Establishment and evaluation of a post caesarean acute pain service in a perinatological center:
retrospective observational study
Zavedení systému léčby pooperační bolesti po císařském řezu v perinatologickém centru a jeho vyhodnocení:
retrospektivní observační studie
Cíl studie:
Cílem této studie bylo zjistit účinnost zavedení institucionalizované léčby pooperační bolesti po císařském řezu.
Typ studie:
Retrospektivní observační studie.
Název a sídlo pracoviště:
Fakultní nemocnice Brno.
Metodika:
Do studie byly zařazeny všechny pacientky, které podstoupily ukončení těhotenství císařským řezem v obdobích říjen 2009 až září 2010 a listopad 2010 až říjen 2011. V pooperačním období byla v předem stanovených intervalech měřena intenzita bolesti Vizuální analogovou škálou (VAS), počet přídavných analgetických požadavků, tlak krve, počet pulzů a byly zaznamenávány komplikace související s aplikací pooperační analgezie. Srovnali jsme hodnoty Vizuální analogové škály a počet přídavných analgetických požadavků ve skupině 212 žen před a 195 žen po zavedení institucionalizované léčby pooperační bolesti v prvních 72 hodinách po císařském řezu.
Výsledky:
Mezi jednotlivými skupinami byl statisticky významný rozdíl v intenzitě bolesti dle Vizuální Analogové škály (p<0,05). Počet přídavných analgetických požadavků od 24 hodin po operaci poklesl pod jeden požadavek ve všech skupinách. Epidurální analgezie byla shledána nejúčinnější metodou pro tlumení pooperační bolesti po císařském řezu v prvních 24 hodinách. V dalším období nebyl mezi jednotlivými analgetickými metodami rozdíl.
Závěr:
Implementace institucionalizované léčby pooperační bolesti po císařském řezu vede v pooperačním období ke snížení hladiny intenzity bolesti.
Klíčová slova:
systém léčby pooperační bolesti, pooperační analgezie, císařský řez, neopioidní analgezie, opioidní analgezie, epidurální analgezie
Authors:
P. Štourač 1,3,4,5; E. Kuchařová 4; I. Křikava 1,3,4; R. Malý 1,4; M. Kosinová 1,3,4; H. Harazim 1,4,5; O. Smékalová 1,4; I. Bártíková 1; R. Štoudek 1,4; Petr Janků 2,4
; Martin Huser 2,4
; K. Wágnerová 2,4; O. Haklová 3; L. Hakl 3,4; D. Schwarz 5; H. Zelinková 5; S. Littnerová 5; Jiří Jarkovský 5
; R. Gál 1,4; P. Ševčík 1,3,4,6
Authors‘ workplace:
Department of Anaesthesiology and Intensive Care Medicine, The University Hospital Brno, Czech Republic, Head of the department prof. MUDr. R. Gál, Ph. D.
1; Department of Obstetrics and Gynecology, The University Hospital Brno, Czech Republic, Head of the department prof. MUDr. P. Ventruba, DrSc., MBA
2; Department of Pain Control, The University Hospital Brno, Czech Republic, Head of the department MUDr. O. Haklová
3; Faculty of Medicine, Masaryk University, Kamenice, Brno, Czech Republic, Dean prof. MUDr. J. Mayer, CSc.
4; Institute of Biostatistics and Analyses, Masaryk University, Kamenice, Brno, Czech Republic, Director doc. RNDr. L. Dušek, Ph. D.
5; Department of Anaesthesiology and Intensive Care Medicine, Faculty Hospital Ostrava, Faculty of Medicine, University of Ostrava, Czech Republic, Head of the department prof. MUDr. P. Ševčík, CSc.
6
Published in:
Ceska Gynekol 2014; 79(5): 363-370
Overview
Objective:
The aim of this study was to determine the efficacy of establishing a Post Caesarean Acute Pain Service.
Design:
Retrospective observational study.
Setting:
University Hospital Brno.
Methods:
We evaluated all patients undergoing delivery via Caesarean Section under anaesthesia in the periods 10/2009 – 9/2010 and 11/2010 – 10/2011. During the postoperative period at predefined times, we measured the Visual Analogue Scale, Additional Analgesic Requests, blood pressure, pulse rate and recorded any complications. We compared the Visual Analogue Scale Score and number of Additional Analgesic Requests in two groups of women, 212 patients before and 195 patients after the establishment of an Acute Pain Service in the first 72 hours after Caesarean Section.
Results:
There was a statistically significant difference in Visual Analogue Scale Score between the groups (p<0.05). The number of Additional Analgesic Requests 24–72 hours after Caesarean Section decreased below one requirement per 24 hours. The most effective analgesic method after Caesarean Section during the first 24 hours postoperatively was epidural analgesia. There was no statistically significant difference 24–72 hours after Caesarean Section between the methods of analgesia used.
Conclusion:
In conclusion, implementation of a Post Caesarean Acute Pain Service led to decrease in Visual Analogue Scale Score postoperatively.
Keywords:
Acute Pain Service, postoperative analgesia, Caesarean Section, non-opioid analgesia, opioid analgesia, epidural analgesia
Introduction
The increase in number of Caesarean Sections (CS, 21%) in the Czech Republic [10] as well as globally [2, 23], makes it a priority to provide adequate postoperative analgesia for these patients. Although a large number of analgesic techniques are available for achieving satisfactory control of postoperative pain, a considerable number of women still complain of moderate to severe postoperative pain after CS [4]. Reported figures from the United States indicate an incidence of modera-te to severe pain in 50-70% of patients [1]. Studies explain this as lack of relevant education of the medical staff and that patients do not consider postoperative pain management important [16]. In one study patients reported good pain control during surgery and in the postoperative period as a priority after CS [3]. CS patients benefit from good analgesia as early mobilisation not only reduces the risk of thrombembolic events but also enables active care of the newborn [15].
A well-established Acute Pain Service (APS) should routinely assess pain intensity level, appropriately respond to the situation and determine whether analgesic interventions have led to adequate pain relief [8]. It is not acceptable to wait for the patients’ complaints or depend on strictly scheduled analgesic medication. Accreditation audits companies like JCI (Joint Commission International) and NIAHO (National Integrated Accreditation for Healthcare Organizations) also focus on postoperative pain management [7].
However, there are relatively few studies relating specifically to postoperative pain management after Caesarean Section [19]. This study describes the experience and results of specific aspects of APS, namely Post Caesarean APS (PCAPS).
The aim of this study was find out influence of Post Caesarean Acute Pain Service establishment on Visual Analogue Scale score level within 72 hours postoperative.
Materials and methods
The Ethics Committee for multicentric trials of the University Hospital in Brno approved this retrospective observational cohort study. Patient informed consent was not required due to the study characteristics (observational study). We obtained informed consent with statistical analysis of ano-nymized patient data. The study was registered on ClinicalTrials.gov (NCT02062320).
Inclusion Criteria
Caesarean Section performed from October 2009 to September 2010 on a group of women before the establishment of PCAPS (PRE-PCAPS group) and from November 2010 to October 2011 on a group after the establishment of PCAPS (POST--PCAPS group). Both groups completed a standardized “Evaluation of postoperative analgesia after Caesarean Section” form.
Primary outcome
Comparison of pain intensity using the Visual Analogue Scale (VAS) during the postoperative period (72 hours) between the PRE-PCAPS and POST-PCAPS groups at predefined times.
Secondary outcomes
Comparison of pain intensity according to postoperative analgesic method and method of anaesthesia for Caesarean Section in both groups. Achievement VAS score under 4 (satisfactory pain relief). Additional Analgesic Requests (AAR) Count. Analgesia related complication rate.
Observed parameters
Maternal age, indication for CS (acute, elective), type and details of anaesthesia for CS, date and end time of CS, information about administering the obstetric analgesia and designed method for postoperative analgesia including a detailed description of application. The VAS score was recorded at rest and during movement (the higher score was recorded) at predefined times: 0, 1, 3, 6, 9, 12, 18, 24, 48 and 72 hours after CS (0 no pain; 10 agonizing pain). All measures were recorded by the medical staff on a structured evaluation form for postoperative pain after Caesarean Section.
Anaesthesia protocol – general anaesthesia
General anaesthesia induction: thiopentone 5 mg.kg-1 IV, suxamethonium 1 mg.kg-1 IV.
General anaesthesia maintaining: sevoflurane up to 1 MAC, N2O up to 50%, sufentanil 15–25 mcg IV, rocuronium 0.3 mg.kg-1 IV or vecuronium 0.03 mg.kg-1 IV.Neuromuscular blockade active reversal: in case of clinical signs of residual neuromuscular blockade, neostigmine 0.03 mg.kg-1 with atropine 0.01 mg.kg-1 IV.
Anaesthesia protocol – spinal anaesthesia
Detection of intrathecal space in L3/4 or L4/5 intervertebral space with spinal needle G26 or G27 and administration of plain bupivacaine 12.5–15 mg.
Anaesthesia protocol – epidural anaesthesia
Detection of the epidural space using the Loss of Resistance method and Tuohy needle G18 in L2/3 or L3/4 intervertebral space. Insertion of epidural catheter G20 and administration of 0.5% bupivacaine 16–18 mL with sufentanil 10 µg.
Analgesic protocol after CS under general anaesthesia
Combination of paracetamol 1000 mg (IV or orally) every 8 hours with diclofenac 100 mg supp. or IM every 12 hours.
Analgesic protocol after CS under spinal analgesia
In case of a VAS>4, paracetamol 1000 mg IV, diclofenac 50 mg IM or metamizole 1.0 g IV were applied as needed.
Analgesic protocol for epidural analgesia
Patient Controlled Epidural Analgesia (PCEA): bupivacaine 0.125% with sufentanil 0.5 mcg/mL; basal rate 6 mL.hour-1, lock-out interval 45 minutes; PCEA bolus 4 mL. In the case of failure to reach a VAS<4 even after bolus application, the basal dose was increased in steps of 2 mL up to a total basal rate of 10 mL.hour-1.
Continual epidural analgesia: bupivacaine 0.125% with sufentanil 0.5 mcg.mL-1; basal rate 6 mL/hour. In the case of failure to reach a VAS<4, the basal dose was increased in steps of 2 mL up to total basal rate 10 mL.hour-1 after 4 mL bolus administration.
Rescue analgesics for every group
Rescue analgesic for breakthrough pain was metamizole IV.
Complications related to analgesiaadministration
During the entire administration of the analgesia, any adverse effects were monitored and reported in association with the administration of the analgesia. These were, in particular, nausea, vomiting, paraesthesia, tingling of the extremities, post-dural puncture headaches, hypotension (systolic blood pressure under 90 mm Hg), hypertension (systolic blood pressure above 160 mm Hg), tachycardia (>100 beats per minute), bradycardia (<50 beats per minute), hypoventilation (breaths under 10 per minute).
Continuous APS service
We focused on pain relief throughout the whole peripartum period. Dial the intrahospital cell phone code was the way to contact the PCAPS team for consultation on all cases of unsatisfactory analgesia (VAS>4) and any other complications related to the analgesia after CS. Two levels of consultations were established (1st full qualified anaesthesiologist; 2nd algesiologist). We offered both outpatient consultations to pregnant women with chronic pain at the Department of Pain Control and at the anaesthesia outpatient clinic of the Department of Anaesthesiology before elective CS.
Statistical Analysis
The data were summarised as means, medians, standard deviation, frequency (MS Excel 2007). We used the Mann-Whitney test to evaluate efficacy between the groups with VAS score as dependent variable. We compared the average VAS score and the AAR count in groups in the first 72 hours after CS using the Kruskal-Wallis ANOVA test. We also used Pearson Chi-square test for categorical variables. We used the software Statistica 10 (StatSoft, Czech Republic). The level of significance chosen was 5%.
Results
We enrolled 212 women into the PRE-PCAPS group (PRE-PCAPS, n=212) and 195 women into POST-PCAPS group (POST-PCAPS, n=195). A basic description of the groups is shown in Table 1.
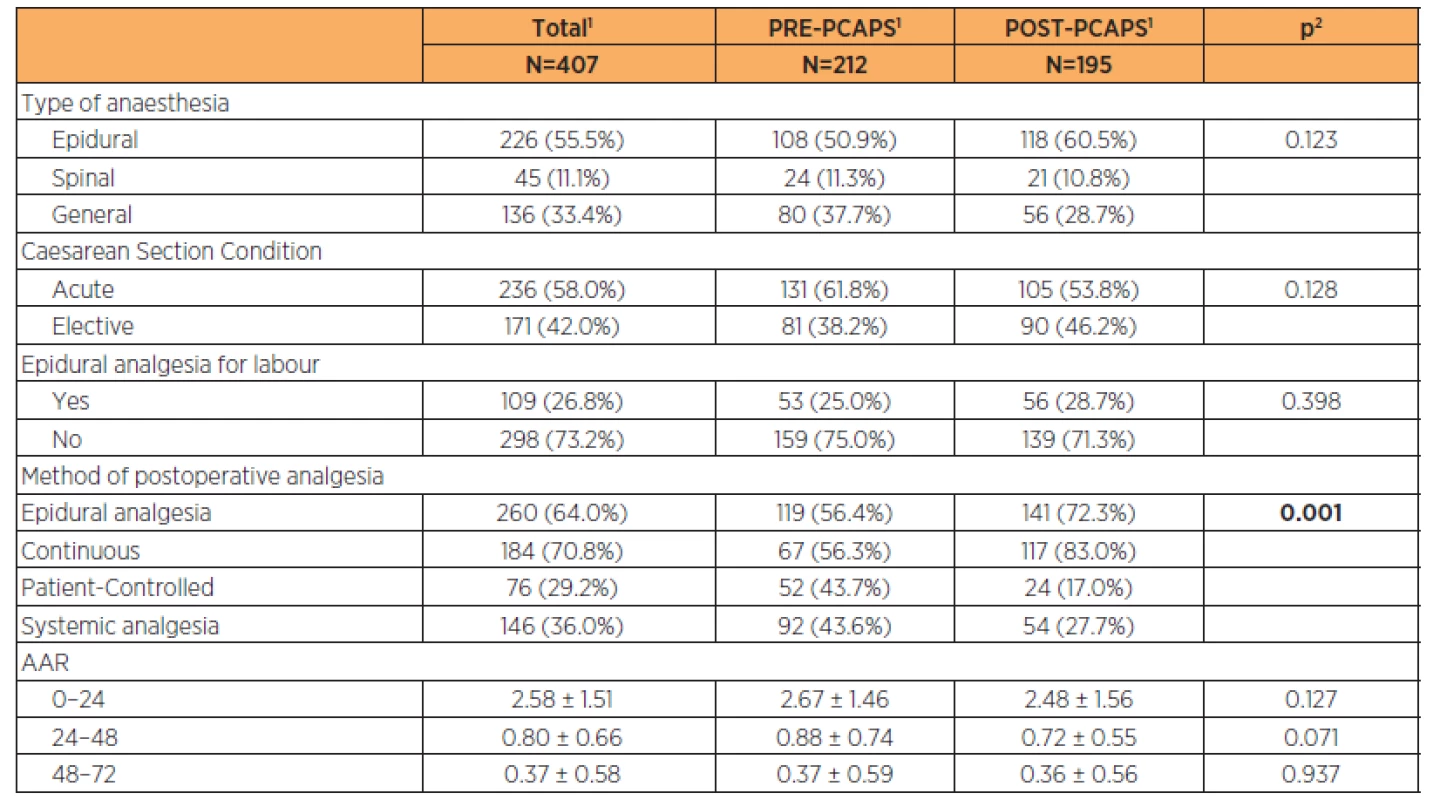
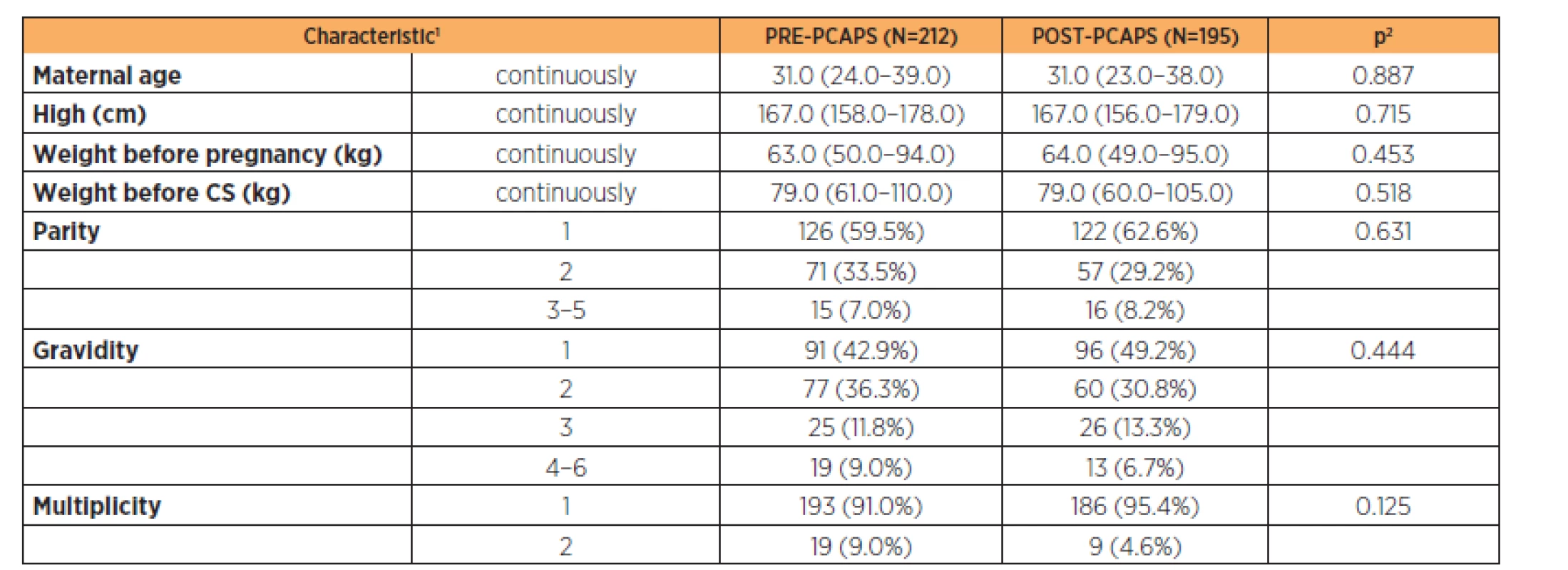
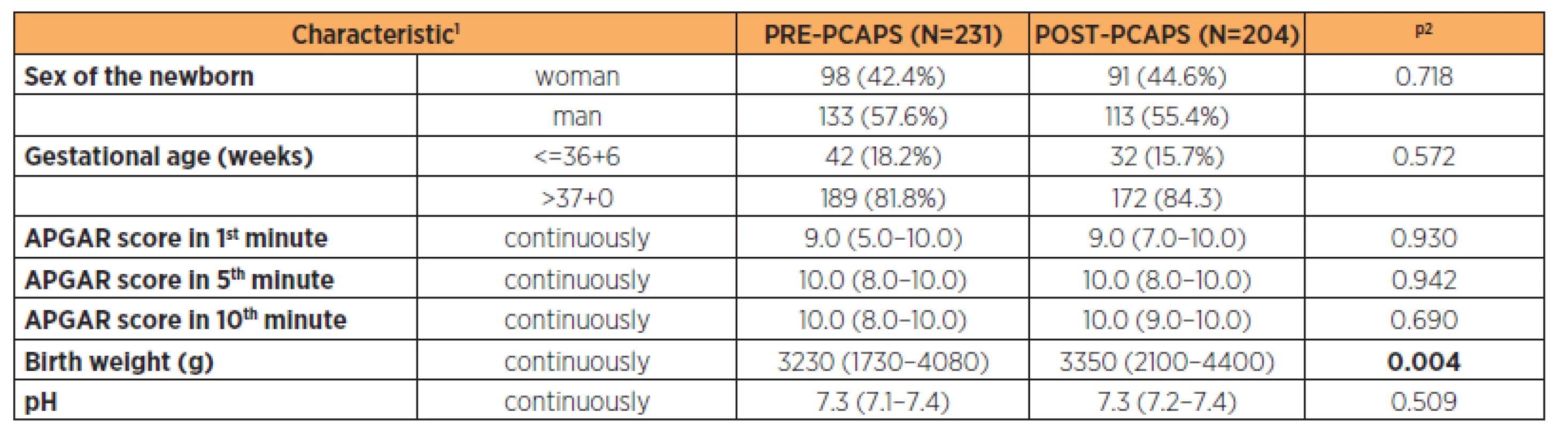
Parturient characteristics are described in Table 2 (the groups were comparable). Newborn characteristics are shown in Table 3. There was a statistically significant difference in the birth weight between groups (3200 g vs 3350 g) but this was not clinically significant.
Only 1 woman in the PRE-PCAPS group had no analgesia in the whole postoperative period. This patient had a VAS score below 3 throughout.
In the PRE-PCAPS group, the PCEA mode of analgesics administration chosen was more frequently epidural catheter compared to the POST-PCAPS (52 women (43.7%) versus 24 (17%), p=0.001).
Comparison of analgesic effectiveness
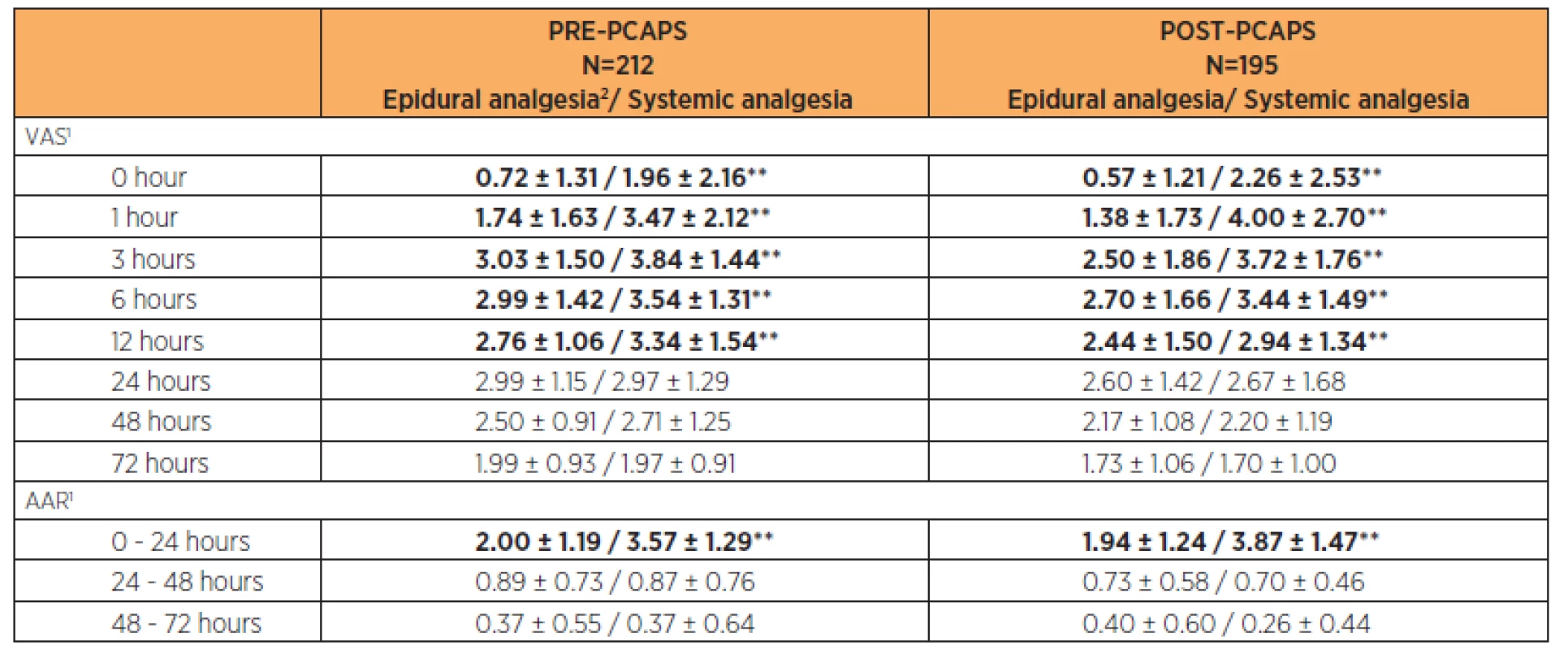
Comparing the VAS score between the two groups, the POST-PCAPS group achieved a lower VAS level. This was statistically significant (p<0.05) from 1 hour postoperatively (Figure 1). There were no differences in AAR administration 72 hours postoperatively (Table 1). In both groups throughout the observed period, we reported an average VAS level below 4 (Fig. 1, Table 4, Table 5). This suggests satisfactory pain relief.

Analgesic effectiveness according to the type of anaesthesia for CS
General anaesthesia (GA), epidural anaesthesia or spinal anaesthesia was used for Caesarean Section. There were statistically significant (p<0.05) differences in pain intensity according to anaesthesia choice at times 0, 1, 3 and 6 hours after CS. It was statistically significant also at 12 hours after CS in the PRE-PCAPS group. The results are shown in Table 4.
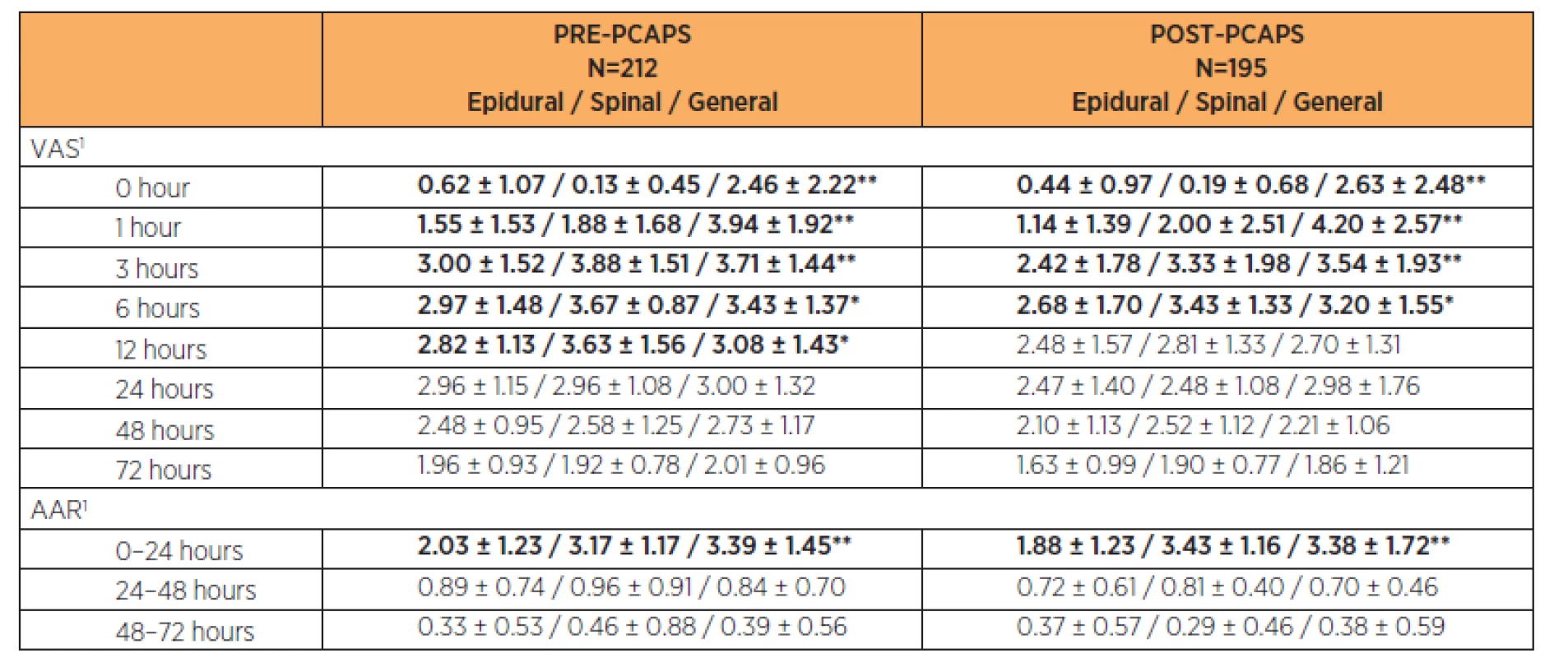
Additional analgesic requests (AAR) within the first 24 hours showed a statistically significant lower number in both groups after epidural anaesthesia (p<0.05). There was no statistically significant difference in number of AARs between particular types of anaesthesia (p>0.05) in the period 24–72 hours after CS. After the 24th hour after CS the number of AAR decreased below one per 24 hours on average in both groups. The results are shown in Table 4.
Analgesic effectiveness according to choice of method of postoperative analgesia
In both PRE-PCAPS and POST-PCAPS groups, we recorded epidural analgesia (PCEA, continuous) and systemic analgesia. For the different types of epidural analgesia, there were no statistically significant differences in analgesic effectiveness throughout the postoperative period (p>0.05). A statistically significant difference (p<0.01) was found between epidural analgesia and systemic analgesia in the period 0–12 hours after CS. The number of AARs was significantly lower for epidural forms of analgesia in the first 24 hours after CS (p<0.01). The results are shown in Table 5.
Complications of postoperative analgesia
There was a low incidence of complications related to postoperative analgesia in both groups. There were no fatal or life-threatening complications. In the PRE-PCAPS group there were 15 complications recorded (7.1%). In the POST-PCAPS group, there were 11 complications (5.6%) with no statistically significant difference between groups. Most often we recorded residual motoric blockade of the lower limbs in the PRE-PCAPS group (6/15, 40%) and post-dural puncture headaches (3/15, 20%). In the POST-PCAPS group this was tingling of the extremities (2/11, 18.2%) and non-specific complaints (7/11, 63.6%).
Discussion
The main finding of this study was confirmation of the analgesic efficacy of post-caesarean acute pain service establishment. This was very likely due to the more systematic approach to pain relief after algesiological audit and the preference for neuroaxial (epidural) blockade for Caesarean Section and subsequent epidural analgesia in the POST-PCAPS group.
The groups were comparable (Table 1 and 2). The only exception was the birth weight in newborns where we can see a statistically significant difference but with no clinical significance. Overall, the gestational age was comparable for both groups (Table 3).
In this study, most CSs were unscheduled (61.8% in PRE-PCAPS, 53.8% in POST-PCAPS). Similar results were reported in the OBAAMA-CZ study (57% unscheduled procedures) [20]. A similar study reported by Samina et al. [19] included only elective CS which was surprising. Another limitation of that study is the absence of comparison of data with administration of anaesthesia and absence of patient evaluation of satisfaction with analgesia.
A good sign, considering the findings of the PRE-PCAPS data audit, is the greater tendency to epidural anaesthesia for Caesarean Section (PRE-PCAPS 50.9%, POST-PCAPS 60.5%). This was followed by a general trend toward epidural analgesia use after Caesarean Section (PRE-PCAPS 56.4%, POST-PCAPS 72.3%). The most frequently used method of epidural analgesia was continuous epidural blockade (56.3% in PRE-PCAPS, 83% in POST-PCAPS; Table 1). The reason for this was simple application with continuous infusion pump and possibility of individualization of the administered dose according to the established protocol. Two PCEA pumps were available in our hospital for the postoperative room of the Department of Obstetrics and Gynecology at the time of PRE-PCAPS part. One was preferably allocated for remifentanil labour analgesia, a very promising new method of labour analgesia in case of contraindication of neuroaxial blockade [21]. Since greater analgesic effectiveness of this form has not been demonstrated, one of the PCEA pumps was dislocated at the delivery room for obstetric analgesia.
In PCAPS after CS in GA we administered a combination of paracetamol, diclofenac and metamizole. This approach was based on the Miranda et al. study [13]. The results of this study supported the clinical use and supra additive effect of a combination of paracetamol and metamizole or paracetamol and diclofenac. There was also successfully used the combination of either tramadol or paracetamol with diclofenac in post-caesarean pain management after spinal anaesthesia in Mitra et al. study [14]. The distribution of the information about the method of anaesthesia and indication for postoperative analgesia through a standard PCAPS form were great advantages. Before PCAPS, establishing analgesics used to be prescribed, often under heavy workload of the obstetric physician, late from a ward round and the administration of analgesia was delayed, which correlates with experience elsewhere. This method can be considered an “Anaesthesiologist Based Acute Pain Service” [17].
Most of the complications associated with applied analgesia were due to the adverse effects of the epidural analgesia. With complete spontaneous reversal of blockade, however, all described problems were resolved. Post-dural puncture headaches were reported in the PRE-PCAPS group in three cases (1.4%), which correlates with the incidence of about 1% in general obstetric practice [5]. Based on the results of the PRE-PCAPS group we determined the maximum diameter of the needle at G26 for spinal anaesthesia in CS. In the POST-PCAPS group there was no reported case of post-dural puncture headache. The significance of this finding is limited by the low incidence of this complication.
Both number of additional analgesic requests and VAS score rapidly decreased in the period 12–24 hours postoperatively up to less than one requirement per 24 hours [4]. Due to this fact and due to a higher incidence of complications associated with epidural catheter, which depends on the length of the implementation, it appears unnecessary to load the epidural catheter with a view to only controlling postoperative pain after CS [18]. This study showed that clinically good pain control can be achieved with a minimum of adverse effects using non-opioid analgesics as well [14]. Fear of the transfer of analgesics into breast milk in the case of its systemic use is certainly understandable. However in the immediate postoperative period when breast-feeding is only starting the exposure dose is small [22]. Most recommendations are to avoid non-opioid analgesics, especially the non-steroidal antiinflammatory drug group (NSAIDs), in the peripartal period. For this reason these recommendations apply to the prenatal period [12].
McDonnell et al. [11] speculated about the “gold standard” in analgesia after Caesarean Section and noted that in the multimodal approach there is no gold standard in the control of postoperative pain management after CS. In reality, compared to many countries, our country rarely uses intrathecal or epidural administration of opioids without local anaesthetic [6]. The preference of Czech hospitals [20], including our own, is a combination of local anaesthetic in low concentration (0.125 % bupivacaine) with opioid to reduce the incidence of complications associated with local anaesthetic (motor block, toxicity) [9] or opioids (respiratory depression, pruritus).
Limitations
The study omitted the relationship between acuteness of the procedure and pain perception and this may be a limitation.
Conclusion
In conclusion, implementation of a Post Caesarean Acute Pain Service led to decrease in the Visual Analogue Scale Score postoperatively.
Acknowledgments
Financial support from the Czech Ministry of Health Internal Grant Agency – project NT 13906-4/2012.
ClinicalTrials.gov ID: NCT02062320.
Martina Kosinová, MD
Department of Anaesthesiology and Intensive Care Medicine
Faculty of Medicine, Masaryk University
The University Hospital Brno
Jihlavska 20
625 00 Brno
Czech Republic
e-mail: mata.kosinova@gmail.com
Sources
1. Apfelbaum, J., Chen, C., Mehta, S., Gan, T. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg, 2003, 97, p. 534–540.
2. Betran, A., Merialdi, M., Lauer, J., et al. Rates of caesarean section: Analysis of global, regional and national estimates. Paediatr Perinat Epidemiol, 2007, 21, p. 98–113.
3. Carvalho, B., Cohen, S., Lipman, S., et al. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg, 2005, 101, p. 1182–1187.
4. Dolin, S., Cashman, J., Bland, J. Effectiveness of acute postoperative pain management. I. Evidence from published data. Br J Anaesth, 2002, 89, p. 409–423.
5. Gleeson, C., Reynolds, F. Accidental dural puncture rate in UK. Obstetric practice. Int J Obs Anesth, 1998,7, p. 242–246.
6. Chen, B., Kwan, W., Lee, C., Cantley, E. A national survey of obstetric post-anaesthesia care in teaching hospitals. Anesth Analg, 1993, 76, Suppl. 43.
7. JCAHO alert gives new recommendations for PCA. Hosp Peer Rev, 2005, 30, p. 24–25.
8. Karlsten, R., Strom, K., Gunningberg, L. Improving assessment of postoperative pain in surgical wards by education and training. Qual Saf Health Care, 2005, 14, p. 332–335.
9. Křikava, I., Jarkovský, J., Štourač, P., et al. The effects of lidocaine on bupivacaine-induced cardiotoxicity in the isolated rat heart. Physiol Res, 2010, 59, Suppl. 1, p. 65–69.
10. Mardešićová, N., Velebil, P. [Epidemiology in Caesarean Section]. Postgraduální medicína, 2010, 2, p. 171–174. [Article in Czech]
11. McDonnell, N., Keating, M., Muchatuta, N., et al. Analgesia after caesarean delivery. Anaesth Intensive Care, 2009, 37, p. 539–551.
12. McWhorter, J., Carlan, S., O‘Leary, J., et al. Rofecoxib versus magnesium sulfate to arrest preterm labor: randomized trial. Obstet. Gynecol., 2004, 103, p. 923–930.
13. Miranda, H., Puig, M., Prieto, J., Pinardi, G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain, 2006, 121, p. 22–28.
14. Mitra, S., Khandelwal, P., Sehgal, A. Diclofenac-tramadol vs. diclofenac-acetaminophen combinations for pain relief after caesarean section. Acta Anaesthesiol Scand, 2012, 56, 6, p. 706–711.
15. Pan, P. Post cesarean delivery pain management. Multimodal approach. Int J Obstet Anesth, 2006, 15, p. 185–188.
16. Rawal, N. 10 years of acute pain services. Achievements and challenges. Reg Anesth Pain Med, 1999, 24, p. 68–73.
17. Ready, L., Oden, R., Chadwick, H., et al. Development of an anesthesiology based postoperative pain management service. Anesthesiology, 1988, 68, p. 100–106.
18. Royakers, A., Willigers, H., van der Ven, A., et al. Catheter-related epidural abscesses—don’t wait for neurological deficits. Acta Anaesthesiol Scand, 2002, 46, p. 611–615.
19. Samina, I., Khurram, S., Faraz, S. Observational study to assess the effectiveness of postoperative pain management of patients undergoing elective cesarean section. J Anaesthesiol Clin Pharmacol, 2012, 28, 1, p. 36–40.
20. Štourač, P. [Obstetric Anaesthesia and Analgesia Month Attributes – real message of anesthetic practice in Czech maternity wards]. Anest Intenziv Med, 24, 2013, 2, p. 81–82. [Article in Czech]
21. Štourač, P., Suchomelová, H., Stodůlková, M., et al. Comparison of Parturient-Controlled Remifentanil with Epidural Bupivacain and Sufentanil for Labour Analgesia: Randomised Controlled Trial. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014, 158, 2, p. 227–232.
22. The American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics, 2001, 108, p. 776–785.
23. Villar, J., Valladares, E., Wojdyla, D., et al. Caesarean delivery rates and pregnancy outcomes: The 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet, 2006, 367, p. 1819–1829.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2014 Issue 5
-
All articles in this issue
- Vaginal breech delivery after 36 week of pregnancy in a selected group of pregnancy – analysis of perinatal results in years 2008–2011
- The influence of breach position of the second twinon perinatal outcomes in vaginal births of bichorial - biamniotic twins after 33rd week of gravidity
- Preeclampsia in pregnancy – prediction, prevention and further management
- Psychosomatic aspects and antipsychotics medication in ethiopathogenesis of endometrial carcinoma
- Genetic aspects of pelvic floor defects and stress urinary incontinence in women
- Incidence and therapy of lymphoceles after pelvic and paraaortic lymph node dissection – our file
- Extramammary Paget´s disease of the vulva – a case report
- Condylomata acuminata (genital warts)
- Herbal therapy during pregnancy – myths and facts
- Eminent gynecologists and obstetricians from Klatovy and surroundings
-
Establishment and evaluation of a post caesarean acute pain service in a perinatological center:
retrospective observational study - Tailoring surgical treatment of cervical precancerosis
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Condylomata acuminata (genital warts)
- Preeclampsia in pregnancy – prediction, prevention and further management
- Vaginal breech delivery after 36 week of pregnancy in a selected group of pregnancy – analysis of perinatal results in years 2008–2011
- Extramammary Paget´s disease of the vulva – a case report
