Advances in the Treatment of Myasthenia Gravis on the Horizon
The research of new drugs for the generalized form of myasthenia gravis has made significant progress in recent years. How is the new specific therapy performing? And are we heading towards a radical change in treatment strategy?
Myasthenia gravis in a nutshell
Myasthenia gravis (MG) is a chronic autoimmune disease caused by antibodies directed against receptors and proteins in the postsynaptic membrane of the neuromuscular junction.
- In most patients, these are antibodies against the acetylcholine receptor (AChR Ab), primarily belonging to the subtypes of immunoglobulins IgG1 and IgG3. Upon binding of the antibody to the AChR, the complement cascade is activated. Activated complement proteins form the so-called membrane attack complex, which directly damages the neuromuscular junction cells.
- A small percentage of patients are diagnosed with positive antibodies against muscle-specific tyrosine kinase (MuSK Ab) or protein 4 related to the LDL receptor (LRP4 Ab). The immunopathogenesis in LRP4 Ab positive patients is the same as in those with AChR Ab positivity. MuSK antibodies are primarily of the IgG4 subgroup and do not activate complement upon binding. Blocking MuSK affects the interaction of postsynaptic proteins, thereby causing a failure of neuromuscular transmission post-synaptically.
The resulting pathological processes lead to blockade of neuromuscular transmission. Clinically, the disease is characterized by fluctuating muscle weakness and fatigability. MG may present with speech disruption, difficulty swallowing or chewing, shortness of breath, ptosis, and/or diplopia, and limb weakness, which significantly impacts the quality of life.
Rise of specific therapy
First-line treatment for MG involves cholinesterase inhibitors, and in case of disease progression or manifestation of weakness in oropharyngeal or respiratory muscles, it is recommended to start corticosteroids, usually combined with other immunosuppressives. Thymectomy is performed in early-onset MG or MG associated with thymoma. A major complication is myasthenic crisis, a life-threatening condition that affects at least one in five patients at some point in their life.
Despite improvements in therapeutic strategies and better prognosis, about 15% of patients cannot maintain adequate disease control using conventional therapeutic approaches. Not just in this group, specifically acting modern biologicals have found their place in recent years - complement inhibitors, neonatal Fc receptor blockers, agents that induce B and T lymphocyte depletion, and others. The advantage of these treatments is a faster effect and generally fewer side effects.
Rozanolixizumab - an Fc receptor blocker
One of these modern approaches to MG treatment targets neonatal Fc receptors. Blocking these reduces the concentration of IgG immunoglobulins, including autoantibodies. IgG immunoglobulins are protected from lysosomal elimination by binding to the neonatal Fc receptor; without this binding, they are degraded at an increased rate. An example of a biologic from this group, whose registration study (MycarinG) was recently completed and is gradually being introduced into practice, is rozanolixizumab.
Methodology, conduct, and goals of the study
MycarinG was a randomized, double-blind, placebo-controlled phase III adaptive study conducted at 81 outpatient sites. Adult patients with generalized MG (Myasthenia Gravis Foundation of America, class II–IVa) with proven AChR Ab or MuSK Ab, an MG Activities of Daily Living (MG-ADL) score ≥ 3 (excluding ocular symptoms), and a Quantitative MG score ≥ 11 were included.
Patients were randomly divided into 3 equally sized groups:
- subcutaneous infusion of rozanolixizumab at a dose of 7 mg/kg once weekly for 6 weeks
- subcutaneous infusion of rozanolixizumab at a dose of 10 mg/kg once weekly for 6 weeks
- placebo administration
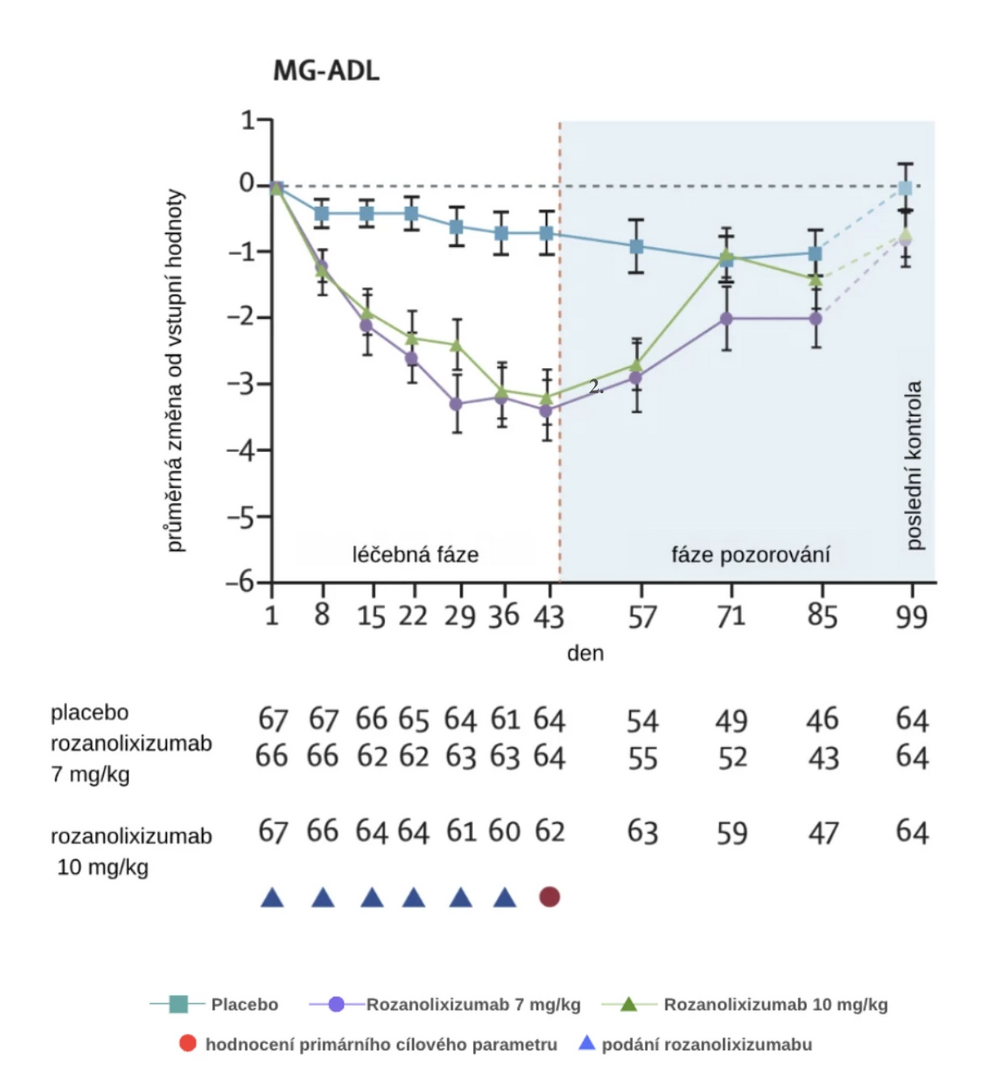
Randomization was stratified according to the presence of AChR and MuSK autoantibodies. The primary efficacy endpoint was the change in MG-ADL score over 43 days, and the safety of the drug was also evaluated.
Results
A total of 200 patients were included in the study between June 2019 and 2021. An effect was demonstrated at both doses given, for patients with AChR Ab and MuSK Ab. In the 7 mg/kg dose group, there was an improvement in the MG-ADL score by an average of 3.37 points (2.59 points more than the placebo group) and in the 10 mg/kg dose group by 3.40 points (2.62 points more than the placebo group).
Treatment-emergent adverse events (TEAEs) occurred in 52 (81%) patients in the 7 mg/kg group, 57 (83%) in the 10 mg/kg group, and 45 (67%) in the placebo group. The most common were headaches (45% in the 7 mg/kg group, 38% in the 10 mg/kg group, 19% in the placebo group), diarrhea (25%, 16%, and 13%, respectively), and fever (13%, 20%, and 1%, respectively). Serious TEAEs were recorded in 5 (8%) patients in the 7 mg/kg group, 7 (10%) in the 10 mg/kg group, and 6 (9%) in the placebo group. No treatment-related deaths were reported.
Conclusion
The research of new drugs for the generalized form of MG has advanced significantly in recent years. A good example is the favorable outcomes of the clinical study with rozanolixizumab, which increases the degradation of antibodies in the blood by blocking the neonatal Fc receptor. The effect was shown in both doses administered (7 and 10 mg/kg/day) and the drug was well tolerated. Additionally, this is not the only new agent in this indication. Thus, the question arises whether in the foreseeable future there might be a radical change in the treatment strategy for the generalized form of MG, including the elimination of chronic corticosteroid use, as has been the case with multiple sclerosis.
(dos)
Sources:
1. Bril V., Drużdż A., Grosskreutz J. et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol 2023 May; 22 (5): 383−394, doi: 10.1016/S1474-4422(23)00077-7.
2. Menon D., Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs 2022; 82 (8): 865−887, doi: 10.1007/s40265-022-01726-y.
3. Piťha J. Refractory myasthenia gravis – clinical characteristics and biological treatment options. Česká a slovenská neurologie a neurochirurgie 2019; 82(5): 490−495, doi: 10.14735/amcsnn2019490.
4. Týblová M. News in the treatment of myasthenia gravis. Neurologie pro praxi 2023; 24 (4): 286−292, doi: 10.36290/neu.2023.057.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.