Memory reserve and memory maintenance in SuperAgers
Paměťová rezerva a údržba paměti u superúspěšně stárnoucích osmdesátníků
Cíl: Dospělí ve věku 80 let a více s epizodickou pamětí jako průměrný šedesátník jsou nazýváni paměťově superúspěšně stárnoucími jedinci (PSÚSJ). Cílem práce je zjistit, zda PSÚSJ mají vyšší paměťovou rezervu nebo stabilnější paměťovou údržbu. Soubor a metodika: Analyzovali jsme kognitivní výkonnost u 46 kognitivně zdravých starších dospělých během 6 let ve 3 časových bodech (T1, T2 a T3). Všichni účastníci měli 80 a více let a měli normální kognitivní výkon v T3. PSÚSJ byli definování výkonem ve spontánním oddáleném vybavení z paměti ve Filadelfském testu učení a paměti (LDFR-PVLT) ≥ devět slov (průměr zdravých osob ve věku 60–64 let) v T3. Logická paměť a PVLT suma pokusů 1–5 byly porovnávány mezi i v rámci skupin. Výsledky: V souboru bylo 20 PSÚSJ (16 žen) a 26 ne-PSÚSJ (11 žen) v T3, což znamená, že více PSÚSJ bylo mezi ženami než muži. V T3 nebyly zjištěny významné rozdíly mezi skupinami ve věku, vzdělání, depresivní symptomatice. PSÚSJ měli významně vyšší PVLT 1–5, ale nikoliv logickou paměť v T1. Jejich kognitivní rezerva byla vyšší pro verbální učení, nikoliv pro logickou paměť ve srovnání s ne-PSÚSJ. U PSÚSJ došlo během 6 let ke zlepšení v PVLT 1–5 ale nikoliv v logické paměti. Závěr: Naše výsledky naznačují, že paměťová rezerva i paměťová údržba hrají roli u PSÚSJ. Rozdíl mezi skupinami byl dán rozdílným učením nikoliv logickou pamětí. PSÚSJ jsou více mezi ženami než muži.
Klíčová slova:
paměť – stárnutí – kognitivní údržba – kognitivní rezerva – odolnost
Authors:
M. Kopeček 1,2; R. Heissler 1; Z. Tichá 3; H. Georgi 3
Authors‘ workplace:
National Institute of Mental Health, Klecany, Czech Republic
1; Department of Psychiatry and Medical Psychology, Third Faculty of Medicine, Charles University, Prague, Czech Republic
2; Prague College of Psychosocial Studies, Prague, Czech Republic
3
Published in:
Cesk Slov Neurol N 2023; 86(6): 391-396
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2023391
Overview
Aim: Memory SuperAgers (SAs) are adults aged 80+ years whose episodic memory is as good as that of sexagenarians. The aim was to determine whether SAs are people with a higher memory reserve or more stable memory maintenance. Sample and method: We analyzed cognitive performance of 46 cognitively healthy older adults over 6 years, measuring them at three time points (T1, T2 and T3). All participants were aged 80+ years and had normal cognitive performance at T3. SAs were defined as persons whose Long Delayed Free Recall score of Philadelphia Verbal Learning Test (LDFR-PVLT) was ≥ nine words (the mean for healthy age group 60–64 years) at T3. Logical memory II (LM II-delayed recall) and PVLT sum of trial 1–5 were compared between and within the groups. Results: In our sample, we found 20 SAs (16 females) and 26 non-SAs (11 females) at T3, meaning more SAs were female than male. At T3, there were no significant differences between the two groups in age, education, or depressive symptoms. At T1, SAs performed significantly better on PVLT 1–5 but not in LM II; their cognitive reserve was thus higher for verbal learning but not for logical memory in non-SAs. Over time, SAs showed improvement in PVLT 1–5 but not in logical memory. Conclusion: We suggest that both memory reserve and memory maintenance play a role in SuperAging. The differences between SAs and non-SAs were more in verbal learning than in logical memory. Our data suggest that women SuperAge more frequently than men do.
Keywords:
memory – aging – cognitive maintenance – cognitive reserve – resilience
Introduction
Pathological cognitive aging has been studied for over a century but resilience to cognitive aging started to be intensively studied only in the last decade [1–3]. Memory problems are a major complaint about cognitive functioning in older adults, which is why the concept of memory SuperAging and successful memory aging had attracted much attention [4]. According to Northwestern University criteria, memory SuperAgers (SAs) are defined as older adults aged 80 years and over who have episodic memory at least as good as the average 60-year--old [5]. Alternatively, we can say that the memory age of SAs is significantly lower than the memory age of their non-SuperAger (non-SAs) peers. What is novel in this definition is the use of a new reference group. The usual normative approach compares peers within the appropriate age groups but such design cannot compare different age groups with respect to one reference group. Several studies tried to discover what is behind the phenomenon of SuperAging. To date, at least four neuroimaging cross-sectional studies found that the SAs’ cerebral cortex was thicker than that of typical older adults [5–8]. In fact, SAs were anatomically indistinguishable from young adults [8]: their hippocampal volume [8–10] and anterior cingulate [7,10] were likewise preserved. These studies confirmed intact brain structure in SAs and confirmed the validity of the concept of memory SuperAging. On the other hand, longitudinal studies found that the longitudinal rate of hippocampal volume atrophy and thinning of the entire cortex did not significantly differ between successful agers and typical older adults [10,11]. In theory, high memory reserve or memory maintenance may both play a significant role in how one becomes a SA.
Cognitive reserve is defined as individual differences in how people process tasks which “allow some to cope better than others with brain pathology” [12,13]. In other words, cognitive reserve can be understood as cognitive resilience to brain pathology, a buffer that can emerge due to academic background (level of education) or the type of occupation. Consequently, the number of years of education completed, the type of occupation, or a measure of general cognitive ability (IQ) are common proxies for cognitive reserve [14]. It is unclear whether cognitive reserve is a general psychological characteristic or whether it is domain-specific. For the purpose of this study, let us assume that memory performance at the beginning of the study is equivalent to the memory reserve and the years of education characterize the general cognitive reserve.
As a concept complementary to cognitive reserve “brain maintenance” was introduced [13,15]. Nyberg et al. [13] define brain maintenance as follows: “Individual differences in the manifestation of age-related brain changes and pathology allow some people to show little or no age-related cognitive decline.” Further research in aging cognition and resilience has led to introduction of “cognitive maintenance”, which is “the degree to which cognitive decline over time is minimized “ and pertains to specific cognitive abilities [16]. We use the term memory maintenance for the degree to which episodic memory decline over time is minimized.
Various studies described maintenance of memory function in sexagenarians [17,18] and octogenarians [19], or the maintenance of executive function in septuagenarians [20], but none used the definition of SuperAging. Recently, a longitudinal observational study found that a group of SuperAging octogenarians showed a trend toward increasing memory performance over a five-year period [10]. So far, no study documented what the main episodic memory characteristic is that predicts if one becomes a SA in their late life. Thus, we ask whether it is solely due to the very high level of memory performance they reached in earlier years (memory reserve) or mainly due to their memory maintenance or whether SAs do show both superior memory reserve and memory maintenance.
To answer the questions, we present three models: i) If only memory reserve plays a role, the differences between SAs and non-SAs identified at the end of the study (T3) will be detected already at the beginning of the observation (T1); ii) If memory maintenance is solely responsible for the differences between SAs and non-SAs, we should observe that memory performance of SAs did not decrease between T1 and T2 resp. T3; iii) A combination of both processes is at work. The answer will help us better understand the general principles of SuperAging. Further, we explore possible differences in demographic variables between the SAs and non-SAs.

Patients and methods
Study design and sample
The current study is based on the data from the Cognitive SuperAging (CoSA) project, conducted in 2018–2020, and the data from the first (T1) and fourth waves (T2) of the National Normative Study of Cognitive Determinants of Healthy Aging (NANOK), its predecessor, which was implemented in 2012–2015 [21]. Only NANOK participants were invited to CoSA. Inclusion criteria for NANOK were: age ≥ 60 years, Czech as the first language, willingness to participate in a four-year study, and absence of cognitively relevant issues in the medical history (e. g., a diagnosis/treatment for a serious neurological disorder, stroke, traumatic brain injury, acute phase of a serious mental disorder, hospitalization for substance abuse, or chemotherapeutic treatment). In 2018 (T3), 113 participants (60% female) who met the inclusion criteria (age ≥ 80 years) and provided informed consent were recruited from NANOK into the CoSA project. For this study, five participants were excluded due to suspected dementia (Mini-Mental State Examination < 23 [22]), four persons were excluded due to age (they declared at recruitment to be 80 years old when they were only nearly 80 years old at the assessment), and one participant was excluded due to the inability to finish the protocol. Additionally, at T3, we excluded 57 participants due to suspected pathological aging, which was defined as –1.5 standard deviation below the mean for age and education band in one of the following areas: memory (Philadelphia Verbal Learning Test – Long Delayed Free Recall [PVLT-LDFR]) [23], executive functions (Trail Making test, part B [TMT-B]) [24], confrontation picture-naming (Boston Naming Test [BNT]) [25,26] or Categorical Verbal Fluency – Animals [27, 28]. The final dataset included 46 participants who were over 80 years old in 2018 (T3) and their performance in neuropsychological tests did not indicate cognitive impairment (Tab. 1).
Procedure and instruments
Memory was assessed with a Story recall from the Wechsler Memory Scale-Revised subtest of Logical Memory II (LM II delayed recall) [29,30] at T1 and T2 (over 3 years). In the LM II, subjects are asked to recall the passages after a 30-min delay.
Episodic memory was measured by Philadelphia Verbal Learning Test (PVLT) at T1, T2 and T3 (over 6 years). In the learning part of PVLT, probands listen and recall a 12-words list five times. The sum of five trials of PVLT (PVLT 1–5) reflects the verbal learning. Short and Long Delayed Free Recall score of Philadelphia Verbal Learning Test (PVLT-LDFR) give us information how many correct words is a participant able to recall immediately after interference of a new 12-words list (short), or after a 30-min delay (long).
Changes (delta) in memory (PVLT-LDFR) and learning performance (PVLT 1–5) were calculated by subtracting the scores from the 2018 and 2012 assessments (T3–T1) or 2015 and 2012 assessments (T2–T1).
The mean Long Delayed Free Recall score of Philadelphia Verbal Learning Test (PVLT-LDFR) was nine words for age group 60–64 in the Czech normative study [23]. The cut score of nine words is concidentally equal to the score in another verbal list delayed recall test (Rey Auditory Verbal learning Test) used in the previous studies on SuperAging [5,10]. In a further step, additional criteria for SuperAging were applied in line with previous studies on the subject: performance on non-memory tasks such as the BNT-30, TMT-B, and Category Fluency – Animals, must be better than –1 standard deviation below the mean for the relevant age and education band [5–8,31]. General cognitive abilities were tested using the Mini-Mental State Examination (MMSE) [21,32]. Depressive symptoms were evaluated by the Geriatric Depression Scale-15 [33,34] and instrumental functional activities by the Functional Activities Questionnaire (FAQ) [35,36].
Statistics
We used parametric and nonparametric statistics based on the results of the Shapiro-Wilk and Kolmogorov-Smirnov tests. The independent sample t-test and the Mann-Whitney U test were used to compare quantitative variables between independent groups of SAs and non-SAs. Paired t-test was used to compare the change of pair variables within the groups and Fisher’s exact test was applied to evaluate nominal variables such as sex. All tests were two-sided; the significance level of P < 0.05 was adopted. All analyses were performed using IBM SPSS 23 (Armonk, NY, USA).

Results
Twenty SAs (16 females) and 26 non-SAs (11 females) were found in our sample at T3. Using the Mann-Whitney U test, we determined that there were no significant differences in age, education, depressive symptoms), MMSE and FAQ between the two groups at T3 (Tab. 1). More females were SAs (80%) than males (Fisher’s exact test; P = 0.016).
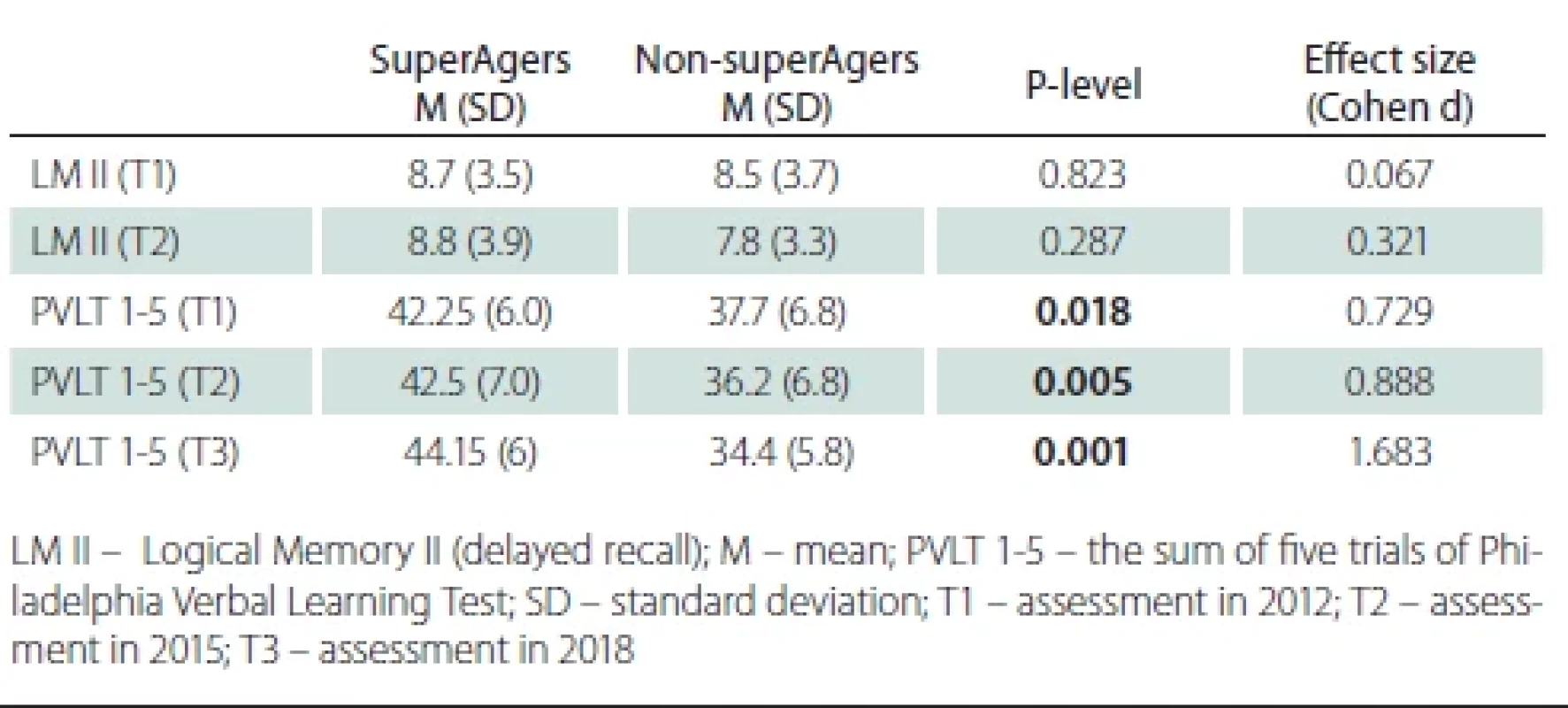
At T1, SAs performed significantly better on PVLT 1-5 (t-test, t = 2.450; P = 0.018) but not in LM II (t-test, t = 0.225; P = 0.823) (Tab. 2); their memory reserve was thus higher for verbal learning but not for logical memory in non-SAs. PVLT 1-5 was higher (t-test, t = 2.985; P = 0.005) in SAs at T2 and T3 (t-test, t = 5.660; P = 0.001). LM II was not different between the groups (t-test, t = 1.078; P = 0.287) at T2.
There were no significant changes in LM II (P = 0.311; P = 0.947) and PVLT 1–5 (P = 0.287; P = 0.845) performance within both groups between T1 and T2 (Tab. 3). A significant decline in PVLT 1-5 (paired t-test, t = –2.273; P = 0.032) was observed between T1 and T3 in non-SAs and no decline was detected in SAs (paired t-test, t = 2.009; P = 0.059).

Discussion
We assessed cognitively healthy adults aged 80+ years who met the criteria for memory SuperAging in 2018 (T3) and compared their memory performance with cognitively healthy peers (non-SAs) during assessments in 2012 (T1), 2015 (T2) and 2018 (T3). Our results showed that SAs had a higher memory reserve in verbal learning than non-SAs, i.e., their performance in the verbal learning was significantly higher 6 years before they attained the SuperAger status. We did not find the higher memory reserve in logical memory. It seems that main advantage of SAs over non-SAs is better verbal learning (Fig. 1).
We did not find a significant memory decline (logical and verbal learning) over the course of the three years, which indicates similar memory maintenance in either group. Nevertheless, we found a significant memory decline in verbal learning in non-SAs and no significant change in verbal learning in SAs after 6 years. These findings support our hypothesis about memory maintenance in SAs. The effect size between SAs and non-SAs in verbal learning at T3 was higher (Cohen’s d 1.68) than at T2 (Cohen’s d 0.89) and T1 (Cohen’s d 0.79), which implies that differences at T3 were influenced not only by memory reserve at T1 but also by better memory maintenance in SAs than in nonSAs. We are not able to decide if the maintenance is specific for verbal learning or logical memory because we did not assessed logical memory at T3.
Our results revealed that both memory reserve and memory maintenance play a role in SuperAging SAs (Tab. 2 and 3).
Years of education, which is a common proxy variable for general cognitive reserve, did not turn out to play a significant role in this study as there was no significant difference in education between the SA and non-SA group. We can speculate that it may be due to the communist era in the Czech Republic (1948–1989), when persons from “wrong” political background, i.e., usually from middle or higher social classes and academia, had limited possibilities to study at universities regardless their intellectual potential.
We observed an effect of sex on SuperAging, which is in line with previously reported results [10,37]. Across their lifespan, women outperform males on verbal memory tests [38–40] and this female advantage may reflect a sex-specific form of cognitive reserve [41].
The present study has several limitations:
1)
Although our exclusion criteria were carefully formulated to minimize the possibility of including individuals with pathological aging, our research sample did not undergo a thorough medical/neurological evaluation, including structural brain imaging, to exclude individuals with incipient or pronounced brain atrophy. On the other hand, we used standard psychometric criteria based on the 2018 assessment to exclude participants with probable mild cognitive impairment (MCI).
2)
SuperAging was defined using PVLT-LDFR, but PVLT sum trials 1–5 were included in the memory tests, which could be interpreted as using dependent tests with PVLT-LDFR – and thus a tautology. This applies also to the evaluation of the crossover between groups at T3, where we defined the two groups. On the other hand, our hypotheses were primary based on differences at T1 and on longitudinal assessments. In theory, a change in memory performance over time within a group can take place in any direction. It can happen that even participants with superior baseline memory will experience a memory decline over a period of 6 years – but this was not the case in our study.
3)
All participants in our study were Caucasian with a shared Central European history, which is why generalizability of the results to other ethnic and cultural groups must not be taken for granted.
4)
We used a retrospective design (looking back at the cognitive evolution of SAs vs. non-SAs). This approach can substantially influence the results. It eliminates patients who converted to MCI or died, and it overestimates the differences in evolution between a SAs and a non-SAs (as those who declined during the follow-up had a much higher chance to be classified as non-SAs than SAs). The future design of study should be prospective.
5)
Our study lacks neuroimaging and other biomarkers data. Such data would substantially increase the overall neuroscientific generalizability of our findings, and would also enable us to broaden the aims and conclusions to the “brain maintenance”. The relationship between brain reserve, cognitive reserve, maintenance, and compensation and their contribution to resilience is the focus of a lively debate within the research community and the concept and definitions are still evolving [16,42].
6)
The retrospective design and a small sample size do not allow us to test if the memory maintenance an inherent part of memory reserve. A larger sample size and prospective design may help to disentangle the role of both mechanisms.
Conclusions
Our study confirms that both memory reserve and memory maintenance play a role in the phenomenon of SuperAging. We replicate the finding that females are more likely to be SAs than males. It seems that the main advantage of SAs over non-SAs is better verbal learning.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). The study protocol was approved by the Institutional Ethical Review Board of the Czech National Institute of Mental Health (reference number: 115/17, date of approval: March 22, 2017) and written informed consent has been obtained from all participants.
Funding
This study was supported from the Czech Science Foundation under grant number 18-06199S.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Sources
1. Kaup AR, Nettiksimmons J, Harris TB et al. Cognitive resilience to apolipoprotein E epsilon4: contributing factors in black and white older adults. JAMA Neurol 2015; 72 (3): 340–348. doi: 10.1001/jamaneurol.2014.3978.
2. Arenaza-Urquijo EM, Przybelski SA, Lesnick TL et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain 2019; 142 (4): 1134–1147. doi: 10.1093/brain/awz037.
3. de Godoy LL, Alves C, Saavedra JSM et al. Understanding brain resilience in superagers: a systematic review. Neuroradiology 2021; 63 (5): 663–683. doi: 10.1007/ s00234-020-02562-1.
4. Nyberg L, Pudas S. Successful memory aging. Annu Rev Psychol 2019; 70 : 219–243. doi: 10.1146/annurev-psych-010418-103052.
5. Harrison TM, Weintraub S, Mesulam MM et al. Superior memory and higher cortical volumes in unusually successful cognitive aging. J Int Neuropsychol Soc 2012; 18 (6): 1081–1085. doi: 10.1017/S1355617712000847.
6. Rogalski EJ, Gefen T, Shi J et al. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cogn Neurosci 2013; 25 (1): 29–36. doi: 10.1162/jocn_a_00300.
7. Gefen T, Peterson M, Papastefan ST et al. Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci 2015; 35 (4): 1781–1791. doi: 10.1523/JNEUROSCI.2998-14. 2015.
8. Sun FW, Stepanovic MR, Andreano J et al. Youthful brains in older adults: preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. J Neurosci 2016; 36 (37): 9659–9668. doi: 10.1523/JNEUROSCI.1492-16.2016.
9. Dekhtyar M, Papp KV, Buckley et al. Neuroimaging markers associated with maintenance of optimal memory performance in late-life. Neuropsychologia 2017; 100 : 164–170. doi: 10.1016/j.neuropsychologia.2017.04. 037.
10. Harrison TM, Maass A, Baker SL et al. Brain morphology, cognition, and beta-amyloid in older adults with superior memory performance. Neurobiol Aging 2018; 67 : 162–170. doi: 10.1016/j.neurobiolaging.2018.03.024.
11. Gardener SL, Weinborn M, Sohrabi HR et al. Longitudinal trajectories in cortical thickness and volume atrophy: superior cognitive performance does not protect against brain atrophy in older adults. J Alzheimers Dis 2021; 81 (3): 1039–1052. doi: 10.3233/JAD-201243.
12. Stern Y. Cognitive reserve. Neuropsychologia 2009; 47 (10): 2015–2028. doi: 10.1016/j.neuropsychologia.2009. 03.004.
13. Nyberg L, Lovden M, Riklund K et al. Memory aging and brain maintenance. Trends Cogn Sci 2012; 16 (5): 292–305. doi: 10.1016/j.tics.2012.04.005.
14. Cabeza R, Albert M, Belleville S et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 2018; 19 (11): 701–710. doi: 10.1038/s41583-018-0068-2.
15. Lindenberger U, Burzynska AZ. Nagel IE heterogeneity in frontal lobe aging. In: Stuss DT, Knight RT (eds). Principles of frontal lobe function. Oxford: Oxford University Press 2013 : 609–627.
16. Kremen WS, Elman JA, Panizzon MS et al. Cognitive reserve and related constructs: a unified framework across cognitive and brain dimensions of aging. Front Aging Neurosci 2022; 14 : 834765. doi: 10.3389/fnagi.2022.834765.
17. Pudas S, Persson J, Josefsson M et al. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci 2013; 33 (20): 8668–8677. doi: 10.1523/JNEUROSCI.2900-12.2013.
18. Dixon RA, de Frias CM. Cognitively elite, cognitively normal, and cognitively impaired aging: neurocognitive status and stability moderate memory performance. J Clin Exp Neuropsychol 2014; 36 (4): 418–430. doi: 10.1080/13803395.2014.903901.
19. Dekhtyar M, Papp KV, Buckley R et al. Neuroimaging markers associated with maintenance of optimal memory performance in late-life. Neuropsychologia 2017; 100 : 164–170. doi: 10.1016/j.neuropsychologia.2017.04. 037.
20. de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: from cognitively elite to cognitively impaired. Neuropsychology 2009; 23 (6): 778–791. doi: 10.1037/a0016743.
21. Štěpánková H, Bezdíček O, Nikolai T et al. National normative study of cognitive determinants of healthy ageing – status report. E-psychologie 2015; 9 (1): 43–64.
22. Štěpánková H, Nikolai T, Lukavský J et al. Mini-mental state examination – Czech normative study. Cesk Slov Neurol N 2015; 78/111 (1): 57–63.
23. Bezdicek O, Libon DJ, Stepankova H et al. Development, validity, and normative data study for the 12-word Philadelphia Verbal Learning Test [czP (r) VLT-12] among older and very old Czech adults. Clin Neuropsychol 2014; 28 (7): 1162–1181. doi: 10.1080/13854046.2014.952666.
24. Bezdicek O, Stepankova H, Axelrod BN et al. Clinimetric validity of the Trail Making Test Czech version in Parkinson’s disease and normative data for older adults. Clin Neuropsychol 2017; 31 (Suppl 1): 42–60. doi: 10.1080/13854046.2017.1324045.
25. Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea & Febiger 1983.
26. Bezdicek O, Rosicka AM, Mana J et al. The 30-item and 15-item Boston naming test Czech version: item response analysis and normative values for healthy older adults. J Clin Exp Neuropsychol 2021; 43 (9): 890–905. doi: 10.1080/13803395.2022.2029360.
27. Morris JC, Heyman A, Mohs RC et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39 (9): 1159–1165. doi: 10.1212/wnl.39.9.1159.
28. Nikolai T, Štěpánková H, Michalec J et al. Verbal fluency tests – Czech normative study for older persons. Cesk Slov Neurol N 2015; 78/111 (3): 292–299. doi: 10.14735/amcsnn2015292.
29. Nikolai T, Stepankova H, Kopecek M et al. The uniform data set, Czech version: normative data in older adults from an international perspective. J Alzheimers Dis 2018; 61 (3): 1233–1240. doi: 10.3233/JAD-170595.
30. Jenčová A, Černochová D. WMS-IIIa – Wechslerova zkrácená paměťová škála. Prague: Hogrefe – Testcentrum 2011.
31. Wechsler D. Wechsler memory scale – third edition abbreviated manual. USA: The Psychological Corporation 2002.
32. Folstein MF, Folstein SE, McHugh PR. “Mini - -mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12 (3): 189–198. doi: 10.1016/0022-3956 (75) 900 26-6.
33. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5 (1–2): 165–173. doi: 10.1300/J018v05n01_09.
34. Heissler R, Červenková M, Kopeček M et al. Geriatric depression scale (GDS-15): Czech normative study. Ceskslov Psychol 2020; 64 (1): 49–65.
35. Pfeffer RI, Kurosaki TT, Harrah CH et al. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37 (3): 323–329. doi: 10.1093/geronj/37.3.323.
36. Bezdicek O, Stepankova H, Martinec Novakova L et al. Toward the processing speed theory of activities of daily living in healthy aging: normative data of the Functional Activities Questionnaire. Aging Clin Exp Res 2016; 28 (2): 239–247. doi: 10.1007/s40520-015-0413-5.
37. Maccora J, Peters R, Anstey KJ. Gender differences in superior-memory superagers and associated factors in an Australian cohort. J Appl Gerontol 2021; 40 (4): 433–442. doi: 10.1177/0733464820902943.
38. Kramer JH, Delis DC, Daniel MH. Sex differences in verbal learning. J Clin Psychol 1988; 44 (6): 907–915. doi: 10.1002/1097-4679 (198811) 44 : 6<907:: AID-JCLP2270440610>3.0.CO; 2-8.
39. Aartsen MJ, Martin M, Zimprich D et al. Gender differences in level and change in cognitive functioning. Results from the Longitudinal Aging Study Amsterdam. Gerontology 2004; 50 (1): 35–38. doi: 10.1159/000074387.
40. Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory 2013; 21 (7): 857–874. doi: 10.1080/09658211.2013.765892.
41. Sundermann EE, Biegon A, Rubin LH et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 2016; 86 (15): 1368–1376. doi: 10.1212/WNL.0000000000002570.
42. Stern Y, Albert M, Barnes CA et al. A framework for concepts of reserve and resilience in aging. Neurobiol Aging 2023; 124 : 100–103. doi: 10.1016/j.neurobiolaging.2022. 10.015.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2023 Issue 6
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Surgical treatment of intracranial aneurysm recurrence after clipping
- Diffuse glioma overview based on the 2021 WHO classification part 1 – adult type
- The effect of chemotherapy on cognitive functions in children with leukemia
- Treatment of sleep disorders with repetitive transcranial magnetic stimulation
- The effects of introducing psychoeducational programs in patients with stroke in post-acute care
- Memory reserve and memory maintenance in SuperAgers
- Polysomnographic findings in men over 55 years of age with narcolepsy type 1
- Z. Adam et al. Monoklonální gamapatie klinického významu a další nemoci
- Zpráva o výročním sjezdu České neurochirurgické společnosti ČLS JEP v Hradci Králové
- Atypical cases of cycloplegia caused by Datura stramonium
- Unexpectedly abnormal PICNIR test and brain SPECT even in the grandson of a patient with dementia
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Diffuse glioma overview based on the 2021 WHO classification part 1 – adult type
- Treatment of sleep disorders with repetitive transcranial magnetic stimulation
- The effects of introducing psychoeducational programs in patients with stroke in post-acute care
- Surgical treatment of intracranial aneurysm recurrence after clipping
