Evaluation of Dietary Habits in the Study of Pancreatic Cancer
Vyhodnocení výživových zvyklostí ve studii karcinomu pankreatu
Východiska:
Karcinom pankreatu je závažnou a rychle progredující diagnózou. Méně je známo o úloze výživy v etiologii karcinomu pankreatu. Studie se zaměřila na roli vybraných výživových zvyklostí u karcinomu pankreatu.
Materiál a metody:
Studie případů a kontrol probíhala v České republice ve třech centrech (Olomouc, Ostrava, České Budějovice) v letech 2006 – 2009. Soubor tvořilo celkem 530 osob (310 případů karcinomu pankreatu a 220 kontrolních osob). Údaje byly získávány od subjektů přímo formou rozhovoru se školeným tazatelem a zaznamenány do standardizovaného dotazníku. Data byla vyhodnocena pomocí hrubého odds ratio (OR) a multivariabilní logistické regrese na 95% CI. Statistická analýza byla provedena za použití softwaru STATA v. 10.
Výsledky:
Velmi silný protektivní efekt byl nalezen u nakládaného zelí (OR 0,32; 95% CI 0,19 – 0,55), brokolice (OR 0,37; 95% CI 0,25 – 0,53), vařené cibule (OR 0,14; 95% CI 0,08 – 0,27), rajčat (OR 0,28; 95% CI 0,13 – 0,60), syrové mrkve (OR 0,33; 95% CI 0,20 – 0,56), vařené mrkve (OR 0,35; 95% CI 0,19 – 0,62). V modelu logistické regrese byl nalezen statisticky významný protektivní vliv u konzumace tří a více porcí vařené zeleniny týdně (OR 0,16; 95% CI 0,05 – 0,55) a vysoké konzumace citrusového ovoce (OR 0,46; 95% CI 0,23 – 0,90).
Závěr:
Studie nalezla signifikantní protektivní vliv konzumace tří a více porcí vařené zeleniny týdně a vysoké konzumace citrusového ovoce u karcinomu pankreatu.
Klíčová slova:
karcinom pankreatu – výživa – riziko – studie případů a kontrol
Práce byla podpořena grantem „Effectivity of secondary prevention for cancer in a general practitioner’s office” from Research Support Foundation, Vaduz a grantem IGA_LF_UPOL_2016_003.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Obdrženo:
5. 11. 2015
Přijato:
9. 1. 2016
Authors:
K. Azeem 1; D. Horáková 1
; H. Tomaskova 2; V. Procházka 3
; O. Shonová 4; Arnošt Martínek 5
; Z. Kyselý 1; V. Janout 1; H. Kollárová 1
Authors‘ workplace:
Department of Preventive Medicine, Faculty of Medicine and Dentistry, Palacky University Olomouc, Czech Republic
1; Department of Epidemiology and Public Health, Faculty of Medicine, University of Ostrava, Czech Republic
2; Department of Internal Medicine II – Gastroenterology and Hepatology, University Hospital Olomouc, Czech Republic
3; Department of Gastroenterology, Ceske Budejovice Hospital, Czech Republic
4; Department of Internal Medicine, University Hospital Ostrava, Czech Republic
5
Published in:
Klin Onkol 2016; 29(3): 196-203
Category:
Original Articles
doi:
https://doi.org/10.14735/amko2016196
Overview
Background:
Pancreatic cancer is serious and rapidly progressing condition. Little is known about the role of diet in etiology of pancreatic cancer. The study focused on the role of selected dietary factors related to pancreatic cancer.
Material and Methods:
The case-control study was performed in the Czech Republic in 2006 – 2009, involving three centers in Olomouc, Ostrava and Ceske Budejovice. It comprised a total of 530 persons, of whom 310 had pancreatic cancer and 220 were controls. Data were obtained directly from each participant in an interview with a trained interviewer and entered into a standardized questionnaire. The data were analyzed using a crude odds ratio (OR) and multivariate logistic regression with an adjusted OR and 95% CI. The statistical analysis was performed with the STATA v. 10 software.
Results:
A very strong protective effect was found in pickled cabbage (OR 0.32; 95% CI 0.19 – 0.55), broccoli (OR 0.37; 95% CI 0.25 – 0.53), cooked onion (OR 0.14; 95% CI 0.08 – 0.27), tomatoes (OR 0.28; 95% CI 0.13 – 0.60), raw carrot (OR 0.33; 95% CI 0.20 – 0.56), cooked carrot (OR 0.35; 95% CI 0.19 – 0.62). In logistic regression model, statistically significant protective associations were found in consumption of more than three portions of cooked vegetables per week (OR 0.16; 95% CI 0.05 – 0.55) and high consumption of citrus fruit (OR 0.46; 95% CI 0.23 – 0.90).
Conclusion:
The study found statistically significant protective effect of consumption of more than three portions of cooked vegetables per week and high consumption of citrus fruit.
Key words:
pancreatic cancer – diet – risk – case-control studies
Introduction
Pancreatic cancer is a serious and rapidly progressing condition. Estimated worldwide incidence in 2012 was 337,872 (178,161 in males and 159,711 in females). Over the same period of time, estimated worldwide mortality was 330,372 (173,827 males and 156,564 females) [1]. Since 2008, the absolute numbers have increased by almost 70,000 of new cases per year and by approximately 70,000 deaths per year. The severity of this cancer is evidenced by the fact that by 2020, the number of new cases globally is predicted to rise to approximately 420,000, and the number of deaths should be about 410,000 per year. The gender difference in incidence rates is insignificant, with a male-to-female ratio of 1.12 : 1 [1]. The Czech Republic is among countries with high incidence and mortality rates. When comparing age-standardized rates (World Standard Population), the Czech Republic has the highest incidence in the world (9.7/ 100,000) and ranks third in mortality, behind Armenia and Hungary (8.7/ 100,000) [1]. In 2012, the absolute number of new cases in the Czech Republic was 2,046 (1,080 males and 966 females), and 1,748 persons died of the disease (919 males and 829 females) [2]. Similarly alarming are survival rates, which are shorter than those in most other diseases, and the trend of increasing incidence, which has doubled since 1977 [2]. Approximately 95% of patients die within the first year, mostly in 4 – 8 months from diagnosis. The 5-year survival rate is below 1%; in only a small proportion of patients eligible for radical surgery (approximately 10 – 20% of cases), the 5-year survival ranges from 3.4% to 10%, with a mean survival of 17 – 20 months [3 – 5]. Another problem is early detection of this serious type of cancer, as only 5% of cases are diagnosed in stage I, and the vast majority of cases (more than 37%) are only detected as stage IV [2].
The risk factors associated with the development of pancreatic cancer are both non-modifiable, such as age, gender, hereditary factors or urbanization, race, and modifiable, such as smoking, nutrition (in particular energy intake and related obesity), alcohol consumption, occupational factors (asbestos, pesticides, etc.) and health status (especially chronic pancreatitis, cholelithiasis and diabetes mellitus). Apart from factors with a positive association which increase the risk for development of pancreatic cancer, there are inversely associated factors with protective effects, in particular nutrition and sufficient physical activity [6].
The objective of the present study was to assess the relationship between nutrition and pancreatic cancer.
Materials and methods
A case-control study was performed in the Czech Republic in 2006 – 2009, involving three centers in Olomouc, Ostrava and Ceske Budejovice. It comprised a total of 530 persons, of whom 310 had pancreatic cancer and 220 were controls. The numbers of pancreatic cancer patients and controls recruited in individual centers are shown in Tab. 1.
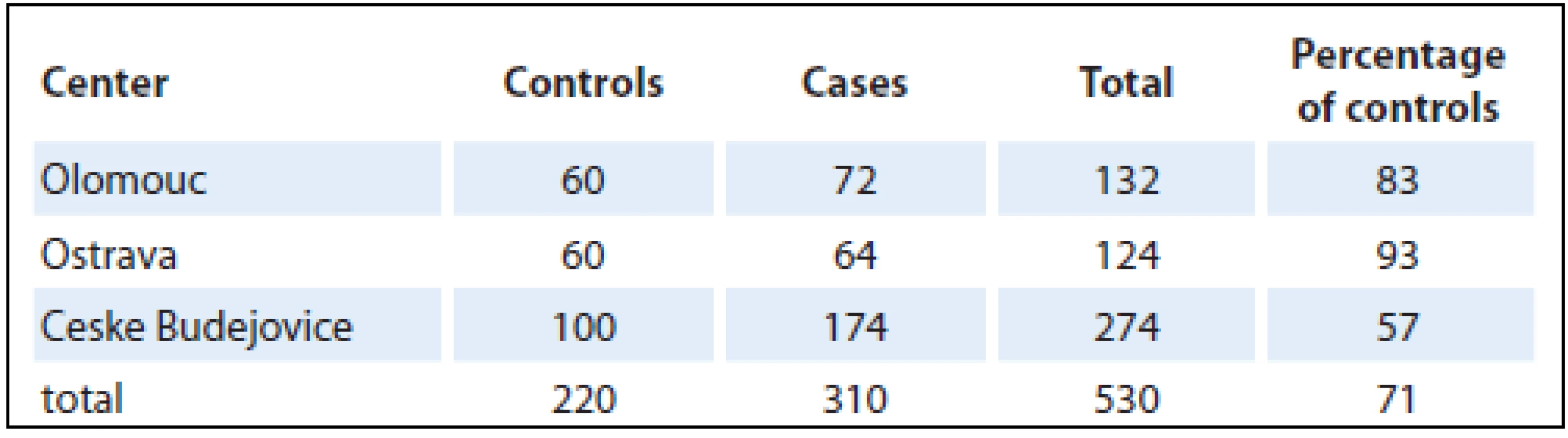
Altogether, there were 303 males and 227 females selected in the centers (University Hospital Olomouc, University Hospital Ostrava, Hospital Ceske Budejovice) from patients newly diagnosed with pancreatic cancer living in the particular region for at least one year (majority of controls – 98% was living in the area for more than 20 years). Controls were addressed in cooperation with selected general practitioners. The population-based control group comprised of individuals from the same regions as cases; their age (± 3 years), gender and health status (individuals without oncologic or life-threatening diagnose) were matched. From general practitioners’ databases, suitable control subjects were selected (more than one control for one case). One randomly selected control from this list was contacted. In case that control subject refused to participate, another suitable control from this list was contacted after that. Control willing to participate was invited for interview (they did not visit the general practitioner, they were selected from registered patients and then invited for interview). In case that the invited control did not attend, control was selected from patients visiting the general practitioner for a minor health condition, routine health examination, mandatory examination required for driving license or workers or preventive purpose. Individuals that agreed to participate were invited for an interview to the study center (participation was high with refusal rate under 10%). Given the difficulties with enrollment of population-based controls, they were fewer in number (71%) than pancreatic cancer patients. Data were obtained directly from each participant in an interview with a trained interviewer and entered into a standardized questionnaire. This contained questions on lifestyle, education, health status, dietary habits and leisure-time physical activity. Also included were questions on body height and weightin different periods of life to calculate BMI. The values for BMI calculations were ascertained for 20 and 40 years of age and two years prior to the disease onset (or, in controls, two years prior to the interview) and at the time of interview. In control group, BMI values were validated from medical records. The analysis was carried out using BMI values two years prior to the disease onset (or the interview in controls), assuming that the weight was not affected by the disease. Each food item was classified into one of six categories according to the frequency of consumption: never (0), less often than once a month (1), 2 – 3 times a month (2), 1 – 2 times a week (3), 3 – 4 times a week (4), 5 – 6 times a week (5). The vegetable and fruit categories and consumption frequencies were combined because of a lack of adequate cell counts. The groups analyzed were: fresh vegetables, cooked vegetables, preserved (pickled) vegetables and fresh fruit. Consumption was categorized as high frequency (category 3 – 5) and low frequency (category 0 – 2). One portion was one piece or approximately 100 grams. The reference group was the lowest frequency.
Given the difficulties with assessing dietary factors and size of the group, the analysis involved responses to the following question: Do you consume the food? Yes/ no. The frequency of consumption was not analyzed as the potential associations are weak and with the group divided into smaller subgroups, the relationship would disappear.
The study was approved by the Ethics Committee of the Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc. All participants gave informed consent prior to their enrollment in the study.
Statistical method
The data were analyzed using a crude odds ratio (OR) and multivariate logistic regression with an adjusted OR and 95% CI. The logistic regression models were adjusted for the most important risk factor with respect to potential confounders. The statistical tests were evaluated at a 5% level of significance. The statistical analysis was performed with the STATA v. 10 software.
Results
The age distribution of cases and controls is shown in Graph 1.

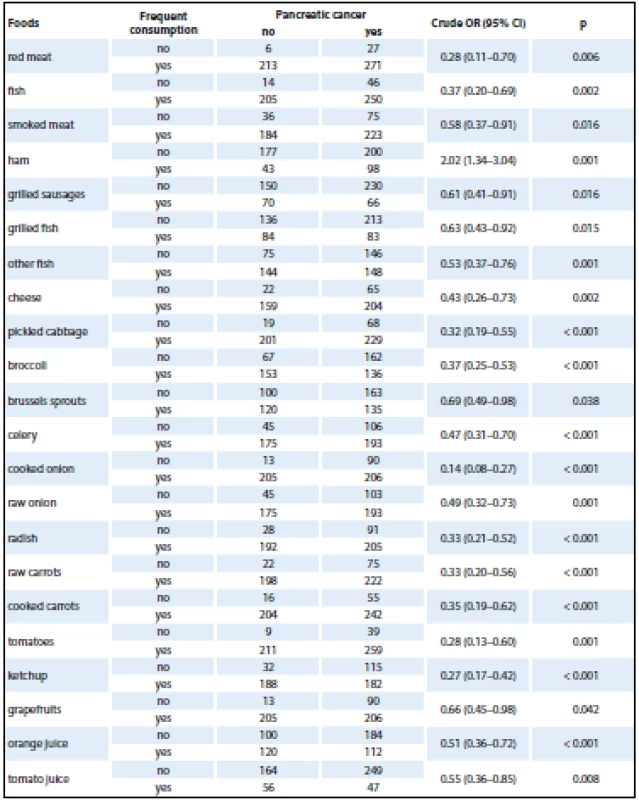
The initial analysis studied the effect of consumption of selected foods using a crude OR (Tab. 2). Only selected foods were included in the analysis, for which statistically significant differences were found with frequency distribution. The analysis revealed a positive association in case of ham consumption. A very strong protective effect was found in pickled cabbage, broccoli, cooked onion, tomatoes and carrot. As each food was analyzed separately, the overall results were provided by logistic regression analysis. The results obtained by logistic regression model analysis, being adjusted for the most important risk factors (age, gender, education, BMI, alcohol, smoking, diabetes mellitus, chronic pancreatitis, physical activity and consumption of selected foods), are shown in Tab. 3. The logistic regression model showed no effect of age, gender or BMI on the development of pancreatic cancer.
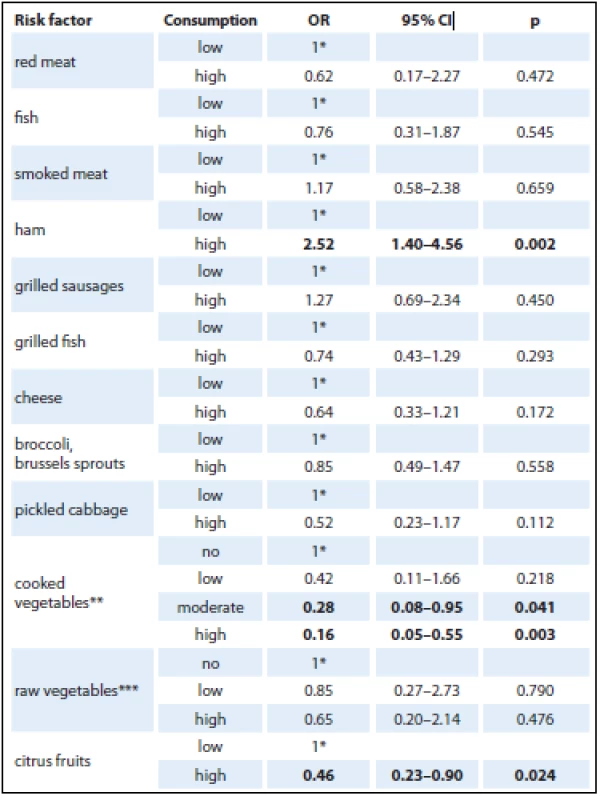
Given the small amount of data obtained for logistic regression model analysis, foods had to be grouped into larger categories (fish, cruciferous vegetables, cooked vegetables, raw vegetables, citrus fruit). Statistically significant protective associations were found in consumption of more than three portions of cooked vegetables per week and high consumption of citrus fruit (Tab. 3).
Discussion
Pancreatic cancer is a serious oncologic disease with a poor prognosis (a 5-year survival of approximately 4%). The main risk factors associated with pancreatic cancer are smoking, nutrition, BMI, alcohol consumption, diabetes mellitus and chronic pancreatitis [7 – 11]. Foods proven to influence pancreatic cancer risk include meat, processed meat, fruit and vegetables. The type and preparation of foods are also important; namely cooking may produce carcinogenic substances.
Red and processed meat
The effect of increased consumption of meat, in particular red and processed meat, has been addressed by numerous studies [12 – 22] and the association is one of the most frequently found ones. Interesting results were reported in a Polish study by Jarosz et al. on the influence of diet on pancreatic cancer between 1960 and 2008. The incidence of pancreatic cancer correlated with the consumption of red meat (correlation coefficients of 0.67 and 0.48 among males and females, resp.) [23].
In a US multiethnic study, pork intake was associated with a 50% increase in risk. The strongest association was with processed meat, with individuals with the highest intake having a 68% increased risk compared with those with the lowest consumption (RR 1.68; 95% CI 1.35 – 2.07; p < 0.01). However, the study failed to find an association between intake of poultry, fish, dairy products, eggs, total fat, saturated fat or cholesterol and pancreatic cancer risk. A statistically significant increase was associated with intake of saturated fat from meat but not from dairy products [24].
In an Italian study, frequent meat consumption was associated with a twofold risk for pancreatic cancer [16]. A Dutch cohort study, on the other hand, did not find any association with intake of red meat, other types of meat, fish or eggs [8,25]. A positive association was observed for the highest consumption of animal proteins [26].
It is not uncommon that increased intake of meat, especially fat meat, is associated with increased consumption of fat and saturated fatty acids. A large NIH-AARP reported increased pancreatic cancer risk with higher consumption of total fat and saturated fats; the strongest association was observed with fat from animal food sources [27]. However, the relationship was not observed in other cohort studies [22,24,25,28]. The positive association with processed meat may be due to carcinogens produced during cooking [24]. The present study showed a 2.5-fold increased OR with higher intake of ham; no relationship was found for the other meat products.
Fish
The association between fish consumption and the incidence of pancreatic cancer varies depending on the type of fish consumed. As in red meat, cooking fish may produce chemicals related to carcinogenesis [29 – 31]. Seven prospective cohort studies found fish consumption to be associated with both incidence and mortality of pancreatic cancer [24,28,29,32 – 35]. High non-fried fish intake was shown to be a protective factor for pancreatic cancer (HR 0.55; 95% CI 0.34 – 0.88; p = 0.045) in the American VITAL Cohort Study [29]. When all preparation methods and shellfish were included in the analysis, no protective effect was observed [29]. Similarly, no association between fish consumption and pancreatic cancer risk was found in the present study.
Cheese
A statistically significant positive association of pancreatic cancer risk with increased saturated fat intake from dairy products (HR 1.19; 95% CI 1.01 – 1.42; p = 0.005) was found in a large prospective NIH-AARP study [27]. The present analysis suggested a protective effect of higher cheese intake but the result was not statistically significant.
Vegetables
Fruit and vegetable consumption is a protective factor for numerous cancers, with varied effects in different tumor locations. The World Cancer Research Fund/ American Institute for Cancer Research Joint Committee [36] as well as the Committee on Medical Aspects of Food and Nutrition Policy [37] concluded that fruit and vegetable consumption clearly has a protective effect on the development of pancreatic cancer. However, outcomes of individual studies are inconsistent. While some cohort studies found an inverse association with vegetable intake, the effect was not confirmed by others [38]. A Finnish study attempted to quantify the effect of high vs. low intake of fruit and vegetables on cancer risk [39]. While their review reported a statistically significant inverse association with high fruit intake (mean RR 0.72; range 0.07 – 0.92), the protective effect of vegetables was just below the level of statistical significance (mean RR 0.80; range 0.32 – 1.03) [39]. An American case-control study on fruit and vegetable consumption and pancreatic cancer risk found protective effects of total fruit (OR 0.57; range 0.37 – 0.86), total vegetable (OR 0.56; range 0.37 – 0.84), dark green vegetable (OR 0.43; range 0.28 – 0.65), deep yellow vegetable (OR 0.58; range 0.39 – 0.86), tomato (OR 0.57; range 0.38 – 0.86) and orange/ grapefruit juice consumption (OR 0.52; range 0.35 – 0.79) [40]. Higher consumption of fruit and vegetables (except potatoes and starchy vegetables) is associated with a lower risk for pancreatic cancer, and the association follows a dose-dependent pattern. Thus, the results support promoting a healthy diet as a pancreatic cancer prevention strategy [40]. An inverse association between high fruit and vegetable intake and pancreatic cancer risk was also reported in a San Francisco case-control study [12] as well as in numerous other studies [13 – 17,41 – 46].
Consumption of cruciferous vegetables has been reported as a protective factor for many cancers. Nearly 50% reduction in pancreatic risk was associated with fresh fruit and cruciferous vegetables in a Canadian study [41]. Regular weekly intake of one or more portions of cruciferous vegetables could protect against pancreatic cancer. Results of a study by Bosetti et al., albeit statistically insignificant, also suggested a potential protective effect (OR 0.84; 95% CI 0.61 – 1.14) [47]. In the present study, however, the protective effect of cruciferous vegetable intake was not confirmed by logistic regression but it was shown by the crude OR.
Fruit
Similarly to vegetables, fruit has been claimed as a protective factor for numerous cancers. The protective effect of fruit and vegetable intake was reported in case-control studies but not in cohort studies [8]. According to a study by Jarosz et al., in 1990 – 2008, pancreatic cancer morbidity correlated with the consumption of fruit (correlation coefficients of −0.62 and −0.50 among males and females, resp. [23]. The aforementioned Canadian case-control study found 49% risk reduction in males consuming high amounts of fruit and vegetables [41]. A negative correlation was observed for diet rich in sugars (mainly derived from fruit) [26]. A European prospective cohort study suggested that higher consumption of fruit and vegetables was not associated with decreased risk of pancreatic cancer [48].
Another protective factor against pancreatic cancer, as stated in some studies, is citrus fruit intake. However, the results are inconsistent, with a statistically significant protective effect being only shown in case-control studies [49]. In accordance with some studies, the present study found a 54% risk reduction using analysis with adjusted OR, and the result was statistically significant.
Although some studies have revealed the risk of developing pancreatic cancer to be lower in people consuming plenty of fruits and vegetables, it will be reasonable to reconsider their interpretation; people who consume fruits and vegetables regularly smoke less. Therefore, there is no surprise in conclusions reached in a study that ‘head to head’ opposed the so-called prudent diet vs. Western diet and proved the same risk of pancreatic cancer in both groups [50].
Several studies have been concerned with specific dietary patterns with respect to pancreatic cancer [24,41,51 – 53]. So far, however, the study results have been rather inconsistent, with a potential benefit of prudent diet being reported in two cohort studies [24,53] and one case-control study [41] but no association being found by four cohort studies [24,51,54] and one case-control study [41]. Diet rich in fruit and vegetables, fish, pulses, whole grains and low-fat foods was associated with approximately 50% reduction of the relative risk of pancreatic cancer in males (OR 0.51; 95% CI 0.31 – 0.84; p = 0.001) and females (OR 0.51; 95% CI 0.29 – 0.90; p = 0.04) [52]. The Western pattern diet, characterized by high intakes of red and processed meat, potato chips, sugary beverages, sweets, high-fat dairy products, eggs and refined grains, was associated with a 2.4-fold increased risk of pancreatic cancer in males (95% CI 1.3 – 4.2; p = 0.008) but not in females [52].
A role may also be played by carcinogenic substances present in the diet, for example heterocyclic amines [55]. Although studies suggest a significant impact of carcinogens and mutagens in average individuals [56], the level of the risk cannot be confirmed by current methods.
It is likely that potential benefits from the diet are due to a combination of food constituents rather than single components acting in isolation. Future efforts need to recognize the integrative nature of dietary exposures and attempt to study nutrients in the larger context of the foods and diets in which they are consumed [22].
Strengths and weaknesses of the study
The case-control study included incident cases of pancreatic cancer and population control group. If prevalent cases were selected, identified factors may be related more to survival with the disease than to development of the disease (incidence).
Design of case-control studies is prone to a variety of biases (selection, interviewer, recall, misclassification, etc.) and thus results interpretation should be carefully considered. Our findings are strengthened by the use of a population control group. The presented analysis was limited by the use of a questionnaire that is information self-reported by subjects enrolled in the study and thus potentially biased. The bias was minimized by the facts that the same type of standardized questionnaire was used for both pancreatic cancer patients and control subjects and that it was filled in by a trained interviewer. Interviewer was not blinded to disease status (blinding was not possible due to character of the disease and place of interview). Recall bias is always a concern in case-control design as cases can recall exposure easier than controls. Recall in control group was limited by the use of data from general practitioner databases. However, in some cases, verification was not possible and we had to rely solely on data reported by the subjects. Relationship between nutrition and risk of cancer can be also modified by confounding factors. Main confounders (obesity, smoking, health status) were assessed together with nutrition and models of logistic regression were used to adjust for these main confounders. In spite of certain limitations, the analysis provided findings consistent with earlier studies and thus suggested potential associations.
Conclusion
Nutrition is one of the most important risk factors for the development of cancer. It is estimated that the incidence rates may be reduced by 30 – 40% if recommended dietary patterns are followed. The present study aimed to assess the role of selected dietary factors with respect to pancreatic cancer. The overall results showed protective effects of moderate and high intake of cooked vegetables and a high consumption of citrus fruit. On the other hand, ham intake resulted in an OR increased by 152%. However, the association should be confirmed in prospective analytical studies.
This study was supported by grant ‘Effectivity of secondary prevention for cancer in a general practitioner’s office’ from Research Support Foundation, Vaduz and by the grant IGA_LF_UPOL_2016_003.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Assoc. Prof. Helena Kollarova, MD, PhD
Department of Preventive Medicine
Faculty of Medicine and Dentistry
Palacky University Olomouc
Hnevotinska 3
775 15 Olomouc
Czech Republic
e-mail: helena.kollarova@upol.cz
Submitted: 5. 11. 2015
Accepted: 9. 1. 2016
Sources
1. Ferlay J, Soerjomataram I, Ervik M et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer 2013. [online]. Available from: www: http:/ / globocan.iarc.fr.
2. Dušek L, Mužík J, Kubásek M et al. Epidemiologie zhoubných nádorů v České republice. Masarykova univerzita 2005. Version 7.0. [online]. Dostupné z: http:/ / www.svod.cz.
3. Hucl T. Karcinom pankreatu. Gastroent Hepatol 2012; 66(5): 350 – 356.
4. Han SS, Jang JY, Kim SW et al. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas 2006; 32(3): 271 – 275.
5. Leffler J. Karcinom pankreatu 2005 současný stav problematiky diagnostiky a léčby. Intern Med Prax 2005; 7(7 – 8): 360 – 363.
6. Bao Y, Michaud DS. Physical activity and pancreatic cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2008; 17(10): 2671 – 2682.
7. Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis 2010; 28(4 – 5): 645 – 656.
8. Nitsche C, Simon P, Weiss FU et al. Environmental risk factors for chronic pancreatitis and pancreatic cancer. Dig Dis 2011; 29(2): 235 – 242. doi: 10.1159/ 000323933.
9. Urayama KY, Holcatova I, Janout V et al. Body mass index and body size in early adulthood and risk of pancreatic cancer in a central European multicenter case-control study. Int J Cancer 2011; 129(12): 2875 – 2884. doi: 10.1002/ ijc.25959.
10. Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012; 51(1): 53 – 63. doi: 10.1002/ mc.20778.
11. Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Sur Oncol 2013; 107(1): 1 – 7. doi: 10.1002/ jso.23149.
12. Chan JM, Wang F, Holly EA. Pancreatic cancer, animal protein and dietary fat in a population-based study, San Francisco Bay Area, California. Cancer Causes Control 2007; 18(10): 1153 – 1167.
13. Olsen GW, Mandel JS, Gibson RW et al. A case-control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health 1989; 79(8): 1016 – 1019.
14. Lyon JL, Slattery ML, Mahoney AW et al. Dietary intake as a risk factor for cancer of the exocrine pancreas. Cancer Epidemiol Biomarkers Prev 1993; 2(6): 513 – 518.
15. Ghadirian P, Nkondjock A. Consumption of food groups and the risk of pancreatic cancer: a case-control study. J Gastrointest Cancer 2010; 41(2): 121 – 129. doi: 10.1007/ s12029-009-9127-2.
16. Polesel J, Talamini R, Negri E et al. Dietary habits and risk of pancreatic cancer: an Italian case-control study. Cancer Causes Control 2010; 21(4): 493 – 500. doi: 10.1007/ s10552-009-9480-2.
17. Anderson KE, Sinha R, Kulldorff M et al. Meat intake and cooking techniques: associations with pancreatic cancer. Mutat Res 2002; 506 – 507 : 225 – 231.
18. Fernandez E, La Vecchia C, Decarli A. Attributable risks for pancreatic cancer in northern Italy. Cancer Epidemiol Biomarkers Prev 1996; 5(1): 23 – 27.
19. Bueno deMesquita HB, Maisonneuve P, Runia Set al. Intake of foods and nutrients and cancer of the exocrine pancreas: a populationbased case-control study in The Netherlands. Int J Cancer 1991; 48(4): 540 – 549.
20. Falk RT, Pickle LW, Fontham ET et al. Lifestyle risk factors for pancreatic cancer in Louisiana: a case-control study. Am J Epidemiol 1988; 128(2): 324 – 336.
21. Cross AJ, Leitzmann MF, Gail M et al. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med 2007; 4(12): e325.
22. Gibson TM, Ferrucci LM, Tangrea JA et al. Epidemiological and clinical studies of nutrition. Semin Oncol 2010; 37(3): 282 – 296. doi: 10.1053/ j.seminoncol.2010.05.011.
23. Jarosz M, Sekuła W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in poland in 1960 – 2008. Gastroenterol Res Pract 2012; 2012 : 682156. doi: 10.1155/ 2012/ 682156.
24. Nothlings U, Wilkens LR, Murphy SP et al. Meat and fat intake as risk factors for pancreatic cancer: the Multiethnic Cohort Study. J Natl Cancer Inst 2005; 97(19): 1458 – 1465.
25. Heinen MM, Verhage BA, Goldbohm RA et al. Meat and fat intake and pancreatic cancer risk in the Netherlands Cohort Study. Int J Cancer 2009; 125(5): 1118 – 1126. doi: 10.1002/ ijc.24387.
26. Lucenteforte E, Talamini R, Bosetti C et al. Macronutrients, fatty acids, cholesterol and pancreatic cancer. Eur J Cancer 2010; 46(3): 581 – 587. doi: 10.1016/ j.ejca.2009.09.024.
27. Thiébaut AC, Jiao L, Silverman DT et al. Dietary fatty acids and pancreatic cancer in the NIH-AARP diet and health study. J Natl Cancer Inst 2009; 101(14): 1001 – 1011. doi: 10.1093/ jnci/ djp168.
28. Michaud DS, Giovannucci E, Willett WC et al. Dietary meat, dairy products, fat, and cholesterol and pancreatic cancer risk in a prospective study. Am J Epidemiol 2003; 157(12): 1115 – 1125.
29. He K, Xun P, Brasky TM et al. Types of fish consumed and fish preparation methods in relation to pancreatic cancer incidence: the VITAL Cohort Study. Am J Epidemiol 2013; 177(2): 152 – 160. doi: 10.1093/ aje/ kws232.
30. Anderson KE, Kadlubar FF, Kulldorff M et al. Dietary intake of heterocyclic amines and benzo(a)pyrene: associations with pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2005; 14(9): 2261 – 2265.
31. Stolzenberg-Solomon RZ, Cross AJ, Silverman DT et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev 2007; 16(12): 2664 – 2675.
32. Zheng W, McLaughlin JK, Gridley G et al. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States). Cancer Causes Control 1993; 4(5): 477 – 482.
33. Stolzenberg-Solomon RZ, Pietinen P, Taylor PR et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol 2002; 155(9): 783 – 792.
34. Larsson SC, Hakanson N, Permert J et al. Meat, fish, poultry and egg consumption in relation to risk of pancreatic cancer: a prospective study. Int J Cancer 2006; 118(11): 2866 – 2870.
35. Lin Y, Kikuchi S, Tamakoshi A et al. Dietary habits and pancreatic cancer risk in a cohort of middle-aged and elderly Japanese. Nutr Cancer 2006; 56(1): 40 – 94.
36. Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/ World Cancer Research Fund, American Institute for Cancer Research 1997. Nutrition 1999; 15(6): 523 – 526.
37. Nutritional aspects of the development of cancer. Report of the Working Group on Diet and Cancer of the Committee on Medical Aspects of Food and Nutrition Policy. Rep Health Soc Subj (Lond) 1998; 48(i – xiv): 1 – 274.
38. Larsson SC, Hakansson N, Naslund I et al. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiol Biomarkers Prev 2006; 15(2): 301 – 305.
39. Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer 2006; 54(1): 111 – 142.
40. Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control 2011; 22(12): 1613 – 1625. doi: 10.1007/ s10552-011-9838-0.
41. Nkondjock A, Krewski D, Johnson KC et al. Canadian Cancer Registries Epidemiology Research Group. Dietary patterns and risk of pancreatic cancer. Int J Cancer 2005; 114(5): 817 – 823.
42. Silverman DT, Swanson CA, Gridley G et al. Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. J Natl Cancer Inst 1998; 90(22): 1710 – 1719.
43. Baghurst PA, McMichael AJ, Slavotinek AH et al. A case-control study of diet and cancer of the pancreas. Am J Epidemiol 1991; 134(2): 167 – 179.
44. Norell SE, Ahlbom A, Erwald R et al. Diet and pancreatic cancer: a case-control study. Am J Epidemiol 1986; 124(6): 894 – 902.
45. Gold EB, Gordis L, Diener MD et al. Diet and other risk factors for cancer of the pancreas. Cancer 1985; 55(2): 460 – 467.
46. Ji BT, Chow WH, Gridley G et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiol Biomarkers Prev 1995; 4(8): 885 – 893.
47. Bosetti C, Filomeno M, Riso P et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol 2012; 23(8): 2198 – 2203. doi: 10.1093/ annonc/ mdr604.
48. Vrieling A, Verhage BA, van Duijnhoven FJ et al. Fruit and vegetable consumption and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2009; 124(8): 1926 – 1934. doi: 10.1002/ ijc.24134.
49. Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Pancreas 2009; 38(2): 168 – 174. doi: 10.1097/ MPA.0b013e318188c497.
50. Dobrila-Dintinjana R, Vanis N, Dintinjana M et al. Etiology and oncogenesis of pancreatic carcinoma. Coll Antropol 2012; 36(3): 1063 – 1067.
51. Michaud DS, Skinner HG, Wu K et al. Dietary patterns and pancreatic cancer risk in men and women. J Natl Cancer Inst 2005; 97(7): 518 – 524.
52. Chan JM, Gong Z, Holly EA et al. Dietary patterns and risk of pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area. Nutr Cancer 2013; 65(1), 157 – 164. doi: 10.1080/ 01635581.2012.725502.
53. Jiao L, Mitrou PN, Reedy J et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 2009; 169(8): 764 – 770. doi: 10.1001/ archinternmed.2009.46.
54. Inoue-Choi M, Flood A, Robien K et al. Nutrients, food groups, dietary patterns, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2011; 20(4): 711 – 714. doi: 10.1158/ 1055-9965.EPI-11-0026.
55. Ericson U, Wirfält E, Mattisson I et al. Dietary intake of heterocyclic amines in relation to socio-economic, lifestyle and other dietary factors: estimates in a Swedish population. Public Health Nutr 2007; 10(6): 616 – 627.
56. Ferguson LR. Natural and human-made mutagens and carcinogens in the human diet. Toxicology 2002; 181 – 182 : 79 – 82.
Labels
Paediatric clinical oncology Surgery Clinical oncologyArticle was published in
Clinical Oncology

2016 Issue 3
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- History of Oncology in Slovakia
- Lynch Syndrome – the Pathologist’s Diagnosis
- Current Possibilities for Predicting Responses to EGFR Blockade in Metastatic Colorectal Cancer
- Changes in the CD8+ Density of Tumor Infiltrating Lymphocytes after Neoadjuvant Radiochemotherapy in Patients with Rectal Adenocarcinom
- Localized Amyloidosis Involving the Nasal Cavity
- Carcinoid of the Appendix Goblet Cells Metastasize to the Orbit – a Clinical Case Report and Review of the Literature
- Evaluation of Dietary Habits in the Study of Pancreatic Cancer
- Screening of Psychological Distress 4.5 Years after Diagnosis in Breast Cancer Patients Compared to Healthy Population
- Clinical Oncology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- History of Oncology in Slovakia
- Lynch Syndrome – the Pathologist’s Diagnosis
- Localized Amyloidosis Involving the Nasal Cavity
- Evaluation of Dietary Habits in the Study of Pancreatic Cancer
