Grunting in a Genetically Modified Minipig Animal Model for Huntington’s Disease – Pilot Experiments
Chrochtání u geneticky modifikovaného zvířecího modelu miniprasat pro Huntingtonovu chorobu – pilotní experimenty
Huntingtonova nemoc (HN) je autozomálně dominantní neurodegenerativní onemocnění charakterizované poškozením volních a mimovolních pohybů, poruchami chování a zhoršením kognitivních funkcí. Společně s hlavními motorickými příznaky byly poruchy hlasu a řeči pozorovány u většiny pacientů s HN. Zvířecí prasečí model je často používán pro výzkum v preklinických studiích. I přes zjevné rozdíly v anatomii artikulačních orgánů mezi prasaty a lidmi lze očekávat stejné trendy u patofyziologických mechanizmů s ohledem na chrochtání i lidskou fonaci. Hlavním cílem této studie bylo proto navržení vhodného experimentu, který umožní získání dostatečně dlouhého záznamu chrochtání od co největšího počtu prasátek. Dalším cílem studie bylo zrealizovaní výsledné verze experimentu na celé databázi a vyhodnocení množství a kvality získaných nahrávek. Databáze použitá pro studii zahrnovala 17 HN transgenních miniprasátek a 16 zdravých sourozenců ze stejných vrhů. Testované varianty experimentu, provedené na části databáze zahrnující čtyři prasnice, byly rozděleni do čtyř podskupin: (a) pozitivní – krmení, (b) pozitivní – zvuková stimulace, (c) negativní – bránění v pohybu, (d) negativní – nepříjemné doteky. Hodnocení kvality získaných nahrávek bylo provedeno s pomocí audio softwaru, ve kterém bylo izolováno čisté prasečí chrochtání a všechny akustické artefakty vymazány. Nejlepších výsledků bylo dosaženo s použitím experimentu při kterém: (i) je záznamové zařízení upevněno na tělo prasátka, (ii) prasátko je ponecháno několik minut o samotě v místnosti, aby se uklidnilo a (iii) osoba vstoupí do místnosti a snaží se nabízet prasátku krmivo zatímco před ním couvá. V důsledku tohoto jednání prasátko následuje osobu s krmením, což je doprovázeno chrochtáním. Dostatečně dlouhé (20 chrochtnutí a více) a čisté nahrávky byly získány od 24 z 33 prasátek (73 %). Závěrem lze tedy říci, že experiment je proveditelný.
Klíčová slova:
Huntingtonova nemoc – chrochtání – transgenní model miniprasat – animální modely – poruchy hlasu a řeči
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
T. Tykalova 1; J. Hlavnicka 1; M. Macakova 2; M. Baxa 2; R. Cmejla 1; J. Motlik 2; J. Klempir 3,4; J. Rusz 1,3
Authors‘ workplace:
Department of Circuit Theory, Faculty of Electrical Engineering, Czech Technical University in Prague, Czech Republic
1; Institute of Animal Physiology and Genetics, AS CR, v. v. i., Libechov, Czech Republic
2; Department of Neurology and Centre of Clinical Neuroscience, 1st Faculty of Medicine, Charles University in Prague, Czech Republic
3; Institute of Anatomy, 1st Faculty of Medicine, Charles University in Prague, Czech Republic
4
Published in:
Cesk Slov Neurol N 2015; 78/111(Supplementum 2): 61-65
doi:
https://doi.org/10.14735/amcsnn20152S61
Overview
Huntington’s disease (HD) is an autosomal-dominant neurodegenerative disorder characterized by the impairment of voluntary and involuntary movements, behavioral disorders and cognitive decline. Besides the main motor symptoms, voice and speech disorders have been documented in a large majority of patients with HD. The animal model of pigs is often used in preclinical studies. Although there are obvious differences in the anatomy of the articulation organs between pigs and humans, the same trends in pathophysiological mechanisms can be expected in both grunting and human phonation. The main aim of the study was therefore to design a suitable experiment that would allow for acquisition of a sufficiently long recording of grunting from as many pigs as possible. The second goal was to perform the final version of the experiment in all available pigs and to evaluate the amount and quality of the acquired recordings. The database consists of 17 HD transgenic minipigs and 16 healthy siblings. Tested variants of the experiment, performed on subgroup of four sows, were divided into four subgroups: (a) positive – feeding, (b) positive – sound stimulation, (c) negative – hindering in movement, (d) negative – unpleasant touch. The evaluation of the quality of the elicited recording was performed using audio software where pure pig grunting was selected and all acoustic artefacts deleted. The best results were reached using the experiment in which: (i) a recording device is put on the pig’s body, (ii) the pig is left alone for few minutes in the pen in order to calm down, and (iii) a person enters the room and tries to offer the pig food while walking backwards. As a result, the pig follows the person and grunts. Sufficiently long (20 single grunts or more) and clear recordings were received from 24 out of 33 pigs (73%). The realisation of the experiment is therefore possible.
Key words:
Huntington’s disease – grunting – transgenic pigs – animal models – voice and speech disorders
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Introduction
Huntington’s disease
Huntington’s disease (HD) is an autosomal ‑ dominant inherited neurodegenerative disorder caused by an expansion in the number of CAG repeats (36 repeats or more) on the short arm of chromosome 4p16.3 in the Huntingtone gene [1,2], which is characterized by uncoordinated body movements, psychological dysfunction and a reduction in cognitive decline resulting in dementia. The prevalence of HD is estimated to be about 4 – 8 subjects for 100,000 people [3] with the onset of the first symptoms typically occurring in the fourth decade of life. From a clinical perspective, HD is primarily manifested by involuntary movements termed as chorea, which may be accompanied by bradykinesia, motor impersistence, and deficits in movement planning, aiming, tracing, and termination [4,5]. Additionally, rigidity or/ and dystonia may occur in some cases as HD progresses. Although the onset of symptoms and the rate of progression may vary, the prognosis implies relentlessdeterioration with significantly reduced life expectancy, as no treatment is currently available to stop disease progression [6].
Animal models of HD
Animal models are crucial in the development, evaluation and validation of new drugs and therapies for neurological disorders. A wide range of HD animal models have been generated to date including nonmammalian animals (Drosophila, C. elegans or zebrafish), rodents, sheep, pigs and non‑human primates (for review see [7].) In general, each of these animal models shows some biochemical and neuronal features similar to HD in humans [7]. In particular, most effort has been put into research of mouse and rat HD models, as these mammals are relatively cheap to maintain and easy to breed. Although the rodents contributed significantly to the understanding of the molecular basis for behavioural and neuronal abnormalities [7 – 9], rodents and humans differ in many ways. For example, HD and other neurodegenerative diseases are age ‑ dependent disorders, yet the lifespans of rodents and humans differ drastically, indicating that aging processes in different species are not identical [10]. In addition, the anatomy, physiology and function of the brain in large mammals are much more complex than those of rodents. From the clinical point of view, the rodents’ small brain size also limits their utility for using non‑invasive imaging methods such as magnetic resonance imaging which are commonly used in human patients [11]. These differences clearly indicate that the anatomy as well as physiological function of monkeys and pigs are much closer to humans than those of rodents, and also explain why large animal models would be better for mimicking the pathological features seen in human patients [10]. Using of transgenic minipigs is also more cost‑effective and raises fewer ethical issues compared to primates.
Voice and speech disorders in human HD and pig animal model of HD
Voice and speech disorders, known as hyperkinetic dysarthria, are a common sign of HD, developing in more than 90% of HD patients in the course of the disease [12]. Typical signs of hyperkinetic dysarthria in HD include voice dysfunction, articulation deficits, irregular loudness variation and abnormalities in speech timing [12 – 16]. Interestingly, slight changes in voice and speech production have also been observed in persons with preclinical stages of HD [13,16]. Voice and speech disorders may thus have the potential to serve as a valuable biomarker of disease onset [13,16] and may help to determine the appropriate time for medical interventions in the preclinical trials focused on neuroprotective treatment.
Although there are obvious differences in the anatomy of articulation organs between pigs and humans, one might expected that similar trends of decreasing voice quality and articulatory undershooting will be observed in both grunting and human phonation due to the same HD‑related pathophysiology mechanisms. For instance, if the imprecise articulation of vowels in human HD patients is characterized by centralization of formant frequencies [12], a similar trend can be expected in HD pig grunting since the articulatory organs (tongue, lips, soft palate, jaw, and pharynx cavity) in both models should have been influenced by the same motor disturbances such as chorea, rigidity or bradykinesia. Indeed, in a previous study [14] investigating phonatory dysfunction in 34 HD patients, a correlation was found between voice deficits and involuntary (rigidity, dystonia, and chorea) components of Unified HD Rating Scale.
Types of grunting in healthy pigs
The previous study [17] investigated the types of vocalization in a group of 67 large white pigs and revealed three distinctive types of pig grunting: (a) single grunts – appear to be associated with investigatory behaviour or contact calls in group, (b) single squeals – may have similar function as single grunts but result from a higher level of arousal and (c) rapidly repeated grunts – appear to have either a greeting or threat function [17]. From acoustical point of view, squeals that are mainly expressed in situations of urgent threat or high stress are high‑frequency callswhile grunts are typically low ‑ frequency calls [18]. Although there is no previous research focused on possible acoustic grunting changes due neurodegenerative disorders in animal pig models, it might be expected that low ‑ frequency calls, i.e. rapidly repeated or single grunts, will be more suitable for acoustic analyses.
Recording experiment
In the previously published literature [17 – 22] focusing mainly on investigation of stresscorrelates or possibility to distinguish different emotional states, several types of experiments were used to obtain pig vocalization. It appears that the most popular experiment involved castration [18 – 20] or isolation of the subject from group mates [21] or piglets from sows [18,20]. Another experiment applied positive stimulations such as nursing grunting or reunion with sows to provoke grunting [18,20,22]. However, most of these experiments are limited to sows and piglets or cannot be repeated frequently, which would limit their practical usage in the preclinical trials where determination of the appropriate time for medical interventions would be of interest.
The aims of the study
The main aim of the study was to design a suitable experiment that would allow acquisition of a sufficiently long recording of grunting from as many subjects as possible and, at the same time, would be applicable to pigs of different ages and genders with the possibility of repeating the experiment several times with the same subject. The second goal was to perform the final version of the experiment with all available pigs and to evaluate the amount and quality of the acquired recordings.
Methodology
Database of pigs
The database consists of 17 HD transgenic minipigs (4 male, 13 female) and 16 healthy siblings (1 male, 15 female). The animals were from the first, second and third generation. The mean age of HD transgenic minipigs group was 32.5 (SD 15.6, range 8 – 60) months while the mean age of healthy minipigs group was 23.7 (SD 16.3, range 8 – 60) months. No significant differences in age were found between the healthy and the transgenic group (p = 0.12). All animals were born and bred at the experimental farm of the Institute of Animal Physiology and Genetics in Libechov [23].
Recording device
Speech samples were recorded using 24bit 96kHz wave/ MP3 recorder (Edirol R ‑ 09HR, Roland, Japanese) and a head ‑ mounted condenser microphone (Beyerdynamic Opus 55, Heilbronn, Germany) which were previously successfully used by our group for the recording and subsequent analyses of voice and speech disorders in persons with HD [12,14]. Grunt signals were sampled at 48 kHz with 16bit resolution. The recording volume acquire was kept constant over the recording procedure in all pigs. The recorder was put into a small cloth case with lockable zippers and an adjustable fabric belt and fastened around the pig’s body so that the recorder was situated under the pig’s belly. The microphone was then fixed with sticking tape on the top of the pig’s head close to its ears. In addition, the cable connecting microphone with recorder was fixed on the pig’s back, so as not to become twisted between the pig’s forelegs. As a result, the microphone was situated approximately 15 to 25 cm from the pig’s snout based on its size. See Fig. 1 for details of recording device fixation.

Proposed versions of the experiment
In previously published literature, mainly experiments including piglets or nursing sows were used [18,20,22] for acquisition of pig vocalizations. However, reliance on the female gender and specific time periods would limit the practical usage of such experiments in the preclinical trials where determination of the appropriate time for medical interventions would be of interest. Moreover, experiments with a strongly negative context are also not feasible, as pigs might not be willing to collaborate if repeating of experiment is required. Therefore we decided to design and test other possible variants of experimental design. For testing purposes, a subgroup of 4 pigs including 2 healthy and 2 transgenic sows from the second generation was used. Tested variants of the experiment were divided into four subgroups:
1. Positive – feeding:
- bowl with fodder brought into the room by well‑known person,
- small titbit (10 raisins) hidden around the room or in a heap of straw,
- bowl with fodder hidden under a large perforated object.
2. Positive – sound stimulation:
- recorded sound of another pig’s grunting playing from loudspeaker,
- recorded sound of barking dog playing from loudspeaker.
3. Negative:
- a known person will enter the room and stay motionlessly facing a wall for 5 min,
- the pig will be left alone in the room for 5 min while behind the door people will speak loudly,
- the pig will be left alone in the room for 5 min while behind the door another pig of the opposite sex or their piglets will be fetched up,
- a person will hinder the pig from strolling freely around the room using big plastic boards,
- the pig will be annoyed by a person (touching ears, scratching on head etc).
4. Another modification of experiment based on acquired experiences.
The final standardised version of the experiment was proposed based on results obtained from the tested variants and performed with all 33 available subjects.
Evaluation of acquired recordings
The evaluation of the amount and quality of elicited recording was performed using the acoustics software Praat [24]. The parts of recordings that included pure pig grunting with no acoustic artefacts (i.e., human speech,smacking or chewing of pigs, stamping of hoofs, tinkling of handle of bucket or other background noise) were separated. In addition, all parts of the recording that were too loud or too quiet with respect to setting of the recording acquire were also deleted. We selected only rapidly ‑ repeated or single grunts; high‑frequency squeals were not involved. To ensure the same experimental condition for all pigs, the gruntings were selected within first 5 min after the actual beginning of the experiment. For the purposes of further analyses, rapidly repeated grunts were recalculated and treated as single grunts; as a result, 30 „single grunts“ per pig were selected if available. Subsequently, the type of presented grunting, the total number of grunts, and duration of recording procedure were calculated for each pig. Finally, the percentage of correctly recorded pigs was assigned.
Statistics
As the Kolmogorov ‑ Smirnov test for independent samples showed that the parameters were normally distributed, the two sample t‑test was used to assess group differences. The Pearson coefficient was calculated to determine correlations between age and number of grunts. The level of significance was set at p < 0.05.
Results
Proposed versions of the experiment
The tested sows did not respond optimally for any of the proposed experiments mentioned in the methodology. In particular, they did not react at all to the person, who brought the bowl with fodder into the room. Searching for raisins hidden around the room resulted in continued smacking but no grunting. Although the pigs were very interested in the bowl with fodder hidden under a large perforated crate, the occasional grunting they performed was also accompanied by disturbing acoustic artefacts in the form of noise caused due to hitting into the box in an effort to lift it, which prevented the practical use of the recordings. Furthermore, the crate was quickly demolished by the pigs.
Positive sound stimulations in the form of another pig’s grunting or a barking dog also resulted in the pig’s reaction; however, the response was only in the form of adjustment of the head and ears in the direction of the sound. In addition, they were interested in sound stimulus only for a short period of time and when they heard the same recording for the third time, they do not react at all.
Negative stimulation turned to be slightly better with respect to grunting. In particular, pigs did not respond to the familiar person ignoring them or to the presence/ occasional grunting of other pig or piglets behind the wooden door, i.e., they did not try to communicate with them. On the other hand, hindering pigs from strolling freely around the room using big plastic boards and annoying them with unpleasant touches indeed resulted in pigs grunting. Pigs were annoyed mainly by touches associated with slapping, scratching or squeezing under the neck and around ears. However, hindering with boards led mainly to single squeals, and single grunts were presented only occasionally. With respect to irritating touches, apart from the single squeals also single grunts relatively often occurred.
Design of final experiment
Due to the disappointing results of the originally proposed experiments, various modifications were subsequently tested. The best results were eventually reached using experiment where: (i) a recording device is put on the pig’s body, (ii) the pig is left alone for a few minutes in the pen in order to calm down, and (iii) a person enters the room and tries to offer the pig fodder while walking backwards. As a result, the pig follows the person and grunts. Food is necessary to offer using the aids that pigs know from everyday life; in our case it was a metal bucket and a plastic scoop of angular shape (Fig. 2). To increase the effectiveness of the experiment (the amount of obtained grunting), it is feasible to use supportive sounds, e. g., clinking of metal handle with the bucket. In addition, it is useful to perform the experiment with hungry pigs (omitting two feeding doses) and with a well‑known figurant (a person from whom the pigs are accustomed to get food). Although the experiment worked successfully even without these supportive conditions, more time was demanded to obtain the required amount of grunting. The presented design of the experiment led mainly to rapidly repeated grunts or single grunts that are thought to be more suitable for acoustic analysis than squeals.

Evaluation of acquired recordings
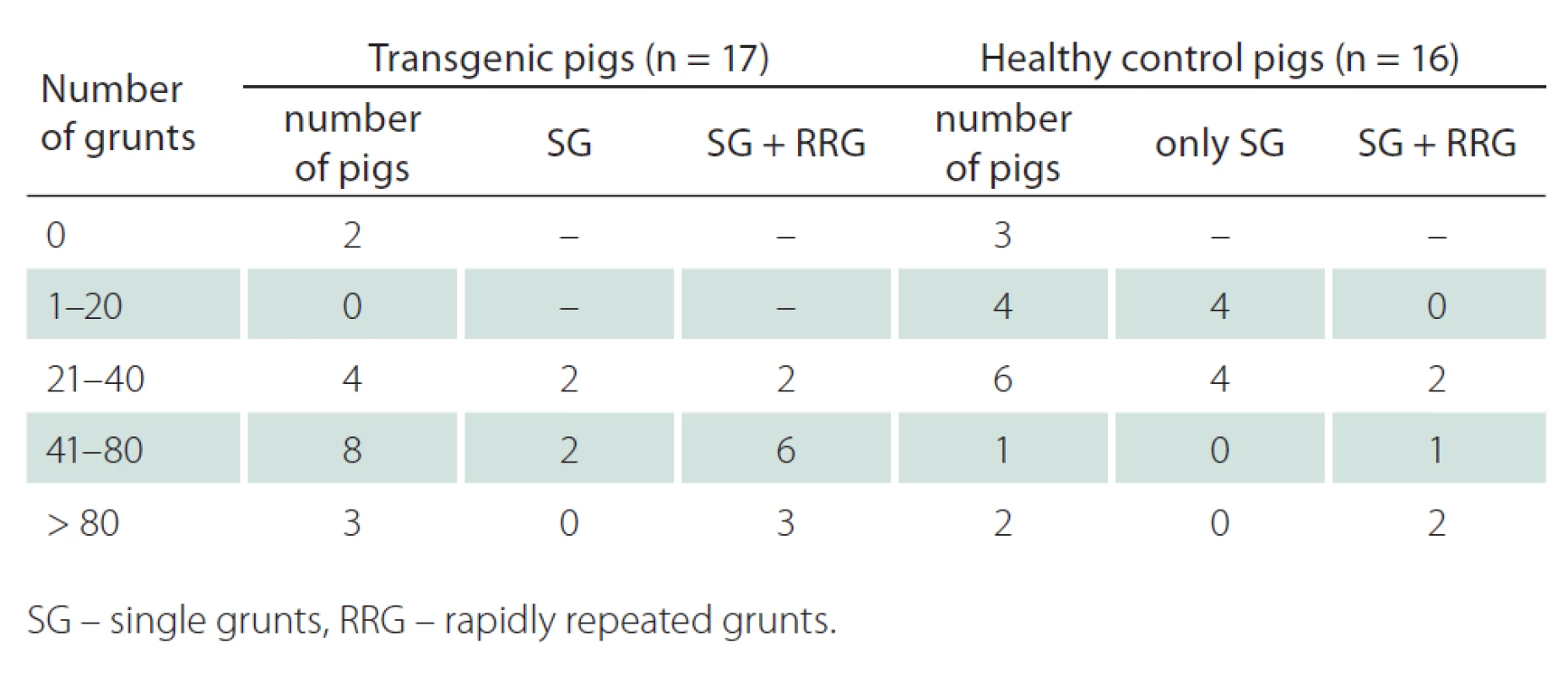
Using the final version of the experiment, we were able to record grunting samples of appropriate quality and amount (at least 20 single grunts) from 24 out of 33 pigs (73%). The mean number of grunts in the HD transgenic group was 53.0 (SD 34.3, range 0 – 120) while the mean number of grunts in the healthy group was 33.5 (SD 41.3, range 0 – 155). Although there were found no significant difference in number of grunts between both groups (p = 0.15), the transgenic pigs tend to grunt more and also often performed rapidly repeated grunts. Interestingly, no correlation was revealed between age and number of grunts (r = 0.002, p = 0.98). Detailed results on the number and type of grunting are depicted in Tab. 1. Considering the duration of the experiment, the average time was 13.5 (SD 3.0, range 9 – 20) min with no differences between groups (p = 0.77).
Discussion
As a part of this study, a standardized version of the experiment based on manipulative offering of fodder to pigs was designed and resulted in a satisfactory amount of grunting in 73% of pigs while only five pigs (15%) remained entirely silent. These results are comparable with other studies, as some silent or motionless behaviours have also been previously reported [17,18,20]. For instance, the study by Tallet et al. [18] which surveys vocalization of 84 piglets from 34 litters using 11 different contexts of emission, reported that in some situations such as nursing only one or two piglets of a litter generally vocalise.
On the contrary, a study by Marchant et al. [17] achieved obviously better results, as they were capable to obtain vocalization from 66 out of 67 gilts using a standard human approach test. Unfortunately, we cannot agree with these findings since we have tried to replicate this kind of experiment in a subgroup of four pigs and did not record almost any vocalization at all. This discrepancy might be caused by the different level of pig socialization with humans, since we observed that our pigs were rather afraid than curious considering presence of an unfamiliar person. Indeed, the lower level of socialization might also be a reasonable explanation why our healthy pigs tend to produce fewer vocalizations than transgenic ones.
Conclusion
With positive motivation using food and appropriate visual and audio stimulations, it is possible to persuade pigs to grunt. Sufficiently long (20 single grunts) and clean recordings were received from 73% of pigs. Our experiment is designed to be applicable to both genders and various ages and thus might be successfully used for acquisition of pig grunting in future research focused on longitudinal investigation of possible disturbances in pigs’ vocalization due to HD.
Acknowledgements
The study was supported by the Czech Science Foundation (GACR 102/ 12/ 2230), Czech Technical University in Prague (SGS 15/ 199/ OHK3/ 3T/ 13) and Charles University in Prague (PRVOUK ‑ P26/ LF1/ 4). This work was also supported by ExAM CZ.1.05/ 2.1.00/ 03.0124.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 5. 10. 2015
Accepted for print: 23. 10. 2015
Ing. Tereza Tykalova
Department of Circuit Theory
Faculty of Electrical Engineering
Czech Technical University in Prague
Technická 2
166 27 Praha 6, Czech Republic
e-mail: tykalter@fel.cvut.cz
Sources
1. Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 1983; 306(5940): 234 – 238.
2. Kremer B, Goldberg P, Andrew SE, Theilmann J, Telenius H, Zeisler J et al. A worldwide study of the Huntington’s disease mutation: the sensitivity and specificity of measuring CAG repeats. New Engl J Med 1994; 330(20): 1401 – 1406.
3. Harper PS. The epidemiology of Huntington’s disease. Hum Genet 1992; 89(4): 365 – 376.
4. Berardelli A, Noth J, Thompson PD, Bollen EL, Curra A, Deuschl G et al. Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord 1999; 14(3): 398 – 403.
5. Paulsen JS. Cognitive impairment in Huntington’s disease: diagnosis and treatment. Curr Neurol Neurosci Rep 2011; 11(5): 474 – 483. doi: 10.1007/ s11910 ‑ 011 ‑ 0215 ‑ x.
6. Lee ST, Kim M. Aging and neurodegeneration. Molecular mechanisms of neuronal loss in Huntington’s disease. Mech Ageing Dev 2006; 127(5): 432 – 435.
7. Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev 2010; 90(3): 905 – 981. doi: 10.1152/ physrev.00041.2009.
8. Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH,Ross CA et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 1997; 90(3): 537 – 548.
9. Reddy PH, Williams M, Charles V, Garrett L, Pike ‑ Buchanan L, Whetsell WO jr et al. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full ‑ length HD cDNA. Nat Genet 1998; 20(2): 198 – 202.
10. Li XJ, Li W. Beyond mice: genetically modifying larger animals to model human diseases. J Genet Genomics 2012; 39(6): 237 – 238. doi: 10.1016/ j.jgg.2012.05.006.
11. Bendixen E, Danielsen M, Larsen K, Bendixen C. Advances in porcine genomics and proteomics ‑ a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics 2010; 9(3): 208 – 219. doi: 10.1093/ bfgp/ elq004.
12. Rusz J, Klempir J, Tykalova T, Baborová E, Cmejla R, Ruzicka E et al. Characteristics and occurrence of speech impairment in Huntington’s disease: possible influence of antipsychotic medication. J Neural Trans 2014; 12(12): 655 – 664. doi: 10.1007/ s00702 ‑ 014 ‑ 1229 ‑ 8.
13. Vogel AP, Shirbin C, Andrew J, Churchyard AJ, Stout JC.Speech acoustic markers of early stage and prodromal Huntington’s disease: a marker of disease onset? Neuropsychologia 2012; 50(14): 3273 – 3278. doi: 10.1016/ j.neuropsychologia.2012.09.011.
14. Rusz J, Klempir J, Baborova E, Tykalova T, Majerova V, Cmejla R et al. Objective acoustic quantification of phonatory dysfunction in Huntington’s disease. PLoS One 2013; 8(6): e65881. doi: 10.1371/ journal.pone.0065881.
15. Skodda S, Schlegel U, Hoffman R, Saft C. Impaired motor speech performance in Huntington’s disease. J Neural Transm 2014; 121(4): 399 – 407. doi: 10.1007/ s00702 ‑ 013 ‑ 1115 ‑ 9.
16. Rusz J, Saft C, Schlegel U, Hoffman R, Skodda S. Phonatory dysfunction as a preclinical symptom of Huntington’s disease. PLoS One 2014; 9(11): e113412. doi: 10.1371/ journal.pone.0113412.
17. Marchant JN, Whittaker X, Brooma DM. Vocalisations of the adult female domestic pig during a standard human approach test and their relationships with behavioural and heart rate measure. Appl Anim Behav Sci 2001; 72(1): 23 – 39.
18. Tallet C, Linhart P, Policht R, Hammerschmidt K, Simecek P, Kratinova P et al. Encoding of situations in the vocal repertoire of piglets (Sus scrofa): a comparison of discrete and graded classifications. PLoS One 2013; 8(8): e71841. doi: 10.1371/ journal.pone.0071841.
19. Cordeiro AF, Naas IA, Medeiros BB, Maia PD, Pereira EM.Energy expenditure in vocalizations of pigs under stress. Engenharia Agricola 2013; 33 : 896 – 901.
20. Tallet C, Spinka M, Maruscakova I, Simecek P. Human perception of vocalizations of domestic piglets and modulation by experience with domestic pigs (Sus scrofa). J Comp Psychol 2010; 124(1): 81 – 91. doi: 10.1037/ a0017354.
21. Schrader L, Todt D. Vocal quality is correlated with levels of stress hormones in domestic pigs. Ethology 1998; 104(10): 859 – 876.
22. Schon PC, Puppe B, Gromyko T, Manteuffel G. Common features and individual differences in nurse grunting of domestic pigs (Sus scrofa): a multi‑parametric analysis. Behaviour 1999; 136 : 49 – 66.
23. Baxa M, Hruska ‑ Plochan M, Juhas S, Vodicka P, Pavlok A, Juhasova J et al. A transgenic minipig model of Huntington’s disease. J Huntingtons Dis 2013; 2(1): 47 – 68. doi: 10.3233/ JHD ‑ 130001.
24. Boersma P, Weenink D. PRAAT, a system for doing phonetics by computer. Glot Int 2001; 5(9 – 10): 341 – 345.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2015 Issue Supplementum 2
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Registry of authors
- 31P MR Spectroscopy of the Testes and Immunohistochemical Analysis of Sperm of Transgenic Boars Carried N‑terminal Part of Human Mutated Huntingtin
- Acyl‑ CoA Binding Domain Containing 3 (ACBD3) Protein in Huntington’s Disease Human Skin Fibroblasts
- Telemetry Physical Activity Monitoring in Minipig’s Model of Huntington’s Disease
- The Effect of Melatonin on Proliferation of Primary Porcine Cells Expressing Mutated Huntingtin
- Buccal Epithelial Cells as Potential Non‑ invasive Materials for the Monitoring of Mitochondrial Disturbances to Track Huntington‘s Disease Progression – a Pilot Study
- The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
- Grunting in a Genetically Modified Minipig Animal Model for Huntington’s Disease – Pilot Experiments
- Different Forms of Huntingtin in the Most Affected Organs; Brain and Testes of Transgenic Minipigs
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- The Libechov Minipig as a Large Animal Model for Preclinical Research in Huntington’s disease – Thoughts and Perspectives
- Buccal Epithelial Cells as Potential Non‑ invasive Materials for the Monitoring of Mitochondrial Disturbances to Track Huntington‘s Disease Progression – a Pilot Study
- Telemetry Physical Activity Monitoring in Minipig’s Model of Huntington’s Disease
- Grunting in a Genetically Modified Minipig Animal Model for Huntington’s Disease – Pilot Experiments
