The Role of Platelets in Tumour Growth
Úloha krevních destiček v rozvoji nádoru
Krevní destičky jako elementy odpovídající v první vlně na poškození cév hrají velmi významnou úlohu v počátečních fázích procesu hemostázy. Zatímco zapojení trombocytů v procesu koagulace je podrobně studováno a popsáno, jejich role v dalších fyziologických a patologických procesech teprve začíná být předmětem zájmu. Krevní destičky obsahují řadu biologicky aktivních molekul a s tím, jak trombocyty adherují na nádorem aktivovaný nebo poškozený endotel, je řada těchto molekul uvolňována do nádorového mikroprostředí, což vede k ovlivnění cévního tonu, reparaci cévy a neoangiogenezi. Destičky pravděpodobně hrají důležitou úlohu v mikroprostředí nádoru, který můžeme považovat za ránu, která se nehojí.
Klíčová slova:
trombocyt – angiogeneze – hojení rány – růst nádoru – metastazování nádoru
Authors:
K. Pilátová 1,2; L. Zdrazilova-Dubska 1,2; G. L. Klement 2,3
Authors‘ workplace:
Department of Laboratory Medicine, Masaryk Memorial Cancer Institute, Brno, Czech Republic
1; Regional Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic
2; Center of Cancer Systems Biology, Steward St. Elizabeth’s Medical Center, Pediatric Hematology Oncology, Tufts University School of Medicine, Boston, MA, USA
3
Published in:
Klin Onkol 2012; 25(Supplementum 2): 50-57
Práce byla podpořena Evropským fondem pro regionální rozvoj a státním rozpočtem České republiky (OP VaVpI – RECAMO, CZ.1.05/2.1.00/03.0101).
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 12. 11. 2012
Přijato: 15. 11. 2012
Overview
Platelets, as initial responders to vascular injury, play a very important role in the initial stages of the haemostatic process. While the role of platelets in coagulation has been well studied and documented, their role in other physiological and pathological processes is just emerging. Platelets contain many biologically active molecules and, as they adhere to sites of tumour activated or injured endothelium, many of these molecules are released into the local microenvironment leading to platelet-mediated effects on vascular tone, repair and neo-angiogenesis. Platelets are likely play important roles in the tumour microenvironment that may be thought of as “a wound that never heals”.
Key words:
blood platelets – angiogenesis – wound healing – tumour growth – neoplasm metastasis
The Hypercoagulable State Associated with Malignancy
Numerous clinical and basic science studies corroborate the importance of thrombosis in cancer development [1–7], cancer progression [8–12], and cancer metastasis [8,13–18]. The association is so well known that a deep vein thrombosis (DVT) in a patient without obvious risk factors triggers a search for an occult cancer. Despite this appreciation of a link between DVT and malignancy [19–21], the underlying biology has not been well characterised. The propensity to develop thromboembolic disease varies with the type of cancer [22], suggesting tumour cell-specific or tumour microenvironment-specific pathways to platelet and fibrin aggregation in tumours. Furthermore in some tumours, such as neuroblastoma, high platelet counts are associated with good prognosis [23], whereas in others (lung, colon, cervical, and breast cancers), the finding of high platelet counts implies poor prognosis [24–26].
Even though the association of hypercoagulability in cancer was first documented by Trousseau in 1865 [27], much work remains before we can use this finding therapeutically. There are some encouraging clinical observations. For example, the use of anticoagulants provides cancer patients with a survival advantage over and above that which would be conferred by the treatment of the DVT alone [28–35]. Unfortunately, large studies of the use of anticoagulants in the cancer population have not led to any significant change in the present management of cancer patients_ENREF_194 [36]. Yet both clinicians and basic scientists appreciate that even in patients not presenting with a cancer-associated thrombosis, the coagulation system is activated and platelet turnover increased. The interplay between platelets, coagulation and cancer is yet to be fully explored.
The Role of Platelet in Angiogenesis
The first scientific evidence suggesting that platelets were necessary for vascular integrity was reported in the late 1960‘s [37]. Organs perfused with platelet poor plasma led to loss of integrity of the endothelial cell layer and haemorrhages, and this effect could be reversed by addition of platelets. Similarly, thrombocytopenia was associated with increase in vascular permeability due to large endothelial wall fenestrations (EC) [38,39]. Based on these and other studies platelets were thought to promote endothelial cell growth [40], even though the mechanism of this trophic effect was unclear.
Platelets contain three types of granules: α-granules, dense granules and lysosomes, but most angiogenesis related proteins are contained in α-granules [41,42]. Tab. 1 lists angiogenesis regulators found in platelets. The presence of proteins with opposing angiogenic functions in platelets suggests that platelets are mediators and their presence can result in different actions depending on the situation. The formation of a clot not only provides a matrix facilitating cell migration, but also leads to a very judicial release of either stimulators or inhibitors of growth. As platelets adhere to activated endothelia or to exposed vascular sub-endothelia, the reciprocal interactions between the cells lead to sequential release of angiogenesis regulators. Platelets in this way serve as potent activators as well as inhibitors of important tissue repair processes such as inflammation [43] and angiogenesis [44].
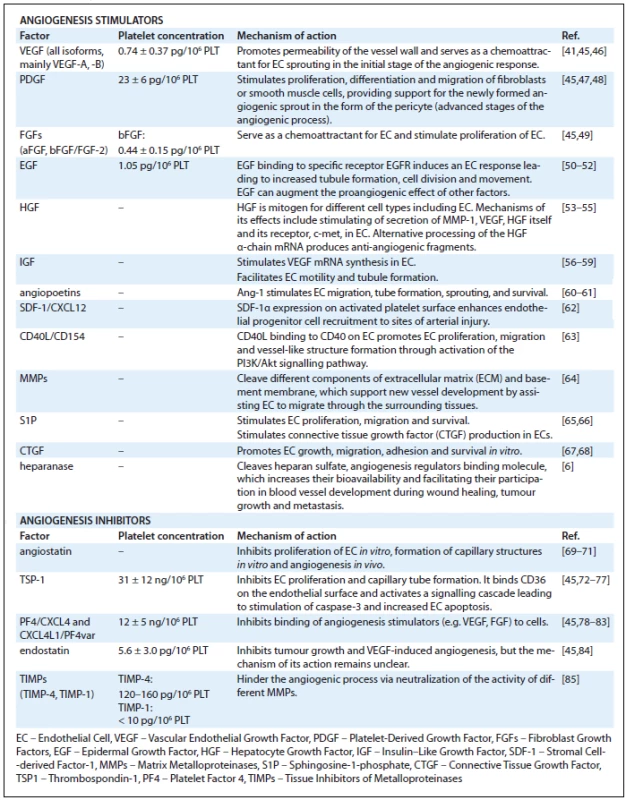
Platelets in Tumour Angiogenesis
A tumour is a community of cells. There are resident cells (fibroblasts, histiocytes, epithelial and mesenchymal cells) that form the tissues, and cells that are recruited to the site in time of injury or malignant growth (mesenchymal progenitors and inflammatory cells). Platelets are mediators of this community.
Primary tumour growth is facilitated by inflammation and angiogenesis not unlike physiological wound healing [86–88]. However, in cancer, the normal physiological processes of dialing-down angiogenesis as scar tissue develops is prevented by the continuous, oncogene-mediated induction of tumour angiogenesis [89–92]. It has been well described that tumour vasculature is immature, unstable and morphologically different from the normal systemic vasculature. While tumour vasculature is often thought of as abnormal, it is better conceptualised as an unpruned, underdeveloped precursor of mature vessels – a continuously expanding, but not maturing, vascular bed.
Platelets play an important role in modulating tumour dynamics. A large body of evidence spanning at least four decades supports the involvement of platelets in cancer [1,2,13]. The process of sequestration of angiogenesis regulators in platelets is an active and highly selective process [41]. An open-ended proteomic comparison of platelets from mice bearing dormant or fast-growing liposarcoma xenografts revealed significant differences in protein profiles between each of these tumour subtypes [41,93,94], as well as differences when platelets of mice bearing either of the tumour types were compared to platelets of non-tumour-bearing sham-operated controls. Despite the open-ended analysis of all proteins present in platelets, the majority of proteins differentially expressed in platelets of tumour-bearing animals and cancer patients were found to be angiogenesis regulators such as VEGF, bFGF, PDGF, PF4, TSP1, MMP9, endostatin, angiopoietin-1 and -2, etc. While the membership in this “platelet angiogenesis proteome”, as well as the concentrations of individual protein members, is fairly stable under physiological conditions [45], it is altered very early in tumour growth [41,93]. The sequestration of angiogenesis regulators in platelets is: i) active because it occurs against a concentration gradient in plasma and ii) highly selective for angiogenesis regulators, as other very abundant proteins, e.g. albumin or fibrinogen, are not taken up by platelets against a concentration gradient. Interestingly, the sequestration of angiogenesis regulators in platelets occurs very early in primary tumour growth [93]. At a time when tumours are not detectable by conventional methods, and long before the tumour burden results in changes in the levels of angiogenesis regulators in plasma or serum, there are detectable changes in platelet levels of angiogenesis regulators [41,93].
Are Platelets Stimulatory or Inhibitory to Tumour Growth?
While postulated many decades ago, the consequences of platelet adhesion to activated endothelium, and their role in early tumour growth and tumour angiogenesis, has been difficult to establish. The main source of the difficulties, similar to the difficulties in establishing their role in wound healing, is the variable method of platelet concentrate preparation. An additional limitation is the animal models, which do not necessarily reciprocate the complexity of platelet receptors and tissue integrins. However, through the use of genetically altered animals, in vivo tracking dyes, and three dimensional in vitro models, some of the interactions between platelets, tumour cells, and other inflammatory cells within the tumour microenvironment are beginning to emerge (Fig. 1). The early literature can be very confusing. For example, there is convincing evidence that platelets enhance the development of metastasis [2,13–15,94–97] and primary tumour growth [2,13,15,98], but some studies advocate that the effect of platelets on primary tumour growth is inhibitory [99–101] and that the inhibition of platelet adhesion leads to promotion of metastasis [102]. Similarly, the most abundant proteins in platelets, e.g. PF4 (Tab. 1) are very potent inhibitors of tumour growth [103–109] and other very abundant platelet-associated proteins such as thrombospondin (TSP1) (Tab. 1), previously thought to be inhibitory to angiogenesis [110], may be augmenting the metastatic process under specific conditions [111–112].
![Fig. 1. Platelets contribution to the regulation of tumour angiogenesis and tumour progression. 1. Coagulation: Stimuli for platelet activation come from endothelial cells, as well as tumour stroma itself (expression of tissue factor, thrombin, ADP etc.). After activation, platelets change their shape, release PMP, α- and dense granule content and trigger coagulation cascade [8,11]. 2. Infl ammation: Chemokines (IL-8, histamine etc.) released by platelets are chemotactic for leukocytes and precursor cells from bone marrow. These cells also regulate the tumour environment by release of growth and angiogenic factors [12]. 3. Angiogenesis: Platelets participate also in regulation of angiogenesis by releasing pro- and anti-angiogenic factors (VEGF, bFGF, PF-4 etc.), as well as by active sequestering of factors from the circulation [9]. 4. Stabilisation of vessel wall: Platelets stabilise the vessel wall and maintain intercellular connections by releasing factors, such as EGF, S1P, ang-1 etc., to prevent haemorrhage at the site of angiogenesis and inflammation [17]. 5. Circulation of tumour cells: Platelets adhered to tumour cells protect them from immune recognition and the cytotoxic effects of NK cell cytokines, which enables survival in the circulation and migration to distant tissue sites [7,17,18]. 6. Adhesion/extravasation: Aggregates of platelets, leukocytes and tumour cells facilitate adhesion of tumour cells to endothelium and subsequent extravasation into distant tissues. Platelets also release factors promoting cell proliferation and increasing permeability of the vessel wall (e.g. VEGF) [6,17].](https://pl-master.mdcdn.cz/media/image/7988c3495535bed9081a7b8a760011eb.jpg?version=1537790377)
One possible explanation for these very contradictory findings may be that platelets are neither inhibitors nor stimulators of tumour growth. Similar to their function in wound healing, they modulate rather than stimulate the malignant process, and the overall result of the platelet effect may depend on the balance of stimulatory and inhibitory signals within the tumour microenvironment. Depending on the reciprocal interaction between the existing host stromal cells, the oncogene-transformed tumour cells, and the recruited progenitors and inflammatory cells, the sum of these communications determines whether the outcome is growth, dormancy, or regression [94]. The final response may be less dependent on platelet numbers than on the specific content of stimulators and inhibitors of angiogenesis in the α-granules of platelets. This content of growth stimulators and inhibitors is continuously modified, a process aided by the short half-life of platelets in circulation (4–7 days in mice and 7–10 days in humans). This theory is informed by the recent finding that there is a higher organization of the opposing angiogenesis-related activities in platelets, enabling a differential release of either stimulators or inhibitors of angiogenesis [113,119]. The stimulators of angiogenesis (e.g., VEGF and bFGF) do not reside in the same granules as the inhibitors (e.g., endostatin) [113].
A widely-held assumption is that platelets degranulate en masse upon activation, and that serum is a good reflection of their content [114–117]. This assumption, which may have hindered the understanding of the reciprocal interaction of platelets and tissues, may not be entirely correct. Angiogenesis regulators associated with α-granules of platelets, unlike the proteins of dense granules, are not indiscriminately released in response to ADP, thrombin or epinephrine [41]. Activation of human platelets with adenosine diphosphate (ADP) stimulates the release of VEGF, but not endostatin whereas, thromboxane A(2) (TXA(2)) releases endostatin but not VEGF [118]. As has been well documented in the setting of gastric ulcers, platelet responses to thrombin are also graded [119–122]. Activation of high-affinity thrombin receptor PAR1 releases stimulators such as VEGF, whereas the low affinity thrombin receptor PAR4 mainly releases inhibitors such as endostatin [121]. Similarly, the increases in acidity and temperature, which are typical in the setting of infection, inflammation, or cancer also change the sequence of release of angiogenesis regulators from platelets [123,124]. This concept may be quite intuitive: if platelets contain both stimulators and inhibitors of angiogenesis, a massive degranulation would be unlikely to provide the sustained and carefully orchestrated signals required for modulation of normal angiogenesis. It is much more advantageous if the majority of angiogenesis regulators sequestered in platelets during early cancer development remain associated with the platelet clot upon coagulation [41]. Some may even be taken up by platelets during activation [125]. This finding may provide some early insights into the mechanisms of tissue//platelet communication. Because the majority of proteins relevant to angiogenesis are retained in the α-granules of platelets, and because the organization of proteins within the α-granules is based on function [113]; the release of angiogenesis regulators, unlike the release of ADP and serotonin from the dense granules, is selective [113,120,121], but also amenable to influences beyond the proteolytic activity of thrombin or environmental influences such as temperature or acidity. In the setting of this new knowledge, a clot, which has been thought of as a simple “plug” to prevent bleeding, now appears to be a sophisticated matrix that is rich in proteins and can regulate angiogenesis and inflammation in a locally-defined, reciprocal fashion.
Platelet-Derived Microparticles in Tumour Progression
Elevation of platelet-derived microparticles (PMP) levels accompanies a number of disorders including cancer, atherosclerosis, sepsis and diabetes [126]. The role of PMP in disease development is unknown but the composition of PMP in the plasma of patients varies considerably depending on the severity of the pathology [127]. The method of cell-cell communication may be dependent on the shedding of PMP upon platelet activation. PMP host a variety of cytokines and growth factors modulating angiogenesis and tissue regeneration. PMP have been shown to promote proliferation of endothelial cells and tubule formation [128] but also survival and proliferation of other cell types [129,130]. Recent evidence suggests that PMP, much like platelets, significantly affect tumour metastasis including modification of angiogenic responses. In gastric cancer, Kim et al showed that PMP levels are better predictors of metastasis than VEGF, IL-6, and RANTES [131]. It has been reported that PMP may serve as chemoattractants to several lung cancer cell lines, activating phosphorylation of ERK and expression of membrane type 1-matrix metalloproteinase [132]. PMP were also shown to stimulate proliferation and adhesion of cancer cells to fibrinogen and EC and enhance the adhesion and chemoinvasion of breast cancer cell lines [130]. PMP can induce secretion of MMP-2 by prostate cancer cells in vitro, facilitating their passage through the collagen that is a major component of extracellular matrix [133] contributing to cancer cell spread.
In summary, the systems biology of cancer is not dissimilar from that of a wound. In general, platelets have a pro-angiogenic effect in the setting of early injury, progressive tumour growth, atherogenesis or chronic inflammation, and an anti-angiogenic effect in the setting of a healing wound, dormant tumours, or receding inflammation [94]. Cancer may be thought of as “a wound that never heals” [86,87].
The work was supported by the European Regional Development Fund and the State Budget of the Czech Republic for Regional Centre for Applied Molecular Oncology (RECAMO, CZ.1.05/2.1.00/03.0101).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Giannoula Lakka Klement, M.D.
Center of Cancer Systems Biology
Steward St. Elizabeth’s Medical Center Pediatric Hematology Oncology
Tufts University School of Medicine
Boston, MA, USA
e-mail: giannoula.klement@tufts.edu
Submitted: 12. 11. 2012
Accepted: 15. 11. 2012
Sources
1. Pearlstein E, Ambrogio C, Gasic G et al. Inhibition of the platelet-aggregating activity of two human adenocarcinomas of the colon and an anaplastic murine tumor with a specific thrombin inhibitor, dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Cancer Res 1981; 41(11 Pt 1): 4535–4539.
2. Gasic GJ, Gasic TB, Galanti N et al. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer 1973; 11(3): 704–718.
3. Donati MB, Falanga A. Pathogenetic mechanisms of thrombosis in malignancy. Acta Haematol 2001; 106(1–2): 18–24.
4. Pinedo HM, Verheul HM, D’Amato RJ et al. Involvement of platelets in tumour angiogenesis? Lancet 1998; 352(9142): 1775–1777.
5. Verheul HM, Pinedo HM. Tumor Growth: A Putative Role for Platelets? Oncologist 1998; 3(2): II.
6. Vlodavsky I, Eldor A, Haimovitz-Friedman A et al. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis 1992; 12(2): 112–127.
7. Zhou J, Sargiannidou I, Tuszynski GP. The role of adhesive proteins in the hematogenous spread of cancer. In Vivo 2000; 14(1): 199–208.
8. Milsom C, Rak J. Tissue factor and cancer. Pathophysiol Haemost Thromb 2008; 36 (3–4): 160–176.
9. Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemos 2004; 30(1): 95–108.
10. Sierko E, Wojtukiewicz MZ. Inhibition of platelet function: does it offer a chance of better cancer progression control? Semin Thromb Hemos 2007; 33(7): 712–721.
11. ten Cate H, Falanga A. Overview of the postulated mechanisms linking cancer and thrombosis. Pathophysiol Haemost Thromb 2008; 36(3–4): 122–130.
12. Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemos 2003; 1(9): 1897–1905.
13. Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. P Natl Acad Sci USA 1968; 61(1): 46–52.
14. Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 2008; 8(8): 1247–1255.
15. Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metast Rev 1984; 3(2): 99–114.
16. Borsig L. Antimetastatic activities of modified heparins: selectin inhibition by heparin attenuates metastasis. Semin Thromb Hemos 2007; 33(5): 540–546.
17. Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 2010; 115(17): 3427–3436.
18. Palumbo JS, Talmage KE, Massari JV et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005; 105(1): 178–185.
19. Connolly GC, Khorana AA. Risk stratification for cancer-associated venous thromboembolism. Best Pract Res Clin Haematol 2009; 22(1): 35–47.
20. Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 2009; 27(29): 4839–4847.
21. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (23 Suppl 1): I17–I21.
22. Khorana AA. Risk assessment for cancer-associated thrombosis: what is the best approach? Thromb Res 2012; 129 (Suppl 1): S10–S15.
23. Berthold F, Sahin K, Hero B et al. The current contribution of molecular factors to risk estimation in neuroblastoma patients. Eur J Cancer 1997; 33(12): 2092–2097.
24. Engan T, Hannisdal E. Blood analyses as prognostic factors in primary lung cancer. Acta Oncol 1990; 29(2): 151–154.
25. Lopes A, Daras V, Cross PA et al. Thrombocytosis as a prognostic factor in women with cervical cancer. Cancer 1994; 74(1): 90–92.
26. Rosenthal MC, Niemetz J, Wisch N. Hemorrhage and thromboses associated with neoplastic disorders. J Chronic Dis 1963; 16 : 667–675.
27. Trousseau A. Phlegmatia alba dolens. Paris: JB Baillere et Fils 1865.
28. Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb 2008; 36(3–4): 195–203.
29. Gerotziafas GT, Papageorgiou C, Hatmi M et al. Clinical studies with anticoagulants to improve survival in cancer patients. Pathophysiol Haemost Thromb 2008; 36(3–4): 204–211.
30. Kakkar AK. An expanding role for antithrombotic therapy in cancer patients. Cancer Treat Rev 2003; 29 (Suppl 2): 23–26.
31. Kakkar AK, Levine MN, Kadziola Z et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol 2004; 22(10): 1944–1948.
32. Kakkar AK, Macbeth F. Antithrombotic therapy and survival in patients with malignant disease. Br J Cancer 2010; 102 (Suppl 1): S24–S29.
33. Kakkar AK, Williamson RC. Thromboprophylaxis in the cancer patient. Haemostasis 1998; 28 (Suppl 3): 61–65.
34. Petralia GA, Lemoine NR, Kakkar AK. Mechanisms of disease: the impact of antithrombotic therapy in cancer patients. Nat Clin Pract Oncol 2005; 2(7): 356–363.
35. Thodiyil P, Kakkar AK. Can low-molecular-weight heparins improve outcome in patients with cancer? Cancer Treat Rev 2002; 28(3): 151–155.
36. Lyman GH, Khorana AA, Falanga A et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25(34): 5490–5505.
37. Gimbrone MA Jr, Aster RH, Cotran RS et al. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature 1969; 222(5188): 33–36.
38. Gore I, Takada M, Austin J. Ultrastructural basis of experimental thrombocytopenic purpura. Arch Pathol 1970; 90(3): 197–205.
39. Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood 1975; 46(4): 567–578.
40. Saba SR, Mason RG. Effects of platelets and certain platelet components on growth of cultured human endothelial cells. Thromb Res 1975; 7(5): 807–812.
41. Klement GL, Yip TT, Cassiola F et al. Platelets actively sequester angiogenesis regulators. Blood 2009; 113(12): 2835–2842.
42. Klement G, Shai E, Varon D. The role of platelets in angiogenesis. In: Michelson A, editor. Platelets. 3rd ed. San Diego, CA: Elsevier/Academic Press 2012.
43. Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011; 11(4): 264–274.
44. Mohle R, Green D, Moore MA et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. P Natl Acad Sci USA 1997; 94(2): 663–668.
45. Peterson JE, Zurakowski D, Italiano JE et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol 2010; 85(7): 487–493.
46. Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 2008; 4(4): 241–246.
47. Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res 1997; 46(1): 4–18.
48. Heldin CH. Simultaneous induction of stimulatory and inhibitory signals by PDGF. FEBS Lett 1997; 410(1): 17–21.
49. Brunner G, Nguyen H, Gabrilove J et al. Basic fibroblast growth factor expression in human bone marrow and peripheral blood cells. Blood 1993; 81(3): 631–638.
50. Nakamura T, Kasai K, Banba N et al. Release of human epidermal growth factor from platelets in accordance with aggregation in vitro. Endocrinol Jpn 1989; 36(1): 23–28.
51. Viloria-Petit A, Crombet T, Jothy S et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res 2001; 61(13): 5090–5101.
52. Lee YM, Bae HM, Lee OH. Synergistic induction of in vivo angiogenesis by the combination of insulin-like growth factor-II and epidermal growth factor. Oncol Rep 2004; 12(4): 843–848.
53. Nakamura Y, Morishita R, Higaki J et al. Expression of local hepatocyte growth factor system in vascular tissues. Biochem Bioph Res Co 1995; 215(2): 483–488.
54. Shima N, Itagaki Y, Nagao M et al. A fibroblast-derived tumor cytotoxic factor/F-TCF (hepatocyte growth factor//HGF) has multiple functions in vitro. Cell Biol Int Rep 1991; 15(5): 397–408.
55. Tomita N, Morishita R, Taniyama Y et al. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation 2003; 107(10): 1411–1417.
56. Karey KP, Sirbasku DA. Human platelet-derived mitogens, II: subcellular localization of insulin like growth factor I to the alpha-granule and release in response to thrombin. Blood 1989; 74 : 1093–1100.
57. Chan K, Spencer EM. Megakaryocytes endocytose insulin-like growth factor (IGF) I and IGF-binding protein-3: a novel mechanism directing them into alpha granules of platelets. Endocrinol 1998; 139 : 559–565.
58. Shigematsu S, Yamauchi K, Nakajima K et al. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J 1999; 46 (Suppl): S59–S52.
59. Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 1994; 145(5): 1023–1029.
60. Caine GJ, Lip GY, Blann AD. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: further evidence for a role of platelets in tumour angiogenesis. Ann Med 2004; 36(4): 273–277.
61. Li JJ, Huang YQ, Basch R et al. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost 2001; 85(2): 204–206.
62. Moore MA, Hattori K, Heissig B. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann NY Acad Sci 2001; 938 : 36–45.
63. Deregibus MC, Buttiglieri S, Russo S. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem 2003; 278(20): 18008–18014.
64. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opon Cell Biol 2004; 16(5): 558–564.
65. Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem 2004; 92(5): 900–912.
66. Markiewicz M, Nakerakanti SS, Kapanadze B. Connective tissue growth factor (CTGF/CCN2) mediates angiogenic effect of S1P in human dermal microvascular endothelial cells. Microcirculation 2011; 18(1): 1–11.
67. Cicha I, Yilmaz A, Suzuki Y. Connective tissue growth factor is released from platelets under high shear stress and is differentially expressed in endothelium along atherosclerotic plaques. Clinical Hemorheol Micro 2006; 35(1–2): 203–206.
68. Yang F, Tuxhorn JA, Ressler SJ et al. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res 2005; 65 : 8887–8895.
69. Jurasz P, Alonso D, Castro-Blanco S et al. Generation and role of angiostatin in human platelets. Blood 2003; 102 : 3217–3223.
70. O‘Reilly MS, Holmgren L, Shing Y et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79 : 315–328.
71. O‘Reilly MS, Holmgren L, Chen C et al. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996; 2 : 689–692.
72. Jaffe EA, Leung LL, Nachman RL et al. Thrombospondin is the endogenous lectin of human platelets. Nature 1982; 295(5846): 246–248.
73. Jiménez B, Volpert OV, Crawford SE et al. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 2000; 6(1): 41–48.
74. Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med 2012; 2(5): a006627.
75. Gupta K, Gupta P, Wild R et al. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 1999; 3(2): 147–158.
76. Dardik R, Solomon A, Loscalzo J et al. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler Thromb Vasc Biol 2003; 23(8): 1472–1477.
77. Dawson DW, Volpert OV, Pearce SF et al. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol 1999; 55(2): 332–338.
78. Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010; 125(4): 292–296.
79. Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin Thromb Hemost 2004; 30(3): 379–385.
80. Chadderton NS, Stringer SE. Interaction of platelet factor 4 with fibroblast growth factor 2 is stabilised by heparan sulphate. Int J Biochem Cell Biol 2003; 35(7): 1052–1055.
81. Kim SH, Kiick KL. Heparin-mimetic sulfated peptides with modulated affinities for heparin-binding peptides and growth factors. Peptides 2007; 28(11): 2125–2136.
82. Hagedorn M, Zilberberg L, Wilting J et al. Domain swapping in a COOH-terminal fragment of platelet factor 4 generates potent angiogenesis inhibitors. Cancer Res 2002; 62(23): 6884–6890.
83. Vandercappellen J, Liekens S, Bronckaers A et al. The COOH-terminal peptide of platelet factor-4 variant (CXCL4L1/PF-4var47-70) strongly inhibits angiogenesis and suppresses B16 melanoma growth in vivo. Mol Cancer Res 2010; 8(3): 322–334.
84. Xu HL, Tan HN, Wang FS et al. Research advances of endostatin and its short internal fragments. Curr Protein Pept Sci 2008; 9(3): 275–283.
85. Radomski A, Jurasz P, Sanders EJ et al. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br J Pharmacol 2002; 137(8): 1330–1338.
86. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315(26): 1650–1659.
87. Dvorak HF, Harvey VS, Estrella P et al. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 1987; 57(6): 673–686.
88. Nagy JA, Brown LF, Senger DR et al. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 1989; 948(3): 305–326.
89. Rak J, Yu JL, Klement G et al. Oncogenes and angiogenesis: signaling three-dimensional tumor growth. J Investig Dermatol Symp Proc 2000; 5(1): 24–33.
90. Rak J, Yu JL, Kerbel RS et al. What do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors? Cancer Res 2002; 62(7): 1931–1934.
91. Rak J, Klement G. Impact of oncogenes and tumor suppressor genes on deregulation of hemostasis and angiogenesis in cancer. Cancer Metastasis Rev 2000; 19(1–2): 93–96.
92. Rak J, Filmus J, Finkenzeller G et al. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev 1995; 14(4): 263–277.
93. Cervi D, Yip TT, Bhattacharya N et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 2008; 111(3): 1201–1207.
94. Almog NK, Klement GL. Platelet Proteome and Tumor Dormancy: Can Platelets Content Serve as Predictive Biomarkers for Exit of Tumors from Dormancy? Cancers 2010; 2(2): 842–858.
95. Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur J Cancer 1996; 32A(14): 2451–2460.
96. Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood 1984; 63(1): 55–63.
97. Yahalom J, Eldor A, Biran S et al. Platelet-tumor cell interaction with the subendothelial extracellular matrix: relationship to cancer metastasis. Radiother Oncol 1985; 3(3): 211–225.
98. Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006; 10(5): 355–362.
99. Daly ME, Makris A, Reed M et al. Hemostatic regulators of tumor angiogenesis: a source of antiangiogenic agents for cancer treatment? J Natl Cancer Inst 2003; 95(22): 1660–1673.
100. Ibele G, Kay N, Johnson G et al. Human platelets exert cytotoxic effects on tumor cells. Blood 1985; 65(5): 1252–1255.
101. Wang Y, Zhang H. Platelet-induced inhibition of tumor cell growth. Thromb Res 2008; 123(2): 324–330.
102. Erpenbeck L, Nieswandt B, Schon M et al. Inhibition of platelet GPIb alpha and promotion of melanoma metastasis. J Invest Dermatol 2010; 130(2): 576–586.
103. Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin Thromb Hemos 2004; 30(3): 379–385.
104. Kolber DL, Knisely TL, Maione TE. Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. J Natl Cancer Inst 1995; 87(4): 304–309.
105. Vandercappellen J, Van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev 2011; 22(1): 1–18.
106. Vandercappellen J, Liekens S, Bronckaers A et al. The COOH-terminal peptide of platelet factor-4 variant (CXCL4L1/PF-4var47-70) strongly inhibits angiogenesis and suppresses B16 melanoma growth in vivo. Mol Cancer Res 2010; 8(3): 322–334.
107. Hagedorn M, Zilberberg L, Wilting J et al. Domain swapping in a COOH-terminal fragment of platelet factor 4 generates potent angiogenesis inhibitors. Cancer Res 2002; 62(23): 6884–6890.
108. Maione TE, Gray GS, Petro J et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 1990; 247(4938): 77–79.
109. Yamaguchi K, Ogawa K, Katsube T et al. Platelet factor 4 gene transfection into tumor cells inhibits angiogenesis, tumor growth and metastasis. Anticancer Res 2005; 25(2A): 847–851.
110. Tuszynski GP, Nicosia RF. The role of thrombospondin-1 in tumor progression and angiogenesis. Bioessays 1996; 18(1): 71–76.
111. Tuszynski GP, Gasic TB, Rothman VL et al. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res 1987; 47(15): 4130–4133.
112. Walz DA. Thrombospondin as a mediator of cancer cell adhesion in metastasis. Cancer Metastasis Rev 1992; 11(3–4): 313–324.
113. Italiano JE Jr, Richardson JL, Patel-Hett S et al. Angiogenesis is regulated by a novel mechanism: pro - and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008; 111(3): 1227–1233.
114. Benoy I, Salgado R, Colpaert C et al. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer 2002; 2(4): 311–315.
115. Caine GJ, Lip GY, Blann AD. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: further evidence for a role of platelets in tumour angiogenesis. Ann Med 2004; 36(4): 273–277.
116. Lee JK, Hong YJ, Han CJ et al. Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol 2000; 17(1): 149–152.
117. Werther K, Christensen IJ, Nielsen HJ. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer 2002; 86(3): 417–423.
118. Battinelli EM, Markens BA, Italiano JE Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 2011; 118(5): 1359–1369.
119. Ma L, Elliott SN, Cirino G et al. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc Natl Acad Sci U S A 2001; 98(11): 6470–6475.
120. Ma L, Hollenberg MD, Wallace JL. Thrombin-induced platelet endostatin release is blocked by a proteinase activated receptor-4 (PAR4) antagonist. Br J Pharmacology 2001; 134(4): 701–704.
121. Ma L, Perini R, McKnight W et al. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci U S A 2005; 102(1): 216–220.
122. Perini R, Wallace JL, Ma L. Roles of platelets and proteinase-activated receptors in gastric ulcer healing. Dig Dis Sci 2005; 50 (Suppl 1): S12–S15.
123. Etulain J, Lapponi MJ, Patrucchi SJ et al. Hyperthermia inhibits platelet hemostatic functions and selectively regulates the release of alpha-granule proteins. J Thromb Haemostasis 2011; 9(8): 1562–1571.
124. Etulain J, Negrotto S, Carestia A et al. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. J Thromb Haemost 2011; 107(1): 99–110.
125. Akerblom B, Lindahl TL, Larsson A. ADP activation induces bFGF binding to platelets in vitro. Ups J Med Sci 2002; 107(3): 165–171.
126. Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev 2006; 20(1): 1–26.
127. Helley D, Banu E, Bouziane A et al. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol 2009; 56(3): 479–484.
128. Kim HK, Song KS, Chung JH et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol 2004; 3 : 376–384.
129. Hayon Y, Dashevsky O, Shai E et al. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J Mol Neurosci 2012; 47(3): 659–665.
130. Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M et al. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion 2006; 46(7): 1199–1209.
131. Kim HK, Song KS, Park YS et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer 2003; 39(2): 184–191.
132. Janowska-Wieczorek A, Wysoczynski M, Kijowski J et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005; 113(5): 752–760.
133. Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer 2009; 124(8): 1773–1777.
Labels
Paediatric clinical oncology Surgery Clinical oncologyArticle was published in
Clinical Oncology

2012 Issue Supplementum 2
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- p63 – an Important Player in Epidermal and Tumour Development
- Detection of Cancer Stem Cell Markers in Sarcomas
- NKT-like Cells are Expanded in Solid Tumour Patients
- Cancer as a Metabolic Disease and Diabetes as a Cancer Risk?
- PThe Regulation of p53 Synthesis
- Protein Quality Control and Cancerogenesis
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- The Role of Platelets in Tumour Growth
- Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
- A Combined Immunoprecipitation and Mass Spectrometric Approach to Determine ΔNp63-Interacting Partners
- Identification and Characterisation of Pro-metastatic Targets, Pathways and Molecular Complexes Using a Toolbox of Proteomic Technologies
- The Biobanking Research Infrastructure BBMRI_CZ: a Critical Tool to Enhance Translational Cancer Research
- Development and Use of Non-FDG PET Radiopharmaceuticals at the Masaryk Memorial Cancer Institute
- New Mechanisms for an Old Drug; DHFR- and non-DHFR-mediated Effects of Methotrexate in Cancer Cells
- Stereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
- Phase I Trial in Oncology – Theory and Practice
- Clinical Oncology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- p63 – an Important Player in Epidermal and Tumour Development
- NKT-like Cells are Expanded in Solid Tumour Patients
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- Phase I Trial in Oncology – Theory and Practice
