Cost effectiveness, the economic considerations of prenatal screening strategies for trisomy 21 in the Czech Republic
Nákladová efektivita a další ekonomické aspekty strategií prenatálního screeningu trizomie 21 v České republice
Cíl:
Provést analýzu nákladové efektivity strategií screeningu trisomie 21 (Downova syndromu) v České republice pomocí modelu rozhodovacího stromu navrženého k vyhodnocení nákladů a potenciálních rizik souvisejících s použitím různých strategií tohoto screeningu.
Metodika:
S použitím rozhodovacího modelu jsme srovnávali nákladovou efektivitu devíti možných strategií screeningu trisomie 21, a to: 1. věk matky 35 let a více v prvním trimestru, 2. věk matky 35 a více v druhém trimestru, 3. druhotrimestrální triple test (AFP, hCG, μE3), 4. měření nuchální translucence, 5. prvotrimestrální biochemický test ze séra (PAPP-A, fβ-hCG), 6. prvotrimestrální kombinovaný test (nuchální translucence, PAPP-A, fβ-hCG), 7. prvotrimestrální kombinovaný test (nuchální translucence, PAPP-A, fβ-hCG) v uspořádání OSCAR, 8. prvotrimestrální kombinovaný test (nuchální translucence, nosní kůstka, PAPP-A, fβ-hCG), 9. prvotrimestrální kombinovaný test (nuchální translucence, nosní kůstka, PAPP-A, fβ-hCG) v uspořádání OSCAR.
Analýza je provedena z perspektivy plátce zdravotní péče s použitím relevantních nákladů a výsledků vztažených ke každé strategii screeningu v kohortě 118 135 těhotných žen vyšetřených ve 12. gestačním týdnu v České republice. S použitím tabulkového procesoru Excel (Microsoft Corporation, Redmond, Wash) byly získány tyto výstupy: celková nákladová efektivita, počet detekovaných plodů s trisomií 21, počet zabráněných narození jedinců s trizomií 21 a počet ztrát euploidních plodů v důsledku invazivních výkonů. Srovnáním strategie 9 a 3 (ta představuje běžnou praxi v České republice) byl rovněž vypočten poměr inkrementálních přínosů a nákladů.
Výsledky:
Analýza ukazuje, že strategie 9 je z hlediska nákladové efektivity nejvhodnější model pro screening trizomie 21. S touto strategií jsou spojeny nejnižší náklady pro zabránění narození jednoho jedince s trizomií 21. Ostatní strategie byly sice méně nákladné, ale měly nižší záchyt trizomie 21 a vyšší počet potratů v souvislosti s invazivními výkony (kromě strategií 6 a 7, které měly stejný počet potratů ve srovnání se strategií 9). Všechny strategie byly levnější ve srovnání se screeningem jen na základě věku matky nad 35 let. Uspořádání OSCAR a zahrnutí vyšetření nosní kůstky činí strategii 9 nejvýhodnější z hlediska nákladové efektivity.
Poměr inkrementálních nákladů a přínosů (náklady na zabránění jednoho dalšího narození jedince s trizomií 21) srovnávající strategie 9 a 3 (běžná praxe v České republice) byl odhadnut na 219 326 Kč. Nelze tedy předpokládat, že široká implementace strategie 9 ušetří náklady, díky nízké falešné pozitivitě je však efektivnější než běžná praxe v České republice (3 580 082 Kč na zabránění jednoho narození jedince s trizomií 21 pro strategii 3 a 2 469 833 Kč pro strategii 9).
Závěr:
V naší analýze se jako nákladově nejefektivnější strategie pro screening trizomie 21 v České republice jeví kombinovaný prvotrimestrální test zahrnující NT, NB, PAPP-A a fβ-hCG.
Klíčová slova:
analýza nákladové efektivity, prvotrimestrální screening, druhotrimestrální screening, věk matky, nuchální translucence (projasnění záhlaví), triple test, OSCAR (one-stop-clinic).
Authors:
I. Dhaifalah 1; O. Májek 2
Authors‘ workplace:
Department of Genetic and Fetal Medicine, University Hospital, Olomouc, Czech Republic
1; Institut of Biostatistics and Analyses, Masaryk University, Brno Czech Republic
2
Published in:
Ceska Gynekol 2012; 77(1): 39-51
Category:
Original Article
Overview
Objectives:
To perform an incremental cost-effectiveness analysis for screening of trisomy 21 (Down syndrome) in the Czech Republic through a decision tree model designed to evaluate the costs and potential risks involved in using different strategies of screening.
Methods and data analysis:
Using decision-analysis modelling, we compared the cost-effectiveness of nine possible screening strategies for trisomy 21 : 1. maternal age ± 35 in first trimester, 2. maternal age ± 35 in second trimester, 3. second trimester triple test (AFP, hCG, μ E3), 4. nuchal translucency measurement, 5. first trimester serum test (PAPP-A, fβ-hCG), 6. first trimester combined (nuchal translucency, PAPP-A, fβ-hCG) not in OSCAR manner, 7. first trimester combined (nuchal translucency, PAPP-A, fβ-hCG) in OSCAR manner, 8. first trimester combined (nuchal translucency, nasal bone, PAPP-A, fβ-hCG) not in OSCAR manner, 9. first trimester combined (nuchal translucency, nasal bone, PAPP-A, fβ-hCG) in OSCAR manner.
The analysis is performed from a health care payer perspective using relevant cost and outcomes related to each screening strategy in a cohort of 118,135 pregnant women presenting around 12 weeks of pregnancy in the Czech Republic. Using a computer spreadsheet Excel (Microsoft Corporation, Redmond, Wash) the following outcomes: overall cost-effectiveness, trisomy 21 cases detected, trisomy 21 live birth prevented and euploid losses from invasive procedures were obtained. The incremental cost-effectiveness ratios were also calculated by a comparison of strategy nine and strategy three (the current practice in the Czech Republic).
Results:
Under the baseline assumptions, the model favors strategy nine as the most cost-effective trisomy 21 screening strategy. This strategy was the least expensive strategy per trisomy 21 cases averted. Although all the other strategies cost less, they all had lower trisomy 21 detection rates and higher numbers of procedure-related losses (except for strategies six and seven, which had same loss rate) compared with strategy nine. All strategies considered were cheaper compared with screening only by maternal age over 35 years. Adding the nasal bone and the OSCAR manner made strategy nine the most cost-effective one.
The incremental cost-effectiveness (cost per additional trisomy 21 case prevented) comparing strategy nine and second trimester triple test (current practice in Czech Republic) yielded an additional baseline cost of 219,326 CZK. This would seem not to save money but due to the low false positive rate the test is less costly than might be expected and it is more cost-effective than the current practice in the Czech Republic (3,580,082 CZK for the current practice and 2,469,833 CZK for our strategy in terms of costs per DS case prevented).
Conclusion:
In our analysis the NT, NB, PAPP-A and fβ-hCG combined test carried out in the first trimester was the most cost-effective screening strategy for trisomy 21 in the Czech Republic.
Key words:
cost effectiveness analysis, first trimester screening, second trimester screening, maternal age, nuchal translucency, triple test, OSCAR (one-stop-clinic).
INTRODUCTION
Cost-effectiveness analysis can be performed and reported in several ways. The cost-benefit analysis translates all outcomes into their costs and is usually reported as a benefit-to-cost ratio. If the strategy is an improvement, the benefits will outweigh the costs, and the ratio will be above 1. Cost-effectiveness is also reported commonly as a cost per outcome, as the cost per diagnosis made, or as disease averted. A specific form of cost-effectiveness analysis that will allow comparison between different medical outcomes is cost-utility analysis. Utility is a measure of an individual’s well-being. In analyses of health and medical interventions, a utility of 1 is typically assigned to perfect health and a utility of 0 is assigned to an outcome of death. The utility of a particular outcome can be multiplied by the time in that state, commonly discounted, to yield quality-adjusted life years (QALYs). The difference between quality-adjusted life expectancies that are achieved in different health states can be compared and, when used in the denominator below costs, yield a cost-utility ratio [1, 2, 3].
A comparison across studies of the cost-effectiveness of ultrasound screening in pregnancy is limited by the major differences in assumptions about detection rates, FPRs, rates of diagnostic procedures, procedure-induced foetal losses and unit costs across countries. While most studies report an average cost per case detected, the differing scale of the programs (i.e. number of screened pregnancies) causes an assessment of the incremental cost compared with existing practice to vary from country to country (even where the technology has the same effectiveness). The incremental cost per extra case detected depends on the number of cases detected, even where there are no economies of scale in the screening program. A further difficulty is the lack of a standardized outcome measure. Studies often report a cost per trisomy 21 pregnancy detected but the cost per outcome is not constant over the level of outcome. As the detection rate rises (with the risk cut off falling), the cost per case detected rises from a certain point due to the number of false positives [4, 5].
In addition, the meaning of a single outcome is questionable if the rate of procedure induced foetal losses rises as the detection rate rises. It is conceivable that two strategies could have the same cost per case detected, even with different numbers of cases detected; procedure-induced foetal loss rates; and rates of unnecessary procedures i.e. false positive rate. Where we are concerned with both cases detected and the consequences of false positive screens, we either have to consider both outcomes together or keep one constant. In one approach, the detection rate is kept constant across interventions and thus the number of cases detected was the same in each strategy for a given sized group of women. Such an approach allows the use of an average cost-effectiveness ratio as a guide to decision making.
An alternative is to keep the FPR constant and to calculate the extra cost per case detected for a given number of procedure-induced foetal losses. Another option which is common in the literature is to present a trade-off between cost, detection and foetal losses. The last approach is more difficult to implement and makes decision making more difficult. There is clinical trial evidence of the effectiveness of ultrasound screening for trisomy 21 in the first trimester, either on its own or in combination with first trimester biochemical screening [5, 6].
Several studies have reported on the economic evaluations of alternative prenatal trisomy 21 screening programs with only few comparing first trimester and second trimester screening directly [7, 8, 9].
Introducing first trimester screening had reduced the rate of diagnostic tests in both high and low risk pregnant women. This had led to a cost saving with high detection rate and a reduction in procedure-related foetal losses. Since many of women coming for screening in the first trimester would have had an ultrasound for other purposes (e.g. for dating of pregnancy), replacing a dating ultrasound with a NT ultrasound in this group of women would greatly reduce costs and improve detection.
METHOD AND DATA ANALYSIS
To perform an incremental cost-effectiveness analysis for screening of trisomy 21 in the Czech Republic a decision tree model was designed to evaluate the cost-effectiveness of nine possible screening strategies for trisomy 21:
- Maternal age ±35 in first trimester.
- Maternal age ±35 in second trimester.
- Second trimester triple test (AFP, hCG, μE3).
- Nuchal translucency measurement.
- First trimester serum test (PAPP-A, fβ-hCG).
- First trimester combined (nuchal translucency, PAPP-A, fβ-hCG) not in OSCAR manner.
- First trimester combined (nuchal translucency, PAPP-A, fβ-hCG) in OSCAR manner.
- First trimester combined (nuchal translucency, nasal bone, PAPP-A, fβ-hCG) not in OSCAR manner.
- First trimester combined (nuchal translucency, nasal bone, PAPP-A, fβ-hCG) in OSCAR manner.
The base line model consisted of pregnant women in the first and second trimester of pregnancy. The analysis is performed from a health payer perspective using relevant cost and outcomes related to each screening strategy in a cohort of 118,135 pregnant women presenting around 12 weeks of pregnancy. The cohort consists of the number of pregnant women in the Czech Republic in the year 2007 according to the Institute of Health Information and Statistics of the Czech Republic (UZIS) and have been chosen to be more close to the reality in our cost estimates.
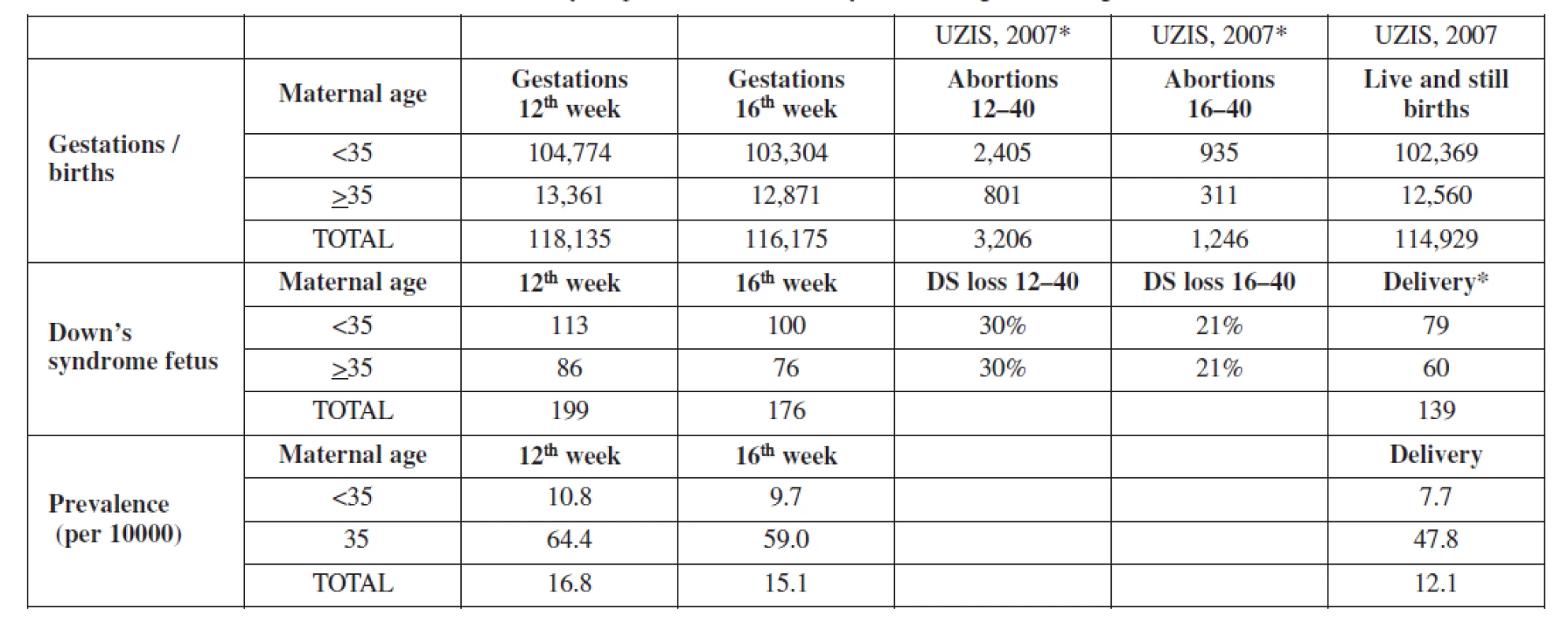
The parameters used in the model were selected for their relevance to clinical practice in the Czech Republic. Risk of trisomy 21 at 12 weeks, 16 weeks, and at term, were calculated as a function of maternal age using previously derived equations and on the bases of the parameters of our population (Table 1). The distinguishing feature between the OSCAR manner and no OSCAR manner (One-Stop-Clinic for Assessment of Risk) is an additional dating scan that will influence the end point of cost analysis. Model parameters of OSCAR were adapted from the publication of Dhaifalah 2011 [10] and are shown in Table 2a,b.


With the use of Excel (Microsoft Corporation, Redmond, Wash), the data were analyzed by experts from the Institute of Biostatistics and Analyses, Masaryk University, Brno to obtain the following outcomes: overall cost-effectiveness, trisomy 21 cases detected, trisomy 21 live birth prevented and euploid losses from invasive procedures. The incremental cost-effectiveness ratios were also calculated by a comparison of strategy nine and strategy three (the current practice in the Czech Republic). Parameters of strategies and costs of this model are summarised in Table 3a,b.


Sensitivity analyses performed on clinical parameters indicate that estimates are quite sensitive to uncertainty in specificity characters, it is necessary to bear in mind that all results are burdened with uncertainty, and the results could change if our estimates are actually incorrect.
Sensitivity and specificity characteristics of each strategy were used to estimate the chances of true - or false-positive and true - or false-negative results. We assumed that patients with negative screen results, regardless of whether they are true or false, did not have invasive prenatal testing and would therefore have rates of a normal neonate or a trisomy 21 neonate as potential outcomes. We assumed that 90% (base case compliance) of patients with positive screening results, regardless of whether they are true or false, accepted invasive testing.
Probability estimates and ranges of the sensitivity, specificity and outcomes characteristics of each strategy were derived from a systematic review of the English literature and the results published for strategy nine by Dhaifalah 2011 [10] specifically for the Czech population. They were calculated by averaging point estimates using the extremes of the reported point estimates (Table 4a,b).


Baseline prevalence of trisomy 21 were estimated to be 16.8, 15.1 and 12.1 (prevelance per 10,000) at first trimester, second trimester and at term in our population. This baseline prevalence was adjusted using a spontaneous loss rate of 30% from 12 to 40 weeks gestation. (Table 1). For the model, we assumed a 100% uptake rate for screening, 90% will accept invasive testing and 100% termination of trisomy 21 when diagnosed.
In the maternal age, strategy one, we assumed that women age 35 years or greater are given the option of having an invasive prenatal diagnostic test and that the sensitivity of maternal age for detecting trisomy 21 is 30% (range 20.45% for sensitivity analysis). The age distributions were varied in the sensitivity analysis to account for the increasing proportion of women of advanced maternal age becoming pregnant. In our cohort 12,7% were aged 35 years or more.
The cost estimates were derived from the up-to-date Všeobecná zdravotní pojišťovna (VZP) health insurance schedule for reimbursement of medical care benefit items (Table 5). The Czech system of reimbursement of medical care fees facilitate for us the calculation of cost estimates. It is a system that contains a number for each item (Cz = kód), what the item contains, and an explanation of its content. The cost of the item is than converted to what we call points (Cz = body) it includes all the necessary costs for any procedures involved (e.g. labour and materials). Later on points are converted into equivalents in Czech currency (koruna) which is used for our final cost estimate.
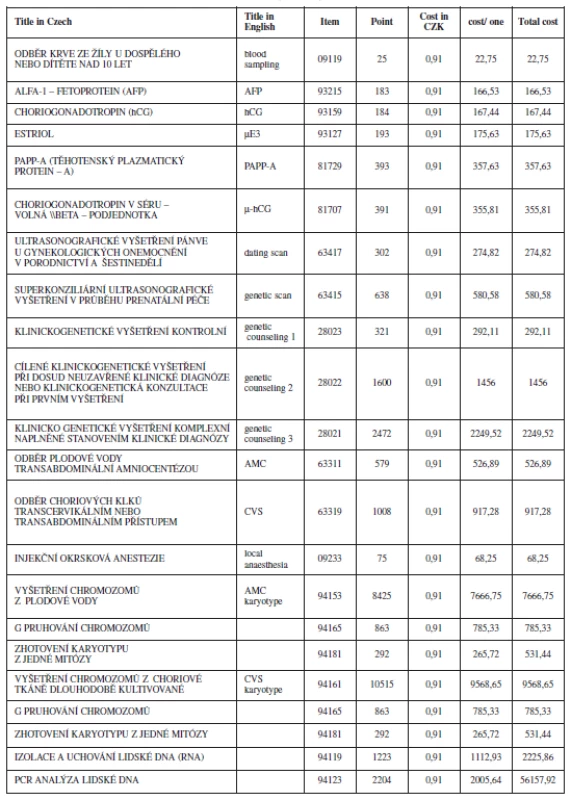
The cost for the NT scan is not yet agreed in our system. For the purpose of this study we used the cost estimate for the genetic ultrasound scan and the reimbursement code assigned to it, based on the current practice of our and other medical centres. The cost estimates and utilities used in the model for the base-line analysis are shown in Table 6.
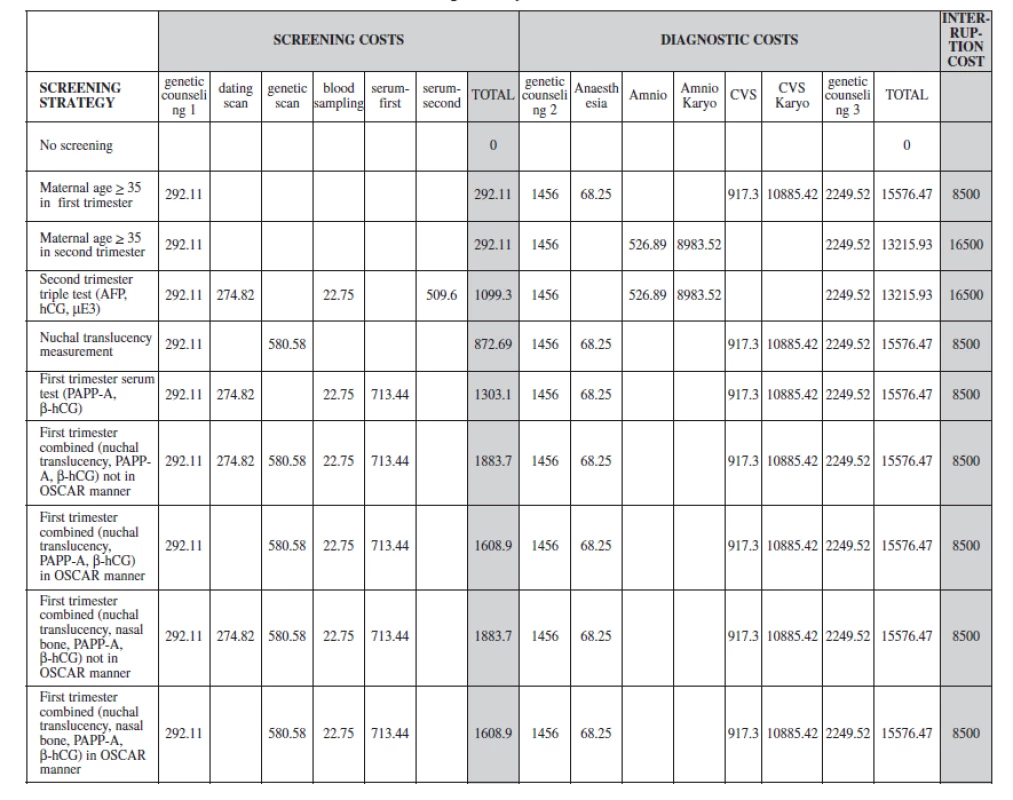
The sensitivity of biochemical screening tests is only accurate with reliable pregnancy dating using ultrasonography. Our model includes the cost for ultrasound dating in the triple test and serum screening in the first trimester. Women who are screen positive for trisomy 21 undergo an invasive diagnostic procedure. The diagnostic procedures include a chorionic villous sampling (CVS) in the first trimester or an amniocentesis in the second trimester. If the diagnostic test result is positive, the woman takes the options of termination taking in consideration the harmful effects from a diagnostic procedure related pregnancy loss and complications from pregnancy termination. The final cost of a strategy will be a sum of the different components of this pathway and we have included these possible consequences in our outcome analyses. We assumed a procedure-related fetal loss rate of 1% after CVS or amniocentesis (Table 4a).
RESULTS
The probabilities and costs were applied to the population of the Czech Republic, in which there are approximately 100,000 births per year. Under the baseline assumptions, the model favours strategy nine as the most cost effective trisomy 21 screening strategy (compared to no screening), as this strategy was the least expensive strategy per trisomy 21 cases averted. Although all the other strategies cost less, they all had lower trisomy 21 detection rates and higher numbers of procedure-related losses (except for strategies six and seven, which had same loss rate) compared with strategy nine.
When we repeated the analysis using strategy three which is the current practice in the Czech Republic with strategy nine, strategy nine remained the most cost-effective strategy. The results of the analysis evaluating clinical outcomes and costs are shown in Table 7. Figure 1 summarizes the incremental cost-effective (CZK/ /trisomy 21 case prevented).
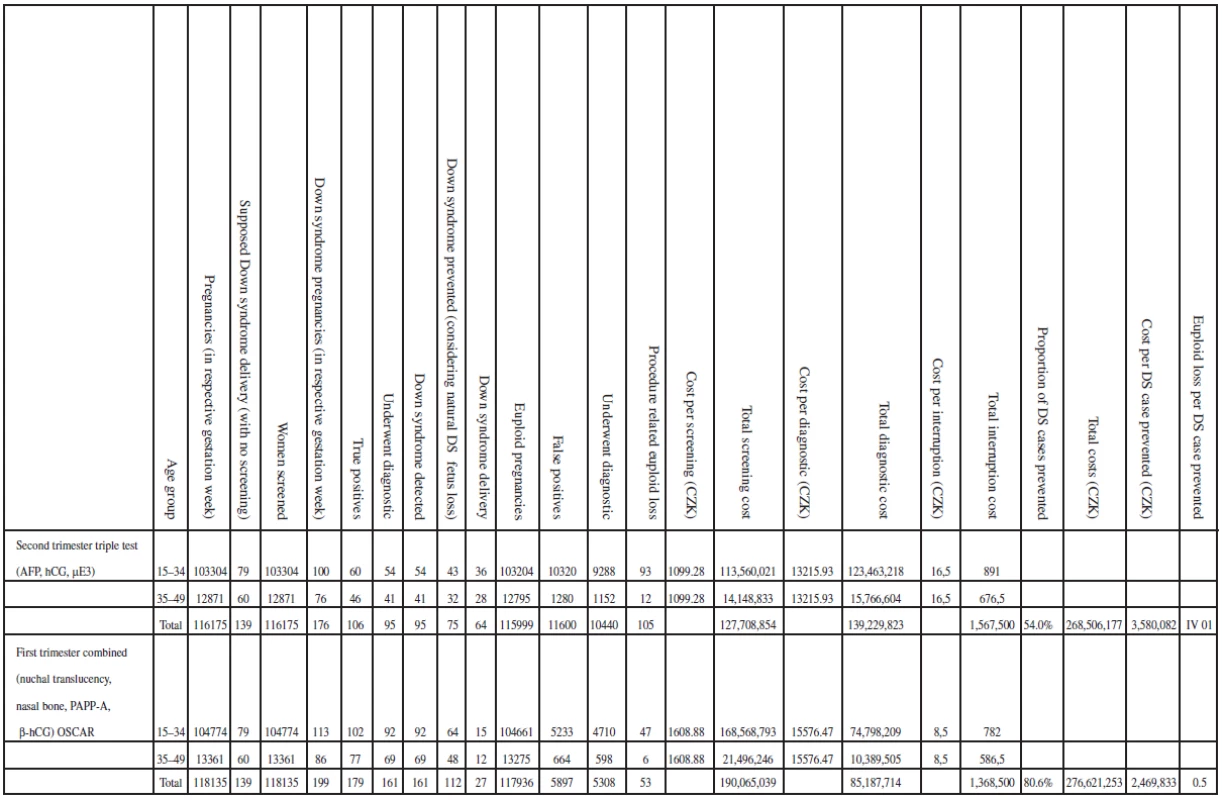

When considering total costs, it seems that age-based are actually cheaper, e.g. 191,864,433 for strategy 1, 268,506,177 for strategy 3, etc.; strategies 3, and 4 are similar to strategy 1 in cost per DS case prevented. Changing from strategy three [Second trimester triple test (AFP, hCG, μE3)] to strategy six [First trimester combined (nuchal translucency, PAPP-A, fβ-hCG)] results in the detection of 44 additional cases of trisomy 21. A change from strategy three to strategy eight [First trimester combined (nuchal translucency, nasal bone, PAPP-A, fbeta-hCG)] detected 53 additional trisomy 21 cases. The maternal age strategy in the first trimester, strategy three and strategy four (Nuchal translucency measurement) were approximately the same and were the most costly per Down syndrome case averted (see Figure 2).

The incremental cost-effectiveness (cost per additional trisomy 21 case prevented) was computed comparing two strategies, strategy nine and second trimester triple test (current practice in the Czech Republic). The computation yielded a baseline cost of 219 326 CZK per additional case of trisomy 21 (Down syndrome) detected by strategy nine (3 580 082 CZK for current practice and 2 469 833 CZK for our strategy). The strategy does not actually seem to save money but it is the most cost-effective. The strategy identified the greatest number of trisomy 21 fetuses (161) and had the lowest ratio of procedure-related loss to trisomy 21 cases that were identified at 0,5 (1,4 for triple test and around 2 for age). At the end point of cost estimate, the strategy costs less to diagnose one case of trisomy 21 (see Table 7 and Figure 1).
Figure 2 presents the incremental cost-effectiveness ratio, the additional cost per additional live born baby with trisomy 21 prevented, by adapting a more effective screening strategy in strategy nine. When the cost of a dating scan was not included in the first trimester examinations, all examinations in the first trimester became more cost-effective. Adding the nasal bone and the OSCAR manner made strategy nine the most cost-effective one. Clinical outcomes and costs from baseline analysis of the cost-effectiveness of trisomy 21 screening, diagnosis and cost per Down syndrome prevented for all the strategies is summarized in Table 8.
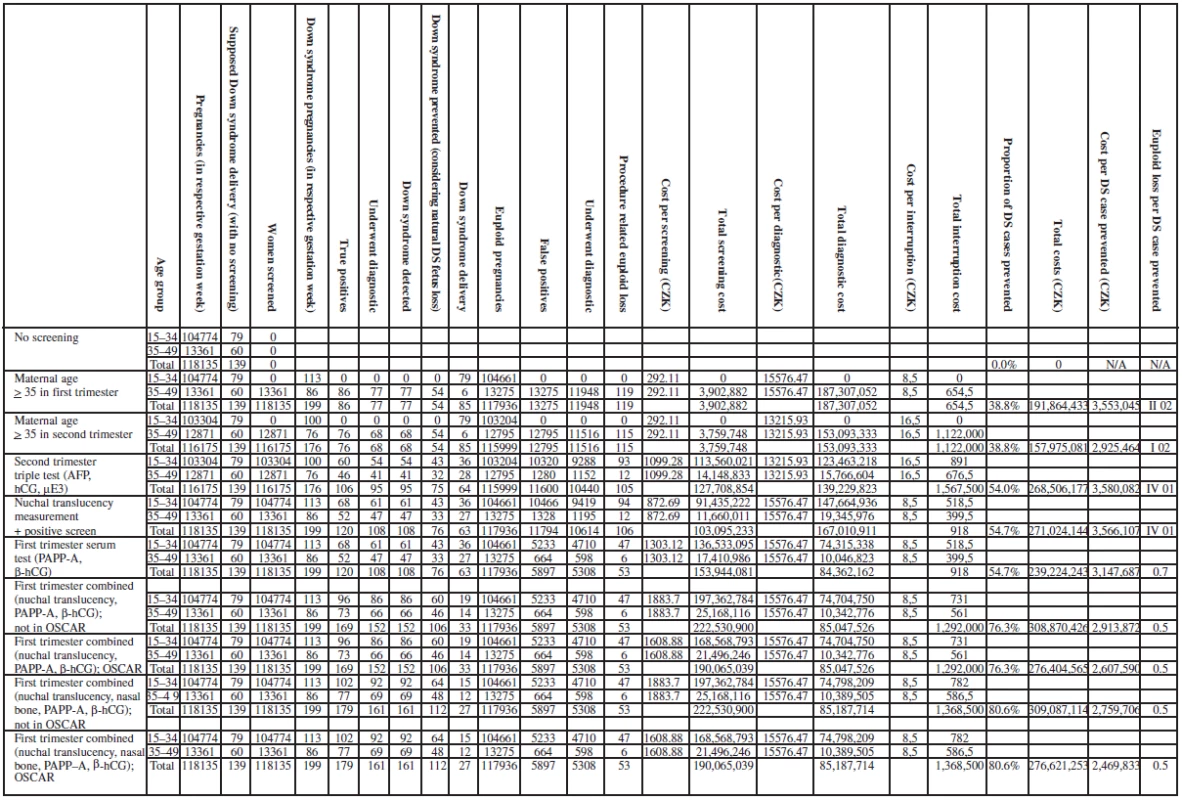
Figure 3 shows the safety-effectiveness of the screening strategies in our analysis. It clearly represents the number of miscarriages caused per additional baby with trisomy 21 prevented by adapting a more effective screening strategy.

The results are very sensitive to any minor change of the parameters and are exposed to a certain degree of uncertainty in specificity estimates. The false positive rate and the detection rate for each of the screening strategy is shown in Figure 4.

DISCUSSION
Many trials have examined the sensitivity and specificity of NT and screening in the first trimester and its impact when adapted as universal screening modality has been determined. The current study finds that the NT and first-trimester serum screen used alone or in combination with other ultrasound markers can be used to diagnose more trisomy 21 foetuses in a cost-effective manner. The model used in this study considers the application of the screening to the entire population of the Czech Republic, where the birth rate is around 100,000 annually.
The other benefits of this method of screening, including improved gestational dating, detection of other chromosomal and other congenital abnormalities, and the general reassurance of the pregnant women of seeing the viable fetus as early as possible, were not estimated.
In our analysis the first trimester, the combined NT, NB, PAPP-A and fβ-hCG test strategy was the most cost-effective screening strategy for trisomy 21. It has the highest detection and lowest false positive rates. The ratio of trisomy 21 cases prevented to euploid pregnancy losses was clearly in favour of this strategy. The analysis also showed that changing from current practice of triple test screening in the second trimester to first trimester combined NT, PAPP-A and fβ hCG test or combined NT, NB, PAPP-A and fβ -hCG test, will result in higher numbers of trisomy 21 cases being prevented and in societal cost savings. Applying these strategies in an OSCAR manner will be even more cost-effective by saving the cost of a separate dating scan.
Our baseline findings agree with some previously published cost-effectiveness studies which showed that first-trimester screening using NT and maternal serum was the most cost-effective strategy. Others found sequential (CVS for women with positive first trimester screening and triple test for the rest) or integrated (combining the first and second trimester results, giving the results after performing the serum triple test) to be the most cost-effective strategy, but they also noted that this strategy was associated with the highest miscarriage rate [41, 42]. In our study the NT was of no more cost-effective than the triple test.
We used Medical care costs in our analysis rather than charges as some investigators have used in previous studies [14, 16]. Using the Medical care National Fee is a more accurate estimate of cost of the service compared with using hospital charges and is, therefore, more representative of the societal burden [16, 41]. In addition our maternal age strategy, incorporating the use of advanced maternal age (35 years) as a screening technique more closely reflected current clinical practice.
We must emphasize that cost analyses involving prenatal screening and diagnosis is based on some underlying assumptions that the clinician or patient must consider in deciding which screening strategy to use and the wellingness of the society to pay [43].
In the Czech Republic, society and the state are willing to pay all of these costs. For this reason and for the purpose of doing our work more economically and more time effectively, strategy nine seems to be the ideal one.
This analysis is the first one in the Czech Republic to research the cost-effective of prenatal diagnosis. There is limited information on the sensitivity and specificity of options of other strategies at the local level and on the current practice. Our analysis was limited to trisomy 21 screening, and the results can be generalized to some other trisomies as well (18 and 13).
This cost-effectiveness analysis determined that the fbeta-hCG or NT, NB, PAPP-A and fβ-hCG strategy is the most cost-effective and it is more effective than the current practice in Czech Republic.
Ishraq Dhaifalah, MD, Ph.D.
Department of Medical Genetic and Fetal Medicine
University Hospital
I. P. Pavlova 6
557 52 Olomouc
Czech Republic
ishraq.dhaifalah@gmail.com
Sources
1. Biagiotti, R., Brizzi, L., Periti, E., et al. First trimester screening for Down’s syndrome using maternal serum PAPP-A and free β-hCG in combination with fetal nuchal translucency thickness. BJOG 1998, 105, p. 917–920.
2. Biggio, JR. Jr., Morris, TC., Owen, J., Stringer, JS. An outcome analysis of five prenatal screening strategies for trisomy 21 in women younger than 35 years. Am J Obstet Gynecol, 2004, 190, p. 721–729.
3. Canick, JA., Kellner, LH. First trimester serum screening for aneuploidy: serum biochemical markers. Semin Perinatol, 1999, 23, p. 359–368.
4. Caughey, AB., Kuppermann, M., Norton, ME., Washington, AE. Nuchal translucency and first tirmester biochemical markers for Down syndrome screening: a costefectiveness analysis. Am J Obstet Gynecol, 2002, 186, p. 1239–1245.
5. Caughey, AB., Kuppermann, M., Norton, ME., Washington, AE. Nuchal translucency and first trimester biochemical markers for Down syndrome screening: A costeffectiveness analysis. Am J Obstet Gynecol, 2002, 187, p. 1239–1245.
6. Cheng, E., Luthy, D., Zebelman, A., Williams, M., et al. A prospective evaluation of a second-trimester screening test for fetal Down syndrome using maternal serum alpha-fetoprotein, hCG, and unconjugated estriol. Obstet Gynecol, 1993, 81, p. 72–27.
7. Cusick, W., Buchanan, P., Hallahan, TW., et al. Combined first-trimester versus second-trimester serum screening for Down syndrome: A cost analysis. Am J Obstet Gynecol, 2003, 188, p. 745–751.
8. DeBiasio, P., Siccardi, M., Volpe, G., et al. First-trimester screening for Down syndrome using nuchal translucency measurement with free b-hCG and PAPP-A between 10 and 13 weeks of pregnancy the combine test. Prenat Diagn, 1999, 19, p. 360–363.
9. deGraaf, I., Pajkrt, E., Bilardo, C., et al. Early pregnancy screening for fetal aneuploidy with serum markers and nuchal translucency. Prenat Diagn, 1999, 19, p. 458–462.
10. Dhaifalah, I., Dusek, L., Šantavý, J. Implementation of One-Stop-Clinic for Risk Assessment of chromosomal abnormalities in the first trimester, evaluating the effectiveness and making it part of our daily praktice. Čes. Gynek, 2011, 76, 4, p. 292–306.
11. Drugan, A., Greb, A., Johnson, MP., et.al. Determinants of parental decisions to abort for chromosome abnormalities. Prenat Diagn, 1990, 10, p. 483–490.
12. Economides, DL., Whitlow, BJ., Kadir, R., et al. First trimester sonographic detection of chromosomal abnormalities in an unselected population. BJOG, 1998, 105, p. 58–62.
13. Finkler, SA. The distinction between cost and charges. Ann Intern Med, 1982, 96, p. 102–109.
14. Gasiorek-Wiens, A., Tercanli, S., Kozlowski, P., et al. Screening for trisomy 21 by fetal nuchal translucency and maternal age: a multicenter project in Germany, Austria and Switzerland. Ultrasound Obstet Gynecol, 2001, 18, p. 645–648.
15. Gilbert, RE., Augood, C., Gupta, R., et al. Screening for Down’s syndrome: effects, safety and cost effectiveness of first and second trimester strategies. BMJ, 2001, 323, p. 423–425.
16. Gold, MR., Siegel, JE., Russell, LB., Weinstein, MC. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996.
17. Haddow, J., Palomaki, G., Knight, G., et al. Prenatal screening for Down.s syndrome with use of maternal serum markers. N Engl J Med, 1992, 327, p. 588–593.
18. Jorgensen, FS., Valentin, L., Salvesen, KA., et al. MULTISCAN, a Scandinavian multicenter second trimester obstetric ultrasound and serum screening study. Acta Obstet Gynecol Scand, 1999, 78, p. 501–510.
19. Kadir, RA., Economides, DL. The effect of nuchal translucency measurement on second trimester biochemical screening for Down’s syndrome. Ultrasound Obstet Gynecol, 1997, 9, p. 244–247.
20. Kellner, L., Weiss, R., Weiner, Z., et al. The advantages of using triple-marker screening for chromosomal abnormalities. Am J Obstet Gynecol, 1995, 172, p. 831–836.
21. Kornman, LH., Morssink, LP., Beekhuis, JR., et al. Nuchal translucency cannot be used as a screening test for chromosomal abnormalities in the first trimester of pregnancy in a routine ultrasound practice. Prenat Diagn, 1996, 16, p. 797–805.
22. Krantz, DA., Hallahan, TW., Orlandi, F., et al. Firsttrimester Down syndrome screening using dried blood biochemistry and nuchal translucency. Obstet Gynecol, 2000, 96, p. 207–213.
23. MacDonald, M., Wagner, R., Slotnick, R. Sensitivity and specificity of screening for Down syndrome with alpha-fetoprotein, hCG, unconjugated estriol, and maternal age. Obstet Gynecol, 1991, 77, p. 63–68.
24. Malone, FD., D’Alton, ME Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol, 2003, 102, p. 1066–1079.
25. Mujezinovic, F., Alfirevic, Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol, 2007, 110(3), p. 687–694.
26. Nicolaides, KH., Snijders, RJ., Cuckle, HS. Correct estimation of parameters for ultrasound nuchal translucency screening [letter]. Prenat Diagn, 1998, 18, p. 519–523.
27. Norgaard-Pedersen, B., Larsen, S., et al. Materna serum markers in screening for Down syndrome. Clin Genet, 1990, 37, p. 35–43.
28. Orlandi, F., Damiani, G., Hallahan, T., et al. First trimester screening for fetal aneuploidy: biochemistry and nuchal translucency. Ultrasound Obstet Gynecol, 1997, 10, p. 381–386.
29. Papageorghiou, AT., Avgidou, K., Bindra, R., et al. One stop clinic for assessment of risk (OSCAR) for trisomy 21 in singleton pregnancies at 11 to 13+6 weeks of gestation. Ultrasound Obstet Gynecol, 2005, 26(4), p. 375–375(1).
30. Phillips, O., Elias, S., Shulman, L., et al. Materna serum screening for fetal Down syndrome in women less than 35 years of age using sloha fetoprotein, hCG, and unconjugated estriol: a prospective 2-year study. Obstet Gynecol, 1992, 80, p. 353–358.
31. Shackley, P. Economic evaluation of prenatal diagnosis: a methodological review. Prenat Diagn, 1996, 16, p. 389–395.
32. Schafer, D., Arnemann, J., Brude, E., Baumann, R. Society must decide about prenatal diagnosis. Hum Reprod, 1995, 10, p. 768–769.
33. Snijders, RJM., Farrias, M., von Kaisenberg, C., Nicolaides, KH. Fetal abnormalities. In Nicolaides, KH. (ed.) Ultrasound markers for fetal chromosomal defects. Carnforth, UK: Parthenon Publishing, 1996. p. 1–62.
34. Spencer, K., Souter, V., Tul, N., et al. A screening program for trisomy 21 at 10.14 weeks using fetal nuchal translucency, maternal serum free ß-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol, 1999, 13, p. 231–237.
35. Spencer, K., Spencer, CE., Power, M., et al. Screening for chromosomal abnormalities in the first trimester using ultrasound and mterna serum biochemistry in a one stop clinic: A review of three years prospective experience. Br J Obstet Gynaecol, 2003, 110, p. 281–286.
36. Stenhouse, EJ., Crossley, JA., Aitken, DA., et al. First-trimester combined ultrasound and biochemical screening for Down syndrome in routine clinical practice. Prenat Diagn, 2004, 24, p. 774–780.
37. Subak, LL., Caughey, AB. Measuring cost-effectiveness of surgical procedures. Clin Obstet Gynecol, 2000, 43, p. 551–560.
38. Tabor, A., Vestergaard, CH., Lidegaard, R. Fetal loss rate after chorionic villus sampling and amniocentesis: an 11-year national registry study. Ultrasound Obstet Gynecol, 2009, 34(1), p. 19–24.
39. Vintzileos, AM., Anath, CV., Smulian, JC., et al. Cost-benefit analysis of prenatal diagnosis for Down syndrome using the British or the American approach. Obstet Gynecol, 2000, 95, p. 577–583.
40. Wald, NJ., Cuckle, HS., Densem, JW., et al. Maternal serum screening for Down.s syndrome: the effect of routine ultrasound scan determination of gestational age and adjustment for maternal weight. Br J Obstet Gynaecol, 1992, 99, p. 144–149.
41. Wald, NJ., Hackshaw, AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn, 1997, 17, p. 821–829.
42. Wald, NJ., Kennard, A., Hackshaw, A., McGuire, A. Antenatal screening for Down’s syndrome. J Med Screen, 1997, 4, p. 181–246.
43. Wald, NJ., Watt, HC., Hackshaw, AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med, 1999, 341, p. 461–467.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2012 Issue 1
-
All articles in this issue
- Urinary tract infections in women – possibilities of differentiated approach in treatment and prevention
- Phytoestrogenes in menopause: working mechanisms and clinical results in 28 patients
- The psychosocial needs of newborn children in the context of perinatal care
- To conclude knowledge about new colposcopic signs – ridge sign and inner border.
- Does the asymptomatic carriage of FV Leiden and FII prothrombin in heterozygous configuration represent the inccreased risk of thrombembolic complications during pregnancy, childbirth and postpartum?
- Risk factors for recurrent disease in borderline ovarian tumors
- Is the hysteroscopy the right choice for therapy of placental remnants?
- Non-invasive monitoring of the timing of early embryo cleavages – objectively measurable predictor of human embryo viability
- Genetic factors in etiology of uterine fibroids
- Invasive cervical cancer incidence in the span from the preventive check-up to the national screening programing in the Czech Republic
- Cost effectiveness, the economic considerations of prenatal screening strategies for trisomy 21 in the Czech Republic
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Is the hysteroscopy the right choice for therapy of placental remnants?
- To conclude knowledge about new colposcopic signs – ridge sign and inner border.
- Risk factors for recurrent disease in borderline ovarian tumors
- The psychosocial needs of newborn children in the context of perinatal care
