increase of drug concentration in the receptor fluid was observed (see Fig. 3A). In contrast, no lag times were observed at HOE model. Instead, high permeation rates were seen during the first three hours followed by the period of successive decrease in permeation rate related to the decrease in the drug depot in the donor medium (see Fig. 3B).

Discussion
Historically, dermal uptake was not considered an important exposure route to chemicals but some compounds were lately shown to overcome the skin barrier fast and in great amounts [42]. With the exception of few model compounds, there is only little information on the percutaneous absorption of hazardous chemicals including the antineoplastic cytotoxic drugs.
According to calculated permeation coefficient, RHE was most permeable by cyclophosphamide (Pmax = 1.2 × × 10–3 cm.h–1) followed by fluorouracil (Pmax = 0.9 × 10–3 cm.h–1). Considering the highest doses applied, which simulated concentrations handled by pharmacy staff during the drug preparation, permeation rates reached up to 30 and 69 µg. cm–2.h–1 at cyclophosphamide and fluorouracil respectively (Tab. 3). Unfortunately, recording the equivalent permeation at the lowest doses was under the analytical detection limits.
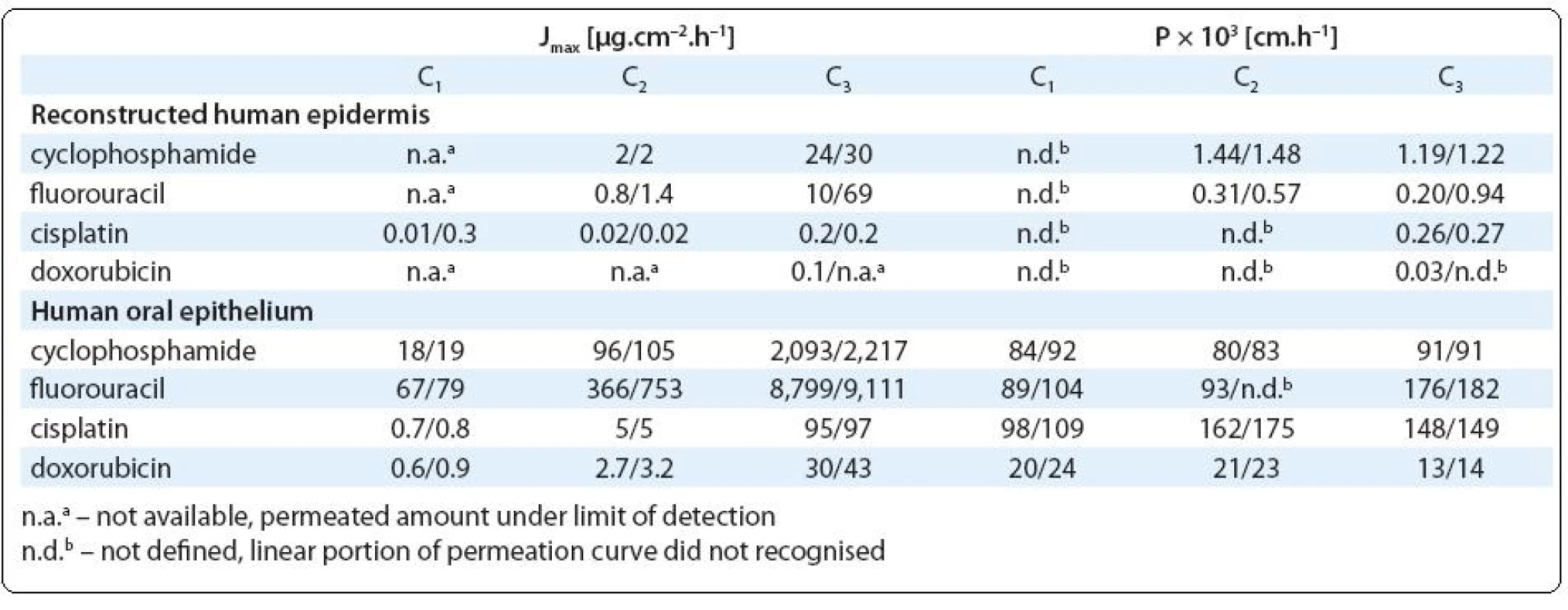
Permeation of cyclophosphamide and fluorouracil through RHE might be related to apparent epidermis cytotoxic damage observed at these experiments (see Fig. 4B; melted or corroded stratum corneum and other effects observed in lower layers of epidermis such as smaller amounts of hypertrophic cells or cells with degenerate nucleus or thickened stratum spinosum layer). Interestingly, relatively high tissue permeation showed also cisplatin (Pmax = 0.3 × 10–3 cm.h–1), which was applied in lower doses (Tab. 2) inducing no pathological changes on RHE.
Although the epidermis permeation by doxorubicin was observed sporadically (Fig. 2A), histology showed cytotoxic effects towards epidermal layers including deformations of the cell shape, cell lyses and broader tissue damage (individual layers of the epithelium including stratum corneum appeared thinner compared to the non-exposed control tissue, Fig. 4C). These deformations might possibly create a barrier, which limited doxorubicin skin permeation. Limited permeation of doxorubicin can be also related to its relatively high molecular weight (MW = 544). Skin absorption of substances with MW > 500 was repeatedly discussed [43,44].
In comparison with the epidermis, much higher transfer was observed in the experiments with oral epithelium, which was readily permeable for cisplatin, cyclophosphamide and fluorouracil (P > 90 × 10–3 cm.h–1). Regardless of the initial concentration, almost 100% of the applied dose permeated the tissue (Fig. 2B). Actually, the permeation was so high that the results can be underestimated due to the significantly decreasing concentration of the drugs in the donor medium. Although in vitro experiments with HaCaT cells indicated minor toxic effects, reconstructed oral epithelium was significantly lysed after higher exposure doses of cyclophosphamide and fluorouracil (Fig. 4E). Approximately 20–40% of the doxorubicin doses permeated through HOE and cell nuclei lyses and tissue necroses were observed (similarly to the skin epidermis, Fig. 4F).
To our best knowledge, our investigations provide some of the first experimental results on the cytotoxic drug permeability through reconstructed human epidermis and oral epithelium. We have shown that all studied antineoplastic drugs can pass fast through oral epithelium. Also the permeation through skin epidermis can not be ruled out, although the stratum corneum provides efficient protection and makes the permeation slower. Permeability coefficients of polar drugs with low molecular weight are close to or exceed 1×10–3 cm.h–1 and they can be recognised as compounds with significant skin penetration [44]. We are aware that two replicates investigated in the present study could not provide statistically fully robust results. In spite of the technical limitations, our study demonstrated very good concordance between both replicated treatments, and the results may further be used for example for exposure modelling using pharmacokinetic models [45].
Previous study demonstrated dermal uptake of cyclophosphamide after examination of urine in volunteers, to whom the drug solutions was applied topically [46]. Another study performed with experimental animals compared cumulative excretion of non-metabolized cyclophosphamide following various routes of administration including intratracheal, dermal, oral and intravenous [32]. In that study, cumulative excretion of cyclophosphamide reached 3–7% of the applied dose after 96 hours, regardless of the application form [32]. The observation, that excretion rate after dermal administration did not vary substantially from oral or intravenous administration, predicts that compounds with permeability coefficients around 1 × 10–3 cm.h–1 can be absorbed up to 100% of the administered dose.
Comparison with the literature is possible also for fluorouracil, which topical administration has been studied in relation to the treatment of psoriasis, actinic keratosis and premalignant and/or malignant conditions of the skin [47]. Previously reported permeability coefficients for fluorouracil varied from 10–5 to 10–4cm.h–1 [47–50]. Tissue permeability measured in our study (Pmax = 9.4 × 10–4 cm.h–1) is particularly well comparable with the permeability of full-thickness rat skin (P = 6.7 × 10–4 cm.h–1) observed by López et al (1996). Based on this agreement we consider the RHE models to be a suitable and appropriate system for percutaneous permeation testing bringing result comparable to other commonly used experimental systems.
Conclusions
In the present study, all evaluated antineoplastic drugs were able to permeate through HOE and RHE but the efficiency of the barrier function of the two tissues varied. Stratum corneum at the epidermis was confirmed a major barrier for permeation. The permeation through thin and hydrated epithelium was relatively easy and fast (up to 100% of the applied dose for hydrophilic drugs during the first 6 hours), while the permeation through more differentiated skin epidermis was much slower. Two of the most frequently used drugs, i.e. cyclophosphamide and fluorouracil penetrated most efficiently, which indicates higher risk of occupational exposure to these compounds. Working conditions in hospital pharmacies with centralized preparation of cytotoxic drugs are usually controlled and workers are well protected. However, there are many hospitals without centralized preparation, where the drugs are prepared by nurses in non-controlled working environment, which may pose higher risk of self-contamination. In addition, nurses and custodians may also be exposed through handling of bed sheets, excrements or vomits of treated patients. Our study brings new insights into the toxicokinetics of widely used antineoplastic agents, and the results may further serve for exposure modelling and critical risk assessment of these hazardous drugs.
Acknowledgement
Authors acknowledge valuable comments on the manuscript from Ass. Prof. D. Valík, MD, Ph.D., from Masaryk Memorial Cancer Institute.
This
work was supported by the Ministry of Education, Czech Republic
projects “CYTO” (grant number 2B06171) and INCHEMBIOL
(VZ0021622412). Cooperation with the co-workers from the
Pliva-Lachema company during the AAS analyses of cisplatin is
acknowledged.
The
authors declare they have no potential conflicts of interest
concerning drugs, products, or services
used in the study.
The
Editorial Board declares that the manuscript met the ICMJE “uniform
requirements” for biomedical papers.
Odraska
Pavel, MSc.
Masaryk
Memorial Cancer Institute
Hospital
Pharmacy
Žlutý
kopec 7 656
53 Brno
Czech
Republic
e-mail:
odraska@mou.cz
Sources
1. Ensslin AS, Huber R, Pethran A et al. Biological monitoring of hospital pharmacy personnel occupationally exposed to cytostatic drugs: Urinary excretion and cytogenetics studies. Int Arch Occup Environ Health 1997; 70(3): 205–208.
2. Hessel H, Radon K, Pethran A et al. The genotoxic risk of hospital, pharmacy and medical personnel occupationally exposed to cytostatic drugs – evaluation by the micronucleus assay. Mutat Res 2001; 497(1–2): 101–109.
3. Pethran A, Schierl R, Hauff K et al. Uptake of antineoplastic agents in pharmacy and hospital personnel. Part i: Monitoring of urinary concentrations. Int Arch Occup Environ Health 2003; 76(1): 5–10.
4. Schneider T, Cherrie JW, Vermeulen R et al. Dermal exposure assessment. Ann Occup Hyg 2000; 44(7): 493–499.
5. Sessink PJM, Bos RP. Drugs hazardous to healthcare workers – evaluation of methods for monitoring occupational exposure to cytostatic drugs. Drug Saf 1999; 20(4): 347–359.
6. Turci R, Sottani C, Spagnoli G et al. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agents: A review of analytical methods. J Chromatog B 2003; 789(2): 169–209.
7. Yoshida J, Kosaka H, Tomioka K et al. Genotoxic risks to nurses from contamination of the work environment with antineoplastic drugs in Japan. J Occupat Health 2006; 48(6): 517–522.
8. Kiffmeyer T, Kube C, Opiolka S et al. Vapour pressures, evaporation behaviour and airborne concentrations of hazardous drugs: Implications for occupational safety. The Pharmaceutical Journal 2002; 268: 331–337.
9. Minoia C, Turci R, Sottani C et al. Application of high performance liquid chromatography tandem mass spectrometry in the environmental and biological monitoring of health care personnel occupationally exposed to cyclophosphamide and ifosfamide. Rapid Commun Mass Spectrom 1998; 12(20): 1485–1493.
10. Sessink PJM, Vandekerkhof MCA, Anzion RBM et al. Environmental contamination and assessment of exposure to antineoplastic agents by determination of cyclophosphamide in urine of exposed pharmacy technicians – is skin absorption an important exposure route. Arch Environ Health 1994; 49(3): 165–169.
11. Odráška P, Gorná L, Doležalová L et al. Monitoring povrchové kontaminace cytotoxickými léčivy v nemocničních lékárnách České republiky. Ceska Slov Farm 2009; 58(5–6): 225–229.
12. Doležalová L, Odráška P, Gorná L et al. Studium kontaminace pracovišť a profesionalní expozice zdravotnických pracovníků zajišťujících přípravu a aplikaci protinádorových léčiv. Prac Lek 2009; 61(3): 117–122.
13. Kromhout H, Hoek F, Uitterhoeve R et al. Postulating a dermal pathway for exposure to anti-neoplastic drugs among hospital workers. Ann Occup Hyg 2000; 44(7): 551–560.
14. Mason HJ, Blair S, Sams C et al. Exposure to antineoplastic drugs in two uk hospital pharmacy units. Ann Occup Hyg 2005; 49(7): 603–610.
15. Sessink PJM, Boer KA, Scheefhals APH et al. Occupational exposure to antineoplastic agents at several departments in a hospital – environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workers. Int Arch Occup Environ Health 1992; 64(2): 105–112.
16. Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health 2005; 78(5): 403–412.
17. Nygren O, Gustavsson B, Strom L et al. Cisplatin contamination observed on the outside of drug vials. Ann Occup Hyg 2002; 46(6): 555–557.
18. Sorsa M, Anderson D. Monitoring of occupational exposure to cytostatic anticancer agents. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. Mutagenicity of Anticancer Drugs 1996; 355(1–2): 253–261.
19. Mader RM, Rizovski B, Steger GG et al. Exposure of oncologic nurses to methotrexate in the treatment of osteosarcoma. Arch Environ Health 1996; 51(4): 310–314.
20. Nygren O, Lundgren C. Determination of platinum in workroom air and in blood and urine from nursing staff attending patients receiving cisplatin chemotherapy. Int Arch Occup Environ Health 1997; 70(3): 209–214.
21. Sessink PJM, Timmersmans JL, Anzion RBM et al. Assessment of occupational exposure of pharmaceutical plant workers to 5-fluorouracil – determination of alpha-fluoro-beta-alanine in urine. J Occup Environ Med 1994; 36(1): 79–83.
22. Sessink PJM, Cerna M, Rossner P et al. Urinary cyclophosphamide excretion and chromosomal aberrations in peripheral blood lymphocytes after occupational exposure to antineoplastic agents. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 1994; 309(2): 193–199.
23. Connor TH, McDiarmid MA. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J Clin 2006; 56(6): 354–365.
24. Connor TH. Permeability of nitrile rubber, latex, polyurethane, and neoprene gloves to 18 antineoplastic drugs. Am J Health Syst Pharm 1999; 56(23): 2450–2453.
25. Wallemacq PE, Capron A, Vanbinst R et al. Permeability of 13 different gloves to 13 cytotoxic agents under controlled dynamic conditions. Am J Health Syst Pharm 2006; 63(6): 547–556.
26. Doležalová L, Odráška P, Gorná L et al. Studie evaporace vybraných cytostatik a propustnosti ochranných rukavic v rámci výzkumu profesní zátěže zdravotnických pracovníků exponovaných cytotoxickým protinádorovým léčivům (projekt cyto). Klin Onkol 2009; 22(5): 218–222.
27. McDevitt JJ, Lees PSJ, McDiarmid MA. Exposure of hospital pharmacists and nurses to antineoplastic agents. J Occup Environ Med 1993; 35(1): 57–60.
28. Poet TS, McDougal JN. Skin absorption and human risk assessment. Chem Biol Interact 2002; 140(1): 19–34.
29. Moody RP, Nadeau B. In vitro dermal absorption of pesticides: VI. In vivo and in vitro comparison with the organophosphorus insecticide diazinon in rat, guinea pig, pig, human and tissue-cultured skin. Toxicol In Vitro 1994; 8(6): 1213–1218.
30. Moody RP, Nadeau B, Chu I. In vivo and in vitro dermal absorption of benzo[a]pyrene in rat, guinea pig, human and tissue-cultured skin. J Dermatol Sci 1995; 9(1): 48–58.
31. Wellner T, Lüersen L, Schaller KH et al. Percutaneous absorption of aromatic amines – a contribution for human health risk assessment. Food Chem Toxicol 2008; 46(6): 1960–1968.
32. Sessink PJM, Vandenbroek PHH, Bos RP. Urinary cyclophosphamide excretion in rats after intratracheal, dermal, oral and intravenous administration of cyclophosphamide. J Appl Toxicol 1991; 11(2): 125–128.
33. Diembeck W, Beck H, Benech-Kieffer F et al. Test guidelines for in vitro assessment of dermal absorption and percutaneous penetration of cosmetic ingredients. Food Chem Toxicol 1999; 37(2–3): 191–205.
34. Gysler A, Konigsmann U, Schafer-Korting M. Tridimensional skin models recording percutaneous absorption. ALTEX 1999; 16(2): 67–72.
35. Netzlaff F, Lehr C-M, Wertz PW et al. The human epidermis models episkin®, skinethic® and epiderm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm 2005; 60(2): 167–178.
36. Garcia N, Doucet O, Bayer M, et al. Characterization of the barrier function in a reconstituted human epidermis cultivated in chemically defined medium. Int J Cosmet Sci 2002; 24: 25–34.
37. Babich H, Borenfreund E. Applications of the neutral red cytotoxicity assay to invitro toxicology. Altern Lab Anim 1990; 18: 129–144.
38. Roguet R, Cohen C, Dossou KG et al. Episkin, a reconstituted human epidermis for assessing in-vitro the irritancy of topically applied compounds. Toxicol In Vitro 1994; 8(2): 283–291.
39. Richardson KC, Jarett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 1960; 35(6): 313–323.
40. Frum Y, Khan GM, Sefcik J et al. Towards a correlation between drug properties and in vitro transdermal flux variability. Int J Pharm 2007; 336(1): 140–147.
41. Zhao L, Fang L, Xu Y et al. Transdermal delivery of penetrants with differing lipophilicities using o-acylmenthol derivatives as penetration enhancers. Eur J Pharm Biopharm 2008; 69(1): 199–213.
42. Wester RC, Maibach HI, Sedik L et al. Percutaneous-absorption of pentachlorophenol from soil. Fundam Appl Toxicol 1993; 20(1): 68–71.
43. Lademann J, Richter H, Jacobi U et al. Human percutaneous absorption of a direct hair dye comparing in vitro and in vivo results: Implications for safety assessment and animal testing. Food Chem Toxicol 2008; 46(6): 2214–2223.
44. Oppl R, Kalberlah F, Evans PG et al. A toolkit for dermal risk assessment and management: An overview. Ann Occup Hyg 2003; 47(8): 629–640.
45. Reddy MB, McCarley KD, Bunge AL. Physiologically relevant one-compartment pharmacokinetic models for skin. 2. Comparison of models when combined with a systemic pharmacokinetic model. J Pharm Sci 1998; 87(4): 482–490.
46. Hirst M, Mills D, Tse S et al. Occupational exposure to cyclophosphamide. Lancet 1984; 323(8370): 186–188.
47. Singh BN, Singh RB, Singh J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. Int J Pharm 2005; 298(1): 98–107.
48. López A, Morant MJ, Guzmán D et al. Skin permeation model of phenylalkylcarboxylic homologous acids and their enhancer effect on percutaneous penetration of 5-fluorouracil. Int J Pharm 1996; 139(1–2): 205–213.
49. Williams AC, Barry BW. Terpenes and the lipid protein partitioning theory of skin penetration enhancement. Pharm Res 1991; 8(1): 17–24.
50. Yamane MA, Williams AC, Barry BW. Effects of terpenes and oleic-acid as skin penetration enhancers towards 5-fluorouracil as assessed with time – permeation, partitioning and differential scanning calorimetry. Int J Pharm 1995; 116(2): 237–251.
Labels
Paediatric clinical oncology Surgery Clinical oncologyArticle was published in
Clinical Oncology

2011 Issue 3
- Metamizole vs. Tramadol in Postoperative Analgesia
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- The Importance of Hydration in Wound Healing
- Obstacle Called Vasospasm: Which Solution Is Most Effective in Microsurgery and How to Pharmacologically Assist It?
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
Most read in this issue
- Gastrointestinal Stromal Tumors
- Selenium and Cancer: from Prevention to Treatment
- Can Cancer Patient in Terminal Stage of Cancer Die with Dignity at Home? And under what Conditions?
- Radiotherapy and the Flow of Data and Documentation
